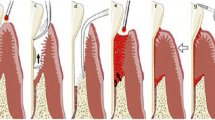
Abstract
Therapy for periodontal and peri-implant disease continues to evolve with new methodologies, medications, and instrumentation added to the conventional armamentarium. Dental lasers have been used both adjunctively and alone in the protocol. Clinical studies and basic investigations have shown that laser photonic energy has been a useful addition to increase the effectiveness and outcomes of treatment of the disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Core MessageTherapy for periodontal and peri-implant disease continues to evolve with new methodologies, medications, and instrumentation added to the conventional armamentarium. Dental lasers have been used both adjunctively and alone in the protocol. Clinical studies and basic investigations have shown that laser photonic energy has been a useful addition to increase the effectiveness and outcomes of treatment of the disease.
1 Introduction
The periodontium is essential for optimal oral function and health. Any inflammation will affect both soft and hard tissue and could lead to loss of those structures. Periodontal disease is an infection whose primary etiologic factor is the oral pathogens existing in the plaque biofilm. Initially the gingiva will become inflamed without attachment loss, and the disease is thus termed gingivitis. With increasing pathogen invasion, there will be loss of connective tissue attachment as well as apical migration of the epithelial tissue with subsequent infection and resorption of the alveolar bone. Chronic periodontitis is a slowly progressing disease and one of the most commonly occurring diseases in middle-aged adults [1].
Moreover, periodontal disease has been linked with other systemic diseases. Oral pathogens can migrate through the inflamed and ulcerated gingival epithelium into the rest of the body. There are suggested clinically important associations between periodontal bacteria and conditions ranging from peripheral artery disease, liver cirrhosis, and chronic kidney disease to other systemic disorders including cardiovascular, respiratory, and osteoarticular problems. These connections highlight the importance of treating this disease because of its implication on general medical health. Interestingly, the reverse association is also important: the patient’s age, smoking habits, and the presence of diabetes can worsen chronic periodontal inflammation.
Putative periodontal pathogens, such as Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis, have long been considered the primary contributors to the disease. However, the red complex especially three species—Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola—are now regarded as the most pathogenic and are prevalent in biofilm. Unfortunately, determining which organisms are important can be a daunting task: there can be several species of pathogens in any one site; some can be more opportunistic than others and may proliferate subsequent to the initial inflammation rather than cause it; and the patient’s immune response can vary.
Peri-implant disease shares the same etiology—pathogenic microorganisms—and the literature is beginning to report significant statistics that indicate many implant sites will develop the disease [2]. For clarity, soft tissue inflammation is termed peri-implant mucositis, whereas implant bone loss is termed peri-implantitis.
The general understanding is that the gold standard for successful treatment of these diseases is gain in clinical attachment level. The root surface should be restored to biocompatibility to reestablish that attachment without the presence of inflammation [3, 4]. There are however other clinical creditable endpoints such as lack of bleeding on probing, complete removal of root accretions, and measureable regeneration of bone, periodontal ligament, and cementum. Of course, the patient’s oral hygiene improvement and reduction of other risk factors can also be considered and are crucial for maintenance of a stable periodontium.
This chapter will be divided into three modalities of treatment utilizing lasers in nonsurgical, surgical, and antimicrobial photodynamic modalities. These methods are separate therapies, but may be combined to produce the best result. A nonsurgical protocol is the first approach, but surgery may follow to help unresolved problems. Photo-activated medications can be a useful addition for either procedure. Since periodontal disease can have episodic progression, one or more of these treatments may be employed for the current stage of the disease.
2 Nonsurgical Periodontal and Peri-implant Disease Laser Therapy
2.1 Description of Non-surgical Therapy
The term «nonsurgical therapy» is defined as a protocol to remove as much calculus as possible, to disrupt or eliminate the biofilm and accompanying microbes, and to reduce inflammation contributing to periodontal and peri-implant disease as initial therapy. After this phase of treatment, the patient’s periodontal condition will be evaluated. Two possibilities then exist: one, the patient will receive periodontal maintenance, and two, a surgical procedure must be performed as a next step.
During initial nonsurgical therapy, it is essential that root/implant accretions be thoroughly removed; indeed conventional periodontal treatment begins with calculus and biofilm removal, using scaling instruments on the tooth surfaces while using carbon fiber or plastic curettes on the implant fixture. For the patient with gingivitis or beginning peri-implant mucositis, that procedure is very straightforward with ease of access. As the severity of the disease increases, root/implant debridement becomes more difficult. Studies have shown that some calculus remains, despite careful root planing or implant debriding; and treatment outcomes may not always be successful with deeper pockets [5,6,7]. Thus surgery would be necessary to access those areas, along with placing regenerative materials.
Another consideration is that conventional ultrasonic and sonic scalers used for subgingival debridement may not be effective to produce a bactericidal effect [8].
This initial therapy is usually performed in the general dentist’s clinic, if immediate referral to a periodontist is not indicated. Within that office setting, a dental hygienist may deliver all or part of the treatment in accordance with the scope of practice and other regulations governing his/her license. During any therapeutic session, the patient must be instructed in an effective oral hygiene regimen. Several appointments may be necessary to complete the initial nonsurgical protocol, and adequate evaluation periods will determine how successful the patient compliance and the practitioner’s efforts have been.
2.2 Laser Wavelengths That Can be Used
Dental lasers are generally used adjunctively for the above-described initial nonsurgical therapy [9]. For purposes of this section, the laser instrument described will have a minimum output of approximately 0.5 W of average power. This is to distinguish it from other lasers used for antimicrobial photodynamic therapy, described in the next section.
Any of the commercially available dental lasers can be utilized for nonsurgical periodontal or peri-implant disease therapy. At this date, the generic types and nominal emission wavelengths include diode (810, 940, 980, and 1,064 nm), Nd:YAG (1,064 nm), Er,Cr:YSGG (2,780 nm), Er:YAG (2,940 nm), and CO2 (9,300 and 10,600 nm.). For treatment of periodontal and peri-implant diseases, all of the above wavelengths can be used for debridement of the soft tissue side of the periodontal pocket; both erbium wavelengths are also currently indicated for calculus removal on the tooth structure. With the exception of Nd:YAG, there are no general contraindications for use of these wavelengths around implant fixtures. Studies have shown that the high peak power emission of the Nd:YAG laser with microsecond pulses caused melting on sandblasted, acid-etched, and titanium plasma-sprayed surfaces of titanium implants [10]. The details are described in ◘ Table 14.1.
2.3 Adjunctive Laser Use
The general principle of adjunctive laser use for periodontal and peri-implant disease therapy is to supplement conventional instrumentation in removing or disrupting the biofilm and calcified deposits. Conventional mechanical therapy of periodontal pockets does not necessarily achieve complete removal of bacterial deposits and toxins. Employing a laser has the potential to improve therapeutic results [11].
All dental lasers produce a temperature rise in the target tissue, which would affect the pathogens and the resulting inflammation. In general, most non-sporulating bacteria, including periodontopathic anaerobes, are readily deactivated at temperatures of 50 °C [12]. Coagulation of the inflamed soft tissue wall of a periodontal pocket and hemostasis are both achieved at a temperature of 60 °C [13]. It should be noted that surgical excision of soft tissue occurs at 100 °C; thus using a laser at these lower temperatures defines a nonsurgical therapy. When erbium lasers are used for calculus removal, the primary interaction occurs when the photonic energy vaporizes the interstitial water of the mineralized matrix at a minimum temperature of 100 °C. However, the rapid pulsing of those lasers used with water spray minimizes any significant temperature rise in the surrounding tissues.
Considering the microbial component, it follows that laser irradiation would have significant potential as an adjunct to traditional scaling instrumentation used on teeth and implants. All of the lasers listed in ◘ Table 14.1 use the photothermal effect capable of strong bactericidal and detoxification effects [14]. In addition, the infected soft tissue in the pocket can be debrided; the lymphatic and blood vessels can also be coagulated to enable healing.
2.4 General Protocol [15]
Following the examination and diagnosis, the clinician should refer to the periodontal charting and perform initial nonsurgical therapy. A suggested protocol is:
-
1.
Prior to any other instrumentation, laser irradiation at low average power is used to reduce the microbial population in the sulcus [16]. This will lower the risk of bacteremia and reduce the aerosolized contaminants during conventional instrumentation. When using the diode, Nd:YAG, and CO2 wavelengths, care should be taken to avoid prolonged laser contact with subgingival calculus and root surfaces. For implant surfaces, care should be exercised with the Nd:YAG beam placement. When using erbium lasers, calculus removal is occasionally performed at the same time with the initial laser irradiation.
-
2.
Appropriate conventional instrumentation is used to perform calculus removal of the tooth or implant surface. Erbium lasers can be used primarily or adjunctively.
-
3.
Decontamination of the pocket epithelium is performed with laser irradiation. The photonic energy interacts with different components of the inflamed soft tissue to disrupt the biofilm and microbial components. The parameters employed produce an average power below that used for excisional surgery, and the clinician should refer to the laser’s operating manual to verify the average power settings. The treatment objective is to aim the laser beam toward the soft tissue with overlapping strokes to ensure that the entire area of the pocket is irradiated. The time needed for this portion of the protocol depends on the pocket anatomy—its shape, depth, and width. Visible debris will accumulate on the contact tip of some lasers or will be flushed out of the pocket with others. Decontamination is complete when fresh bleeding emanates from the pocket.
-
4.
To ensure coagulation and sealing of the blood capillaries and lymphatic vessels, laser energy is used. Generally this occurs in a short time, and the last beam placement will be at the entrance to the pocket. In more shallow pockets, the decontamination procedure may produce the desired hemostasis without any additional irradiation. After the laser is turned off, digital pressure will help readaptation of the tissue to the tooth, especially in deeper pockets. In more shallow pockets, the decontamination procedure may this step will help the initial healing.
-
5.
The patient is given postoperative and oral hygiene instructions. There should be minimal tissue manipulation of the treated area so that the fibrin clot is not disrupted. Very gentle brushing and flossing should be performed for 2 days. Spicy and crunchy foods should be avoided for at least 1 day. Gentle rinsing with warm salt water three times a day should soothe the tissues in a short period of time, and only mild discomfort should be expected. Subgingival irrigation must be avoided.
2.5 Treatment Planning
The above protocol of initial therapy should be followed for every patient manifesting periodontal or peri-implant disease. The extent of the disease will be determined during the periodontal exam, charting, and diagnosis. When planning treatment, several points should be considered:
-
The patient’s physical limitations such as posture or temporomandibular joint disease
-
The patient’s pain sensitivity during the procedure and medications necessary to control it, ranging from topic and local anesthetics to sedation
-
The patient’s systemic health along with any risk factors that would affect the treatment outcome
-
The number of pockets to be treated and the anatomy of each
-
The amount and tenacity of debris and biofilm to be disrupted/removed
-
Any restorations or occlusal problems that need attention and could compromise access or success of the therapy
-
The patient’s ability to continue adequate oral hygiene techniques
The severity of the disease will determine the appointment schedule both for therapy and the patient’s tolerance for treatment. Most importantly, the treatment plan must be customized for each patient. Some cases of gingivitis may only require full-mouth debridement and disinfection and can be accomplished in two appointments, including polishing. Other advanced conditions with excessive deposits and biofilm may necessitate that only a few teeth are to be treated in each visit.
The length of each appointment can also vary. Generally speaking, moderate generalized disease would be treated with hourly visits in each area of the disease. Some clinicians divide the mouth into quadrants for therapy; others choose to treat all of the deeper pockets first. The latter approach has an advantage in that those pockets with more disease can be retreated with steps 3 and 4 on subsequent appointments, especially if some inflammation remains after the first session. To ensure those pockets receive maximum debridement, the laser can be used again during the other therapy visits.
Locally delivered chemotherapeutic agents, such as minocycline hydrochloride, doxycycline hyclate, and chlorhexidine gluconate, may be placed in pockets to help biofilm suppression. They are most effective after the biofilm has been disrupted by the debridement procedure. As such, those additions should be performed after the last laser treatment. Antimicrobial photodynamic therapy should also be considered, as discussed in the next section of this chapter.
The patient’s oral care skills must be continually assessed and reinforced in this protocol. If the presence of biofilm is not minimized, the intended healing will not progress. An assessment appointment should be scheduled approximately 4 weeks after the completion of the initial therapy.
Following the initial nonsurgical therapy, the next appointment 3 months later will assess both the patient’s home care and the periodontal status. Expected outcomes are inflammation reduction or absence, healthier tissue tone, and reduced pocket depths without bleeding. Minimum force should be used during probing in this period, since the attachment apparatus is easily disrupted. Normal detailed probing can be performed at the 6-month post therapy appointment. Reevaluation can continue at 3-month intervals, with careful assessment of how the disease is resolving. Supportive therapy to preserve the improved clinical attachment and minimum inflammation can continue. This will probably include additional debridement and laser decontamination, along with the patient’s daily oral hygiene regimen.
2.6 Clinical Cases
◘ Figure 14.1 shows a diode laser used in a shallow-inflamed periodontal pocket. ◘ Figure 14.2 depicts the adjunctive use of an Nd:YAG laser. ◘ Figure 14.3 shows the adjunctive use of an Er,Cr:YSGG laser for initial treatment of periodontitis (clinical case courtesy of Dr. Rana Al-Falaki). ◘ Figure 14.4 demonstrates the use of a diode laser for adjunctive treatment of peri-implant mucositis.
a Preoperative view of a 6 mm pocket wit bleeding on probing. b After hand and ultrasonic scaling, an Nd:YAG laser is used with a 400 μm fiber and an average power of 1.8 W (30 mJ/pulse and 60 Hz) and directed toward the soft tissue side of the pocket. c Three-month postoperative view showing pocket depth reduction and lack of inflammation
a Preoperative view of an 8 mm pocket with bleeding on probing. b Preoperative radiograph of the pockets. After ultrasonic removal of the calculus, an Er,Cr:YSGG laser was used with a 500 micron diameter radial firing tip at an average power of 1.5 W (50 mJ, 30 Hz) with a pulse duration of 60 microseconds for debridement. c Seven-month postoperative probing shows significant pocket depth reduction without bleeding on probing. d Seven-month postoperative radiograph depicts a more stable periodontium (Clinical case courtesy Dr. Rana Al-Falaki)
a Preoperative view of a 7 mm pocket with bleeding on probing around an implant. b After careful conventional debridement of any calculus, an 810 nm diode laser with a 400 micron tip was used with an average power of 0.4 CW emission directed toward the soft tissue and away from the implant fixture. c Six-month postoperative view showing pocket depth reduction and lack of inflammation
2.7 Considerations About Laser Use in Initial Nonsurgical Therapy
In any laser-tissue interaction, the absorption of the photonic energy depends on many factors, as discussed in Chap. 3. For periodontal and peri-implant therapy, those same factors are at work in a very limited space—the periodontal pocket and surrounding structures. Therefore, the following points are important:
-
Each wavelength will have different interaction on the various tissue components. For example, the near-infrared wavelengths are easily scattered and are only absorbed by inflammation. Their depth of penetration in sulcular fluid can be significant, which means that the energy could travel beyond the intended target tissue [17]. On the other hand, erbium lasers can be used efficiently to remove subgingival calculus, although it is not very selective; and cementum on the root surface can also be removed [18].
-
The current literature indicates that lasers are generally safe for treatment of peri-implant mucositis, but some precaution should be exercised [19, 20]. As mentioned previously, Nd:YAG laser usual emission mode produces very short pulse durations and a very high peak power per pulse. Those high powers have been known to damage titanium surfaces. Erbium lasers have the same pulse durations but produced no surface alterations with low energy density use. Understanding of those differences is necessary for the clinician to choose the appropriate wavelength for beneficial treatment.
-
The laser parameters must produce low average power to minimize ablation of healthy tissue. Each laser instrument has specific operating instructions for this procedure with suggested settings, and these should be used as a guide to begin the therapy.
-
Each laser has a specific handpiece and emission device—for example, an optical fiber tip or a small tube. The clinician should ensure that the laser beam is aimed as precisely as possible toward the intended target tissue. For example, diode photonic energy will be readily absorbed by dark calculus causing a significant temperature rise; so, the tip should be angled toward the soft tissue. Likewise, when using an erbium laser for calculus debridement, the tip should be as parallel to the tooth axis to avoid excessive cementum removal.
-
As granulation tissue is removed, it may accumulate around the laser tip or tube. Those should be checked and cleaned often to avoid concentration of the energy in the debris.
-
Proper case selection is important and continuing evaluation must be performed. If areas of disease do not respond to the nonsurgical approach, then subsequent surgical therapy will be necessary.
2.8 Acronyms for Nonsurgical Initial Periodontal and Peri-implant Therapy
Clinicians may find various acronyms in the operating manuals of different laser instruments or in scientific literature. The intent of these terms is the same—to provide the first phase of treatment. Such terms as LAD/LABR (laser-assisted decontamination/laser-assisted bacterial reduction), LAPT (laser-assisted periodontal therapy), and LCPT (laser-assisted comprehensive treatment) can give specific additional details about the protocol. Some companies have legally protected their acronyms:
-
REPaiR (regenerative Er,Cr:YSGG periodontitis regimen) uses the company’s Er,Cr:YSGG laser for sulcular debridement and root surface cleaning.
-
WPT™ (wavelength-optimized periodontal therapy) uses the company’s Nd:YAG and Er:YAG to remove the diseased epithelial lining and to debride the root surface calculus, respectively.
Whichever terminology or abbreviations are used, various laser wavelengths can add beneficial results for the treatment of periodontal and peri-implant disease.
2.9 Selected Literature Review for Lasers in Nonsurgical Therapy
The following is a sampling of the literature describing various wavelengths used adjunctively for nonsurgical therapy:
-
Qadri et al. [21] showed that the adjunctive use of a diode laser (800–980 nm) with scaling and root planing (SRP) is more effective in treatment of moderate chronic periodontitis than when SRP is used alone.
-
Lerario et al. [22] used mechanical debridement with the adjunctive use of a diode laser (810 nm) for peri-implant disease and demonstrated greater reduction of probing depth and bleeding on probing than conventional treatment.
-
Martelli et al. [23] showed that adding Nd:YAG laser to conventional treatment found significant and long-term effectiveness in improving clinical and bacteriological measurements.
-
Al-Falaki et al. [24] assembled case report series and reported pocket depth and inflammation reduction in the nonsurgical treatment of peri-implant disease using the Er,Cr:YSGG laser.
-
Schwarz et al. [25] reported that the Er:YAG therapy resulted in significant reduction of the BOP score and improvement of the clinical attachment gain in the nonsurgical treatment of periodontitis.
-
Zhao et al. [26] in a meta-analysis highlighted a significant attachment gain using the Er:YAG laser and SRP when compared to SRP alone.
-
Yan et al. [27] in a meta-analysis showed that the Er:YAG laser as an alternative to mechanical debridement could provide some short-term additional benefit.
Summary: Nonsurgical Laser Therapy
In summary, the adjunctive use of lasers for initial, nonsurgical periodontal therapy must follow specific protocols, and the treatment must be continually evaluated to determine if surgery is necessary. This therapy is well accepted by patients, and it may contribute to their improved home care. Well-designed scientific studies are always required to support the evolving reported benefits of the procedure.
3 Surgical Therapy for Periodontal and Peri-implant Disease
3.1 Description of Surgical Therapy and Laser Wavelengths That Can be Used
After initial therapy is completed, periodic evaluations and maintenance appointments follow. There can be challenges to complete debridement and disinfection of periodontal or peri-implant pockets which range from anatomic complexity of the defects to the patient’s inability to maintain good oral hygiene. Moreover, the clinical attachment level and pocket depth produced by initial therapy may still be inadequate for optimum health. Thus, some surgical intervention will be occasionally necessary.
◘ Table 14.2 lists the details of laser wavelengths that can be used for surgical therapy. The small diameter delivery system including curved tips can especially aid in access to infrabony and furcation pockets.
In this section, surgical therapy will be divided into two sections. The first is a flapless technique and the second is the more conventional protocol where a flap is reflected and then repositioned.
3.2 Flapless Periodontal And Peri-implant Surgery
3.2.1 Description of Flapless Surgery
There are two current flapless techniques that are considered to be surgical but without employing any open flap technique for debridement. These fulfill the concept of a minimally invasive procedure, but could have limitations because of limited access to the entire diseased area of the periodontium around the root or the implant. One, termed LANAP® is an acronym for laser-assisted new attachment procedure and uses a proprietary Nd:YAG instrument. The other is termed laser-assisted comprehensive pocket treatment (LCPT) where any erbium laser can be employed.
3.2.2 Laser Assisted New Attachment Procedure (LANAP®)
The LANAP® protocol entails a specific step, single session treatment, shown in ◘ Fig. 14.5. After verifying the pocket depth, the laser selectively removes the pocket’s epithelial lining. The root surfaces are debrided with conventional scaling instruments, and then blunt dissection is performed at the osseous crest. The laser is then used to obtain hemostasis and to form a fibrin clot so that the loose gingival tissue can be approximated back to the tooth. Occlusal adjustments are performed and postoperative instructions given. This procedure has generated case report studies that offer histologic evidence of new connective tissue attachment, new cementum, and new alveolar bone [28, 29]. The Nd:YAG wavelength (1,064 nm) is usually safe when using appropriate parameters for pocket irradiation. However, the photonic energy has a potential of deep tissue penetrability, so care must be taken to avoid thermal damage to the underlying tissues.
Graphic depiction of LANAP® using the pulsed Nd:YAG laser. a Bone sounding to determine pocket depth. b Under local anesthesia, typically a 360 μm optic fiber delivers 3.6–4.0 W average power at a pulse duration of 100–150 μsec to selectively remove the diseased epithelial lining of the pocket, denature pathologic proteins, and create bacterial antisepsis. c The root surface accretions are removed with piezo ultrasonics and conventional instruments. d Blunt dissection with a conventional dental instrument is used to modify the osseous contour at the alveolar crest and perform intramarrow penetration to gain access to stem cells and growth factors. e Using the same fiber but with a pulse duration of 550–650 μsec, the laser energy performs hemostasis; establishes a thick, stable fibrin clot; activates growth factors; and upregulates gene expression. f The gingival tissue is pressed toward the tooth to secure it without sutures. g Occlusal adjustments are performed to eliminate improper contacts and to allow for passive eruption. h Shows anticipated healing in an environment conducive to true regeneration of new cementum, new periodontal ligament, and new alveolar bone (LANAP® is a patented and registered trademark of Millennium Dental Technologies, Inc., Cerritos, Calif., USA) (Graphic reproduced with permission from Millennium Dental Technologies)
◘ Figure 14.6 shows a clinical case of moderate periodontitis in the maxillary anterior sextant. The LANAP® protocol is used for successful treatment (Clinical case courtesy of Dr. Raymond Yukna)
a Clinical view of the anterior facial region of a patient with acute moderate periodontitis. b Clinical view of the lingual anterior region. c Pretreatment periodontal probe chart showing pockets and mobility on all the anterior teeth. Each horizontal line represents a 2 mm increment, and the red markings indicate bleeding on probing. Mobility is indicated with Roman numerals on the incisal view icon at the top of the chart. The patient was treated with the LANAP® protocol as described in ◘ Fig. 14.4. d Three-year facial postoperative view. e Three-year lingual postoperative view. Note the reduction in inflammation. f Three-year postoperative periodontal probe chart shows significant pocket depth and mobility decrease (Clinical case courtesy Dr. Raymond Yukna)
The same company has an identical procedure for treatment of peri-implant disease, termed LAPIP™ (laser-assisted peri-implantitis protocol.) The laser emission is reduced so that much less average power is applied around the implant structure. The fiber is aimed as parallel as possible to the long axis of the fixture to avoid the metal absorbing the energy and thus overheating as well as to minimize any reflected photons off the surface.
3.2.3 Laser-Assisted Comprehensive Pocket Therapy (LCPT)
LCPT (laser-assisted comprehensive pocket therapy) uses erbium lasers with wavelength emissions of 2,870 or 2,940 nm [30]. As noted previously, these lasers can be used for soft tissue and calculus removal. In addition, they are indicated for use in contouring osseous tissue. Thus they can be useful for debridement of both granulation tissue and bone defects in moderate to deep periodontal pockets, depending on the accessibility. The procedural steps are shown in ◘ Fig. 14.7. After assessing the pocket, the laser and hand instrumentation is used for root surface debridement. That is followed by removal of the epithelial and diseased connective tissue of the lining of the gingival pocket as well as diseased osseous tissue. The treatment objective is thorough decontamination of the whole pocket as well as enhance of bleeding from bone surface, including bone marrow-derived cells which are a major source of mesenchymal stem cells. The parameters used will not affect hemostasis in the bone; on the contrary, the procedure should enhance bleeding which would be advantageous for tissue regeneration. There may also be some biostimulatory effects from the low-level laser penetration into the surrounding tissues. The next step is laser ablation of the external gingival tissue at the pocket entrance. The epithelium and occasionally a layer of connective tissue are removed. The pocket depth is automatically reduced with this small-dimension gingivectomy, and the exposure of the connective tissue will delay the migration of the epithelium into the pocket while the attachment is being reestablished. That procedure will cause some gingival recession, but the primary benefit of pocket healing will be realized. The last step is to ensure adequate coagulation for a stable blood clot to seal the pocket entrance. The erbium laser is used in a noncontact mode without water spray to achieve this.
A graphic depiction of LCPT (laser-assisted comprehensive pocket therapy) using an Er:YAG laser. a The pocket depth is assessed. b Subgingival calculus is removed with both the laser (utilizing a water spray) and conventional instrumentation so that the diseased root surface is decontaminated and detoxified. c The laser is used to remove the diseased epithelial and connective tissue lining of the pocket. d The osseous tissue is also debrided by the laser using a water spray to promote bleeding from the bone, and the resulting healthy tissue is shown. e Some of the laser irradiation can offer biostimulation to the surrounding intrasulcular tissue. (f) The outer epithelium and some connective tissue are removed to delay epithelial migration into the healing pocket. This gingivectomy does produce some gingival recession. g The laser is used in a noncontact mode without a water spray to ensure hemostasis and to produce a stable blood clot to protect the entrance to the pocket, as well as to stimulate the outer surface of the periodontium. h Shows the new attachment and pocket depth reduction (Graphic modified from Aoki et al. [30] with permission © copyright 2015 John Wiley and Sons A/S)
This procedure can also be used for the treatment of peri-implant mucositis or the initial stages of peri-implantitis.
◘ Figure 14.7 is a clinical case of Er:YAG (2,940 nm) laser-assisted LCPT shown in ◘ Fig. 14.6. Deep pockets and an infrabony defect are treated. There is radiographic evidence of osseous healing and new attachment at 1-year postoperatively (◘ Fig. 14.8).
a Deep pockets are present on the lateral incisor. The one measures 8 mm with bleeding on probing. b The radiograph shows the vertical bony defect (black arrow). c Immediate postoperative view showing a stable blood clot. The Er:YAG laser was used with an 600 μm curved tip at 1.0 W average power (50 mJ/pulse at 20 Hz) with a water spray to remove the inflamed soft tissue in the pocket and adjunctively with a curette to debride the root surface. Then the inner epithelial wall and the osseous defect were also debrided. The outer epithelium was recontoured to delay gingival down growth and a stable clot was formed. The latter procedure was performed without a water spray. d One-year postoperative view shows good healing with a slight loss of the gingival papilla. e The radiograph confirms the osseous defect has filled in (black arrow) (Clinical case and details courtesy of Dr. Koji Mizutani, and modified from citation [9] with permission © copyright 2016 John Wiley and Sons A/S)
3.3 Osseous Periodontal Surgery Employing a Flap
Osseous surgery, during which bone is removed, recontoured, and/or reshaped, is one of the major periodontal surgical procedures. Optimum bone anatomy will help establish and maintain clinical attachment, shallow pockets, and physiologic gingival architecture—all of which are critical for long-term stability of periodontal tissue.
At the time of writing, no studies are available for the 9,300 nm wavelength, but there are manuscripts showing that both the Er,Cr:YSGG (2780) and Er:YAG (2,940 nm) [31, 32] wavelengths are effective in ablation of bone tissue with minimal thermal damage. In addition, the healing assessment of those lasers performing an osteotomy is at least comparable to conventional instrumentation [33] and may be advantageous for faster and improved outcomes [34, 35].
The correct laser wavelength delivered through a small diameter tip can offer more precision and better access than mechanical instruments. Conventional surgical instruments usually need a wider area of access compared to the laser with its irradiation confined to the end of the tip. Thus more precision is possible.
Bone grafting procedures with appropriate membranes may be used in areas where the defect cannot be properly contoured. A laser produces minimal thermal damage resulting in a new osseous surface with good vascularity and a lack of smear layer, which should aid in successful bone augmentation [32].
◘ Figure 14.9 shows the use of an Er:YAG laser for open flap surgery on a 9 mm deep pocket on the distal of the mandibular right cuspid. This pocket remained after initial therapy. The flap was elevated and the laser was used to remove the granulation tissue and debride the root surface. The osseous defect was also debrided, and no grafting material was placed. The inner surface of the flap was irradiated for debridement, and sutures were placed. Eight-year postoperatively there was significant pocket depth reduction and clinical attachment gain.
a, b After initial therapy, a 9 mm pocket with bleeding on probing remains on the distal of the mandibular right cuspid shown clinically and radiographically. c, d A flap is elevated and granulation tissue fills the pocket when viewed from the buccal and lingual aspects. e An Er:YAG laser is used with an 80 degree 400 micron tip at 1.2 W average power (40 mJ per pulse at 30 Hz) with a saline water spray for debridement. f, g Shows the clean vertical bone defect with no thermal damage from the laser energy. No bone augmentation material was placed. h The laser is used with the same parameters to decontaminate and stimulate the gingival flap tissue. i The flap is sutured in place. j Eight-year postoperative view showing healthy gingival tissue with some recession. In fact, there was 7 mm of pocket depth reduction and 5 mm of clinical attachment gain. k Eight-year postoperative radiograph (Case photos and details modified from Aoki et al. [30] with permission © copyright 2015 John Wiley and Sons A/S)
◘ Figure 14.10 demonstrates a similar open flap procedure where the Er,Cr:YSGG laser was used on a 11 mm pocket on the mesial of the maxillary right first premolar that did not respond to initial therapy. After raising a flap, the laser was used to debride the root surface, the pocket epithelium, and the osseous defect. No bone graft material was placed and the flap was sutured. An 8-month analysis showed pocket depth reduction and attachment gain with slight gingival recession (clinical case courtesy of Dr. Rana Al-Falaki).
a Radiograph showing 11 mm pocket on the mesial of the maxillary first premolar that remained after initial therapy. b After the subgingival calculus was removed with ultrasonic instrumentation, a flap is raised and the osseous defect is explored with the periodontal probe. c Immediate postoperative view of the debrided infrabony pocket. An Er,Cr:YSGG laser was used with a 600 micron diameter contact tip. To remove the granulation tissue from the soft and hard tissue, an average power of 1.5 W (50 mJ, 30 Hz) was used with 50% air and 40% water spray in the short pulse mode. Subsequently, the smear layer was removed from the root surface and osseous tissue with an average power of 0.75 W (15 mJ, 50 Hz) with 50% water and 40% air. d View of the sutured flap. e Eight-month postoperative photo with periodontal probe, demonstrating good reattachment with slight gingival recession. f Eight-month postoperative radiograph showing a more stable periodontal condition with bone regeneration (Clinical case courtesy of Dr. Rana Al-Falaki)
3.3.1 Surgical Therapy for Peri-implantitis
Debridement and detoxification of the implant surfaces as well as the diseased tissues surrounding implant fixtures is the primary objective for the treatment of peri-implantitis. Many laser wavelengths have been studied for their ability to efficiently debride implant surfaces. The diode, carbon dioxide, and erbium instruments generally do not cause any surface damage to implants [36,37,38]. The precaution is that high average power settings can generate heat on the peri-implant tissues and/or directly affect the titanium fixture. Thus appropriate parameters and techniques must be employed during the surgical session.
After debridement of the surrounding tissues and decontamination of the implant itself, bone augmentation materials and appropriate membranes can be placed in the defect to enhance regeneration. Clearly, good bone vascularity is important to achieve, and proper laser parameters can accomplish this. As mentioned, lasers should allow for beneficial bone healing following surgery along with a biocompatible implant fixture.
◘ Figure 14.11 demonstrates how an Er,Cr:YSGG laser for peri-implantitis therapy. After reflecting a flap, the osseous defect was filled with granulation tissue, and the laser debrided the soft and hard tissues in the area. A bone graft and membrane were placed and the flap was sutured in place. Six months later, the periodontal health was restored (clinical case courtesy of Dr. Rana Al-Falaki).
a Preoperative view of peri-implantitis around a maxillary posterior implant with an 8 mm pocket. b After the flap is reflected, the extent of the defect, filled with granulation tissue, can be seen. c Immediate postoperative view of the debridement therapy. An Er,Cr:YSGG laser was used with a 600 micron diameter contact tip. The granulation tissue was removed from the soft and hard tissue with an average power of 2.0 W (66 mJ, 30 Hz) with 70% water and 50% air, angling the tip away from the implant surface. Then the implant was debrided at an average power of 1.25 W (25 mJ, 50 Hz) with 70% water and 50% air. Lastly, the internal surface of the flap was decontaminated at any average power of 0.75 W (15 mJ, 50 Hz) with 50% water and 40% air. Note the good vascularity of the osseous tissue. d Bone grafting material is placed immediately to fill the area. e The flap is sutured in place. f Six-month postoperative view of the healed periodontium with no inflammation (Clinical case courtesy of Dr. Rana Al-Falaki)
◘ Figure 14.12 shows the use of an Er:YAG laser for treatment of severe peri-implantitis. The implant fixture shown had no mobility despite the significant lack of labial supporting bone. A decision was made to attempt regenerative therapy although the prognosis was extremely guarded. The large defect contained large amounts of granulation tissue along with underlying infected bone. The Er:YAG laser debrided all of the tissues, and then bone grafting material and a membrane were placed. The flap was sutured. A 3-month radiograph shows new bone growth with a lack of inflammation.
a Preoperative view of a draining fistula from an area of peri-implantitis. b The radiograph shows extensive disease including a large area of apical bone loss (yellow circle). c A flap is raised showing the extent of the inflammatory granulation and infected osseous tissue. d The Er:YAG laser was used with a 1,300 micron sapphire tip with an average power setting of 8.4 W (700 mJ per pulse at 12 Hz) with a water spray directed at the granulation tissue. Then, using the same tip and water spray, the parameters were changed to an average power of 3 W (150 mJ per pulse at 20 Hz) for debridement of the implant surface and removal of the infected bone. e The site was filled with a xenograft bone substitute and covered with an absorbent bilayer membrane. f The flap was sutured. g A healing cap was placed 2 weeks postoperatively. h The 3-month radiograph shows good bone regeneration and stable bone tissue (Some case details courtesy Dr. Avi Reyhanian)
Summary: Flapless Periodontal and Peri-Implant Surgery
In summary, dental lasers can be used in a surgical approach for treatment of periodontal and peri-implant diseases with benefits such as precision and enhanced visibility during debridement. In addition, osseous tissue can be predictably ablated and contoured. Future research should emphasize proper power settings and describe the details of the protocol. Laser application for bone ablation is becoming a very useful modality for developing the various usages on periodontal and implant therapy. Preventing thermal damage during following laser treatment is critical for optimal wound healing.
4 Antimicrobial Photodynamic Therapy in Management of Periodontal and Peri-implant Disease
4.1 Photodynamic Therapy
Photodynamic therapy (PDT) is a new approach in killing or eliminating pathogens and uses light of a specific wavelength to activate a nontoxic photoactive dye (photosensitizer) in the presence of oxygen to produce cytotoxic products [39, 40]. Various terms are used for PDT such as photoactivated chemotherapy (PACT), photodynamic disinfection (PDD), light-activated disinfection (LAD), photodynamic inactivation (PDI), and photoactivated disinfection (PAD) and antimicrobial photodynamic therapy (aPDT) in different studies and literature. Among these terms, aPDT is the most accepted one for antimicrobial purposes [41, 42].
The successful outcome of PDT critically depends on three elements: photosensitizer, light source, and oxygen (◘ Figs. 14.1 and 14.13).
5 Photosensitizer
A photosensitizer is a chemical compound, which, when activate by an appropriate wavelength, forms a highly reactive oxygen species which results in cell death.
The photosensitizers should have some characteristics including:
-
Existing as nontoxic and chemically pure compound
-
Having the ability to stain the target
-
Be economical and easily available
-
Possess a short interval between administration of the drug and peak accumulation in the tissue
-
Have a short half-life
-
Be rapidly eliminated from normal tissue
-
Have activation at specific wavelength
-
Possess the ability to produce the huge amount of cytotoxic products
-
Have the ability to act on a wide range of microorganism [43, 44]
The most applicable photosensitizers which used in dentistry for antimicrobial procedures are described below.
5.1 Toluidine Blue O
Toluidine Blue O (TBO) is a cationic blue coloring agent used for histological staining. It can also be applied for differential diagnosis between benign and malignant precancerous leukoplakia. It can be activated by wavelength of 635 nm. It can act on both gram-positive and gram-negative bacteria due to its physical and chemical properties and hydrophilic characteristics, and it showed attraction to the mitochondria which has negative charge. TBO can bind to LPS of the outer cell envelope in gram-negative bacteria and the teichuronic acid residues of the outer wall in gram-positive bacteria [45,46,47].
5.2 Methylene Blue
Methylene blue (MB) is used for selective coloring in histology. It is a hydrophilic compound with positive charge. This photosensitizer can be applied for both gram-positive and gram-negative bacteria. It can penetrate through the porin channels in the outer membrane of gram-negative bacteria and interacts with the anionic macromolecule lipopolysaccharide creating MB dimmers which have role in the photosensitization process. It has a peak absorption at wavelength of 660 nm [48, 49].
5.3 Indocyanine Green
Indocyanine green (ICG), a green coloring agent, has recently been introduced as photosensitizer. The mechanism of this photosensitizer is somehow different from other ones. The effect of ICG is mainly that of photothermal therapy (PTT) rather than photochemical reaction. This anionic photosensitizer can be activated by 810 nm but its absorption critically depends on the dissolving medium, the chemical bonds of plasma proteins, and its concentration [44, 50].
5.4 Curcumin
Curcumin is a yellow-orange pigment isolated from Curcuma longa L. which is mostly used as a spice. It has some therapeutic effects on liver diseases, wounds, and inflamed joints, as well as for blood purification and microbial effects. Curcumin has shown no toxic effects on a number of cell cultures and animal studies. It has a broad absorption peak in the 300–500 nm range (maximum 430 nm) and produces strong phototoxic effects. Therefore, curcumin has the capability to be used as a photosensitizer. Easy handling, low cost, and efficacy make this photosensitizer more popular [51,52,53].
6 Light Source
In photodynamic therapy procedure, the light source coincides with maximum absorption of the photosensitizer used. The light source for aPDT can be classified into three types:
-
1.
Broad-spectrum lamps
-
2.
Light-emitting diode lamps (LED)
-
3.
Lasers
Among the different sources, lasers have some characteristics that make them superior compared to other sources. Monochromaticity which allows the laser to interact with photosensitizer due to matching with its peak absorption results in elimination of unnecessary tissue heating by bandwidths not effective in PDT reaction [54, 55].
In dentistry, most of the photosensitizers are activated by wavelengths between 630 and 700 nm. Currently, with the introduction of new photosensitizer such as ICG, infrared wavelength like 810 nm is also used which has more penetration depth. On the other hand, blue light LED (400–500 nm) which coincidences with curcumin can be a suitable option due to its availability in all dental offices for curing of dental resin composites and capability in creating free radicals more efficiently compared to red light. LEDs are more cost effective and compact in comparison to lasers [56, 57].
7 Mechanism of Photodynamic Therapy
When a photosensitizer is activated by an appropriate wavelength, electrons are transferred from a lower level of energy to a higher one which is called the triplet state. Then, the energy is transferred to a biomolecule or to oxygen which leads to the production of cytotoxic species. These products damage the cellular plasma membrane or DNA. Both consequences lead to cell death [58, 59].
The transfer of electrons in activated photosensitizer can be done in two pathways including transfer to the neighboring molecule (type-1 reaction) or to oxygen (type-2 reaction) to produce reactive oxygen species (ROS) like singlet oxygen and other radicals like hydroxyl radical. Although, the two pathways can have a role on bacterial killing, type 2 by producing highly reactive singlet oxygen is detected as the main pathway in killing bacteria (◘ Figs. 14.2 and 14.3). This mechanism is totally different from that of antibiotics; hence, the resistance of bacterial strain is not likely, due to acting on multiple targets inside the bacteria [60, 61] (◘ Figs. 14.14 and 14.15).
It’s important to note that antioxidant enzymes produced by bacteria may protect against some oxygen radicals but not singlet oxygen which makes aPDT more effective procedure. Singlet oxygen has a short lifetime in biological system (≤0.04 μs) and a short radius of action (0.02 μm) that make its action localized without affecting distant cells [62, 63].
One of the main concerns during photodynamic therapy is the photosensitivity of bacteria which seems mainly related to the charge of the photosensitizer used. The neutral or anionic sensitizer binds effectively to gram-positive bacteria. It also binds to some degree to the outer membrane of gram-negative bacteria [64]. The porous layer of peptidoglycan and lipoteichoic acid outside the cytoplasmic membrane of gram-positive species allow the photosensitizer to cross into the cell. On the other hand, gram-negative bacteria have an inner cytoplasmic membrane and an outer membrane separated by the peptidoglycan-containing periplasm which acts as a physical barrier between cells and its environment [65]. The binding of negatively charged photosensitizer to gram-negative bacteria may be improved by linking the photosensitizer to a cationic molecule [66] .
The success rate of photodynamic therapies depend on the type, dose, incubation time, and localization of the photosensitizer, the availability of oxygen, the wavelength of light (nm), the light power density, and the light energy fluency. Limitations of this treatment which should be taken into account are the low-oxygenated environment and the diffusion ability of the photosensitizer and light to be used [67].
8 aPDT in Periodontital and Peri-implant Disease
It is now established that the application of lasers in management of periodontal diseases can offer some benefits. Using a high-powered laser for antimicrobial purposes raises some concerns like irreversible thermal damage to surrounding periodontal tissues, thermal coagulation, carbonization, and root necrosis [68]. Therefore, aPDT was developed, which is a noninvasive method which uses low power to overcome these limitations. In this technique, the bacteria are selectively targeted without damaging the neighboring tissue [69].
8.1 Procedure
-
Assessing periodontal clinical parameters:
-
The clinical parameters are collected before treatment. The record of parameters were as follows: (a) bleeding on probing (BOP), (b) clinical attachment level (CAL), (c) plaque index (PI), (d) probing pocket depth (PPD), (e) full-mouth plaque score (FMPS), and (f) full-mouth bleeding score (FMBS).
-
-
Treatment:
-
After educating oral hygiene instructions, the patients receive full-mouth scaling and root planing (SRP), and the photosensitizer solution is applied to the bottom of the periodontal or peri-implant pocket and gingival sulcus with the use of syringe. Following this, the pocket is exposed to the laser light due to protocol moving from bottom of pocket to coronal. Special safety glasses are provided to the patients, operator, and dental assistant to prevent possible eye damage by the laser irradiation. The procedure can be repeated in the same manner for following weeks due to treatment plan.
-
Bacteria can penetrate into epithelial cells and connective tissue during periodontal diseases. P. gingivalis and A. actinomycetemcomitans can infiltrate the epithelial barrier in to periodontal tissues in this case; aPDT can be a solution for eliminating them [70]. Sulcular epithelium has increased penetration of photosensitizer due to non-keratinized pattern. The uptake of photosensitizer in epithelial cells is dependent on incubation time (the interval between applying photosensitizer and laser irradiation). So, there should be a few minutes waiting time after applying photosensitizer before starting laser irradiation [71, 72].
Photodynamic therapy has some advantages like detoxification of endotoxins such as lipopolysaccharides which inhibit the production of pro-inflammatory cytokines. Also, it can reach to deep or limited access sites without the need for flap surgery in some cases; there is no need to anesthetize the area, and there is no need to prescribe antibiotics. In addition there is a low risk of bacteremia, which is useful for at-risk patients (those with cardiovascular diseases, diabetes, and immunosuppression) [73].
Furthermore, aPDT increases tissue blood flow in microcirculatory system and reduces venous congestion in gingival tissues.
In assessing different studies, controversial results are obtained due to the different wavelengths of laser and the type of photosensitizer type used.
Bassir et al. assessed photo-activated disinfection using LED and TBO as an adjunct in the management of patients with moderate to severe chronic periodontitis. The study concluded that at 1 and 3 months, PDT showed significant improvements with regard to all clinical parameters compared to baseline but did not have additional effects on clinical parameters in patients [74].
On the other hand, Prasanth et al. in evaluation of aPDT by methylene blue and 655 nm diode laser in management of chronic periodontitis concluded that aPDT has an important role in improving clinical outcomes obtained by SRP, and single application of aPDT resulted in effective gingival inflammation and pocket depth reduction over a period of 6 months. The group also suggested that the procedure be repeated at frequent intervals [75].
Monzavi et al. tried to test the efficacy of adjunctive aPDT with ICG compared with scaling and root planing (SRP) alone in chronic periodontitis treatment (◘ Fig. 14.5). The PDT group yielded higher improvements in bleeding on probing (BOP) and full-mouth bleeding score (FMBS) rather than the control group after 1- and 3-month follow-up examinations. After 3 months, the patients that received PDT showed 0% of BOP score, while the control group displays 48% of BOP positive [76]. Boehm and Ciancio found that rapid and significant uptake of ICG into periodontal pathogens that are activated by 810 nm diode laser resulted in significant killing of A. actinomycetemcomitans and Porphyromonas gingivalis [77].
The effect of ICG is mainly photothermal therapy rather than photochemical (80% photothermal and 20% photochemical). In addition, the peak absorption of ICG is close to available soft tissue diode lasers (808 nm), compared to the peak absorption of methylene blue and toluidine blue O which are at 660 nm and 635 nm, respectively. The higher penetration of 810 nm diode laser compared to other wavelengths with an easy insertion of the fiber-optic applicator allows an easier access into deep pockets. Besides, the photothermal effects of ICG accompanied by photochemical effects make this photosensitizer important for eradication of pathogens in deep periodontal pockets or in the periodontal treatment of non-reachable sites (i.e., furcation or invaginations). Therefore, ICG with an 810 nm diode laser can be considered as a promising candidate for adjunctive periodontal treatment [78].
8.2 Clinical Cases
◘ Figure 14.16 illustrates the use of Toluidine Blue O for treatment of a periodontal pocket. A LED source of photonic energy activates the chemical.
◘ Figure 14.17 depicts the use of indocyanine green for treatment of a periodontal pocket. An 810 nm laser was used to activate the photosynthesizer.
◘ Figure 14.18 illustrates the use of methylene blue for the treatment of a periodontal pocket. A proprietary visible red laser (632 nm) was used to activate the photosynthesizer. Clinical case courtesy of Dr. Steven Parker.
a Facial view of the periodontitis condition of the lower anterior area. b After scaling of the pockets, the methylene blue solution is applied. A visible diode laser activates the photosensitizer. c Immediate posttreatment view of the site. d A 1-month posttreatment photo showing tissue health with no inflammation (Clinical case courtesy of Dr. Steven Parker)
◘ Figure 14.19 shows the application of antimicrobial photodynamic therapy for management of peri-implantitis in a 50-year-old woman. In this case, 7 mm pocket depth and bleeding on probing were observed. After manual debridement, the phenothiazine chloride as photosensitizer was applied in the implant sulcus. After 3 min, the excess photosensitizer was rinsed, and diode laser (670 nm) with output power of 75 mW was irradiated for 1 min. In follow-up of 8 months, pocket depth of 3 mm and absence of bleeding on probing were observed.
a Facial view of peri-implantitis of the lower right cuspid. b The lingual view of the implant area with a discharge of exudates. c Preoperative radiograph showing the periodontal defect. d The application of phenothiazine chloride inside the pocket. e Facial view 31 months later showing excellent periodontal health. f Lingual view 31 months later of the healed area. g Radiograph of the treated implant, 31 months later (Clinical case courtesy of Dr. Chen-Yeng Wang, modified from citation [9] with permission © copyright 2016 John Wiley and Sons A/S)
Angular bony defects at both mesial and distal parts were observed before treatment, but 8 and 19 months later, bone gain at both sides was detected, and 31 months later, maintenance of the bone level at mesial and distal aspects was approved (case details provided by Dr. Chen-Ying Wang) [9].
9 Considerations During a PDT Therapy
The effective treatment of periodontal problems must include proper oral hygiene instructions, which consist of a combination of daily tooth brushing, interdental cleaning, and, when necessary, use of chemotherapeutic agents [e.g., mouthwash]. Thus, the patient’s compliance with those instructions is fundamental for the success of the treatment of periodontal and peri-implant disease.
Moreover, the application of some photosynthesizers like methylene blue can stain the teeth if the appropriate concentration is not used. It was suggested that MB in concentrations below 100 μg/ml reduces the chance of tooth discoloration [79]. Pourhajibagher et al. in evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells came to this conclusion that to avoid cytotoxicity, the concentration of the sensitizers and laser irradiation time are essential for aPDT. They also observed that the optimum concentration of ICG as photosensitizer for aPDT should be at least 1,000 μg/ml with 30 or 60s irradiation time by 810 nm diode laser [80].
Summary: Antimicrobial Photodynamic Therapy
In summary, there are still limited data from clinical studies with controversial results in application of PDT as an adjunctive treatment for management of periodontitis and peri-implantitis. In patients with chronic periodontitis, SRP and PDT have shown higher short-term clinical improvements like probing depth or bleeding on probing compared to SRP alone. But in aggressive periodontitis, there is not sufficient evidence to replace systemic antibiotic by PDT. Also, limited evidence exists to consider PDT as an alternative to local antibiotics in peri-implantitis.
Conclusion
Clinicians continue to search for and learn about novel methods to aid in the treatment of periodontal and peri-implant diseases. Various benefits such as pocket depth reduction, gain of clinical attachment, and improved wound healing are reported in the scientific studies. However, the use of the laser for these therapies generates controversial discussion in the literature. Nonetheless, adjunctive or alternative use of dental lasers, both direct minimally invasive surgical or nonsurgical procedures as well as in photochemical activation, are becoming part of the practitioner’s armamentarium.
References
Eke PI, et al. Predicting periodontitis at state and local levels in the United States. J Dent Res. 2016;95(5):515–22.
Lee DW. Periodontitis and dental implant loss. Evid Based Dent. 2014;15(2):59–60.
Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1(1):443–90.
Cobb CM. Lasers in periodontics: a review of the literature. J Periodontol. 2006;77(4):545–64.
Kepic TJ, O’Leary TJ, Kafrawy AH. Total calculus removal: an attainable objective? J Periodontol. 1990;61(1):16–20.
Rabbani GM, Ash Jr MM, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52(3):119–23.
Sherman PR, Hutchens Jr LH, Jewson LG. The effectiveness of subgingival scaling and root planing. II. Clinical responses related to residual calculus. J Periodontol. 1990;61(1):9–15.
Schenk G, Flemmig TF, Lob S, Ruckdeschel G, Hickel R. Lack of antimicrobial effect on periodontopathic bacteria by ultrasonic and sonic scalers in vitro. J Clin Periodontol. 2000;27(2):116–9.
Mizutani K, Aoki A, Coluzzi D, et al. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000. 2016;71:185–212.
Schwarz F, Aoki A, Sculean A, Becker J. The impact of laser application on periodontal and peri-implant wound healing. Periodontol 2000. 2009;51:79–108.
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y. Application of lasers in periodontics: true innovation or myth? Periodontol 2000. 2009;50:90–126.
Russell AD. Lethal effects of heat on bacterial physiology and structure. Sci Prog. 2003;86(part 1–2):115–37.
Knappe V, Frank F, Rohde E. Principles of lasers and biophotonic effects. Photomed Laser Surg. 2004;22(5):411–7.
Aoki A, Sasaki K, Watanabe H, Ishikawa I. Lasers in non surgical periodontal therapy. Periodontol 2000. 2004;36:59–7.
Coluzzi DJ, Convissar RA. Laser Periodontal Therapy. In:Atlas of laser applications in dentistry. Hanover Park: Quintessence Publishing Co, Inc; 2007. p. 25–9.
Gutknecht N, Radufi P, Franzen R, Lampert F. Reduction of specific microorganisms in the periodontal pocket with the aid of an Nd:YAG laser: an in vivo study. J Oral Laser Appl. 2002;2:175–80.
Coluzzi DJ. Fundamentals of lasers in dentistry; basic science, tissue interaction, and instrumentation. J Laser Dent. 2008;16(spec, issue):4–10.
Aoki A, Ando Y, Watanabe H, Ishikawa I. In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser. J Periodontol. 1994;65(12):1097–106.
Kreisler M, Gotz H, Duschner H. Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2, and GaAIAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants. 2002;17(2):202–11.
Romanos GE, Gutknecht N, Dieter S, Schwarz F, Crespi R, Sculean A. Laser wavelengths and oral implantology. Lasers Med Sci. 2009;24(6):961–70.
Qadri T, et al. Role of diode lasers (800–980 nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: a systematic review. Photomed Laser Surg. 2015;33(11):1–8.
Lerario F, et al. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of a diode laser: preliminary clinical study. Lasers Med Sci. 2016;31(1):1–6.
Martelli FS, et al. Long-term efficacy of microbiology-driven periodontal laser-assisted therapy. Eur J Clin Microbiol Infect Dis. 2016;35(3):433–1.
Al-Falaki R, Cronshaw M, Hughes FJ. Treatment outcome following use of the erbium, chromium:yttrium, scandium, gallium, garnet laser in the non-surgical management of peri-implantitis: a case series. Br Dent J. 2014;217:453–7.
Schwarz F, Sculean A, Georg T, et al. Periodontal treatment with an Er:YAG laser compared to scaling and root planing. A controlled clinical study. J Periodontol. 2001;72(3):361–7.
Zhao Y, Yin Y, Tao L, Nie P, Tang Y, Zhu M. Er:YAG laser versus scaling and root planing as alternative or adjuvant for chronic periodontitis treatment: a systematic review. J Clin Periodontol. 2014;41(11):1069–79.
Yan M, Liu M, Wang M, Yin F, Xia H. The effects of Er:YAG on the treatment of peri-implantitis: a meta-analysis of randomized controlled trials. Lasers Med Sci. 2015;30(7):1843–53.
Yukna RA, Carr RI, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27(6):577–87.
Nevins M, Kim SW, Camelo M, et al. A prospective 9 month human clinical evaluation of Laser-Assisted New Attachment Procedure (LANAP) therapy. Int J Periodontics Restorative Dent. 2014;34(1):21–7.
Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68:217–69.
Kang HW, Oh J, Welch AJ. Investigations on laser hard tissue ablation under various environments. Phys Med Biol. 2008;53(12):3381–90.
Kimura K, Yu DG, Fujita A, et al. Effects of erbium, chromium:YSGG laser irradiation on canine mandibular bone. J Periodontol. 2001;72(9):1178–82.
Lewandrowski KU, Lorente C, Schomacker KT. Use of the Er:YAG laser for improved plating in maxillofacial surgery: comparison of bone healing in laser and drill osteotomies. Lasers Surg Med. 1996;19(1):40–5.
Pourzarandian A, Watanabe H, Aoki A, et al. Histological and TEM examination of early stages of bone healing after Er:YAG laser irradiation. Photomed Laser Surg. 2004;22(4):355–63.
Mizutani K, Aoki A, Takasaki AA, et al. Periodontal tissue healing following flap surgery using an Er:YAG laser in dogs. Lasers Surg Med. 2006;38(4):314–24.
Tosun E, Tasar F, Strauss R, et al. Comparative evaluation of antimicrobial effects of Er:YAG, diode, and CO2 lasers on titanium discs: an experimental study. J Oral Maxillofac Surg. 2012;70(5):1064–9.
Romanos GE, Everts H, Nentwig GH. Effects of diode and Nd:YAG laser irradiation on titanium discs: a scanning electron microscope examination. J Periodontol. 2000;71(5):810–5.
Stubinger S, Etter C, Miskiewicz M, et al. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er:YAG, carbon dioxide, and diode laser irradiation. Int J Oral Maxillofac Implants. 2010;25(1):104–11.
Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontol 2000. 2011;55(1):143–66.
Pandey RK, Zheng G. Porphyrins as photosensitizers in photodynamic therapy. In: Kadish KM, Smith KM, Guilard R, editors. The porphyrin handbook. Boston: Academic Press; 2000. p. 157–230.
Parker S. The use of diffuse laser photonic energy and indocyanine green photosensitizer as an adjunct to periodontal therapy. Br Dent J. 2013;215(4):167–71.
Rajesh S, Koshi E, Philip K, Mohan A. Antimicrobial photodynamic therapy: an overview. J Indian Soc Periodontol. 2011;15(4):323–7.
Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86(8):694–707.
Chiniforush N, Pourhajibagher M, Shahabi S, Bahador A. Clinical approach of high technology techniques for control and elimination of endodontic microbiota. J Lasers Med Sci. 2015;6(4):139–50.
Seghatchian J, Struff WG, Reichenberg S. Main properties of the THERAFLEX MB-plasma system for pathogen reduction. Transfus Med Hemother. 2011;38(1):55–64.
Sridharan G, Shankar AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16(2):251–5.
Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656.
Wainwright M, Giddens RM. Phenothiazinium photosensitisers: choices in synthesis and application. Dyes Pigments. 2003;57:245–57.
Prento P. A contribution to the theory of biological staining based on the principles for structural organization of biological macromolecules. Biotech Histochem. 2001;76:137–61.
George S, Hamblin MR, Kishen A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem Photobiol Sci. 2009;8(6):788–95.
Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
Dovigo LN, Pavarina AC, Ribeiro AP, et al. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem Photobiol. 2011;87(4):895–903.
Araújo NC, Fontana CR, Bagnato VS, Gerbi ME. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med Sci. 2014;29(2):629–35.
Salva KA. Photodynamic therapy: unapproved uses, dosages or indications. Clin Dermatol. 2002;20(5):571–81.
Biel MA. Photodynamic therapy in head and neck cancer. Curr Oncol Rep. 2002;4:87–96.
Grant WE, Hopper C, Speight PM, Bown SG. Photodynamic therapy, an effective, but non selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71:937–42.
Takasaki AA, Aoki A, Mizutani K, et al. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000. 2009;51:109–40.
Ochsner M. Photodynamic therapy in squamous cell carcinoma. J Photochem Photobiol B. 2001;52:42–8.
Sharman WM, Allen CM, Van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999;4(11):507–17.
Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549–53.
Cieplik F, Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;5:405.
Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumors. J Photochem Photobiol B. 1997;39(1):1–18.
Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34(12):1515–20.
Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755–67.
Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):522–7.
de Melo WC, Avci P, de Oliveira MN, et al. Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti Infect Ther. 2013;11(7):669–93.
Chrepa V, Kotsakis GA, Pagonis TC, Hargreaves KM. The effect of photodynamic therapy in root canal disinfection: a systematic review. J Endod. 2014;40:891–8.
Saxena S, Bhatia G, Garg B, Rajwar YC. Role of photodynamic therapy in periodontitis. Asian Pac J Health Sci. 2014;1(3):200–6.
Moslemi N, Soleiman-Zadeh Azar P, Bahador A, Rouzmeh N, Chiniforush N, Paknejad M, Fekrazad R. Inactivation of Aggregatibacter actinomycetemcomitans by two different modalities of photodynamic therapy using Toluidine blue O or Radachlorin as photosensitizers: an in vitro study. Lasers Med Sci. 2015;30(1):89–94.
Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52(1):68–83.
Soukos NS, Ximenez-Fyvie LA, Hamblin MR, et al. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42(10):2595–601.
Raghavendra M, Koregol A, Bhola S. Photodynamic therapy: a targeted therapy in periodontics. Aust Dent J. 2009;54 Suppl 1:S102–9.
Giannelli M, Pini A, Formigli L, Bani D. Comparative in vitro study among the effects of different laser and LED irradiation protocols and conventional chlorhexidine treatment for deactivation of bacterial lipopolysaccharide adherent to titanium surface. Photomed Laser Surg. 2011;29(8):573–80.
Bassir SH, Moslemi N, Jamali R, Mashmouly S, Fekrazad R, Chiniforush N, Shamshiri AR, Nowzari H. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: a pilot double-blind split-mouth randomized clinical trial. J Clin Periodontol. 2013;40(1):65–72.
Prasanth CS, Karunakaran SC, Paul AK, Kussovski V, Mantareva V, Ramaiah D, Selvaraj L, Angelov I, Avramov L, Nandakumar K, Subhash N. Antimicrobial photodynamic efficiency of novel cationic porphyrins towards periodontal Gram-positive andGram-negative pathogenic bacteria. Photochem Photobiol. 2014;90(3):628–40.
Monzavi A, Chinipardaz Z, Mousavi M, Fekrazad R, Moslemi N, Azaripour A, Bagherpasand O, Chiniforush N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: a randomized clinical trial. Photodiagnosis Photodyn Ther. 2016;14:93–7.
Boehm TK, Ciancio SG. Diode laser activated indocyanine green selectively kills bacteria. J Int Acad Periodontol. 2011;13(2):58–63.
Chiniforush N, Pourhajibagher M, Shahabi S, Kosarieh E, Bahador A. Can Antimicrobial Photodynamic Therapy (aPDT) enhance the endodontic treatment? J Lasers Med Sci. 2016;7(2):76–85.
George S, Kishen A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J Biomed Opt. 2007;12(3):034029.
Pourhajibagher M, Chiniforush N, Parker S, Shahabi S, Ghorbanzadeh R, Kharazifard MJ, Bahador A. Evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells: an in vitro photocytotoxicity investigation. Photodiagnosis Photodyn Ther. 2016:15:13–18. pii: S1572-1000(16)30052-7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Coluzzi, D.J., Aoki, A., Chininforush, N. (2017). Laser Treatment of Periodontal and Peri-implant Disease. In: Coluzzi, D., Parker, S. (eds) Lasers in Dentistry—Current Concepts. Textbooks in Contemporary Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-319-51944-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-51944-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51943-2
Online ISBN: 978-3-319-51944-9
eBook Packages: MedicineMedicine (R0)