Abstract
A two-dimensional LC (2D-LC) method was coupled to an ion mobility–high-resolution mass spectrometer (IM–MS), which enables the separation of complex samples in four dimensions [2D-LC, ion mobility (IM) and mass spectrometry (MS)]. This approach works as a continuous multiheart-cutting LC-system, using a long modulation time of four minutes, in comparison to comprehensive two-dimensional liquid chromatography, which allows the complete transfer of most of the first dimension peaks to the second dimension column without fractionation. Hence, each compound delivers only one peak in the second dimension, which simplifies the data handling even when ion mobility as a third and mass spectrometry as a fourth dimension are introduced. The analysis of different complex samples, such as a plant extract from Hediotys diffusa and Scutellaria barbata, a waste water inflow, and a biocoal sample, was shown. The results of the four-dimensional separation method demonstrate that with the same column combination and the same solvents and gradients, that means without method optimization, totally different samples can be separated with outstanding separation power. Each sample was spiked with cyclophosphamide and ifosfamide and the ion suppression was determined by comparison of the peak area in the complex samples and in pure water after analysis of these samples with a 1D-LC and a 2D-LC approach. It is shown that the 2D-LC method allows an external calibration for the spiked compounds in the plant and waste water sample because of the higher separation power in comparison with 1D-LC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex samples in the field of metabolomics, lipidomics, environmental, and food analysis are often analyzed by liquid chromatography coupled to a high-resolution mass spectrometer (HRMS). But, there are three major problems in the analysis of complex samples with LC–MS, which make an analysis very difficult. At first, with a high-resolution mass spectrometer (HRMS), a fantastic mass resolution with high accurate mass is possible, which allows us to calculate a sum formula. Unfortunately, it is not possible to construct the structure only with the sum formula and usually more as one, often tens or sometimes hundreds of compounds show the same sum formula, which do not allow an identification of the analytes in the sample. Second, the separation power of a one-dimensional LC (1D-LC) is often not sufficient for a baseline separation of complex samples. This leads to coelutions, which means that several, often tens or more, compounds are in the ion source at the same time. Because of this coelution ion, suppression takes place—especially with an electrospray ion source (ESI), which makes a quantitative analysis more difficult. Furthermore, the coelution leads to mixed MS spectra, increasing also the problems in spectra interpretation. The third problem is that the method development for complex samples is very difficult and takes time.
The use of comprehensive two-dimensional liquid chromatography (LC × LC), coupled to a HRMS, can solve the second problem of coelution due to the high separation power of LC × LC. But the problem of identification and method development cannot be solved with LC × LC.
To identify analytes, the combination of ion mobility with HRMS could be a possible way. The coupling of IM to mass spectrometry started in the 1960s and experienced a strong development since the 1990s [1, 2]. The first commercially available IM–MS was the Synapt HDMS system, a quadrupole/traveling-wave IMS/TOF (TWIMS) system introduced in 2006 by Waters [3]. Since 2014, the Agilent 6560 (IM-qTOF-MS), a system using drift time IMS (DTIMS), is available [4]. IMS separates ions according to their size/shape-to-charge ratio and therefore has the ability to measure collision cross-sections (CCS or Ω) of the ions [5]. In comparison to TWIMS, where an analyte-dependent calibration has to be performed to determine CCS because a non-uniform electric field is used [6], DTIMS allows to calculate CCS directly from the observed drift times using the Mason–Schamp equation [7, 8] and only an analyte-independent calibration is necessary for calculation of CCS with the single-field method [9]. Several values for CCS, which are specific for certain substances, are available in the literature for different peptides [10,11,12,13], N-glycans [6], a few drug-like compounds [5], metabolites [14], lipids [15] or different biomolecules [16].
The combination of a one-dimensional LC with ion mobility and mass spectrometry (IM–MS) is a powerful tool, but to reduce ion suppression caused by coelution and increase peak capacity the combination of LC × LC with IM-MS would be much better. This is theoretically possible, but there is a big challenge for data evaluation because currently no available software can simplify the data into a readable plot. In LC × LC, after the first chromatographic dimension, each substance would be fractionated in 3–4 parts and each of these fractions is then injected onto the second dimension column before being transferred to the IM-MS instrument, which leads to very complex data in four dimensions. To evaluate complex samples after an LC × LC-IM-MS analysis, a feature analysis considering all four separation dimensions is needed and it is not clear whether and if so, when such a software will be available. To overcome this and to simplify data evaluation with available software when IM as a further separation dimension is introduced, our group recently developed a method (here called LC + LC) based on a continuous multiheart-cutting method with a long modulation time (4 min), realizing that peaks are mostly not—or for a few compounds only one time—modulated so that most of the compounds are only observed in a single second dimension chromatogram [17, 18]. This allows us to view the data of a 2D-LC separation on a single time axis like in a simple 1D chromatogram.
Here, we will present that this four-dimensional separation method shows an outstanding separation power, which allows a generic approach for totally different complex samples. The powerful separation will be realized by a two-dimensional chromatography, which is based on a continuous multiheart-cutting approach (LC + LC). After the chromatographic separation an orthogonal gas phase electrophoretic separation, the ion mobility spectrometry in a drift tube, follows. The measured drift time allows the calculation of the collision cross-section via the Mason–Schamp equation [5]. As a fourth separation dimension and the final detector, a high-resolution time-of-flight mass spectrometer (qTOF-MS) was used. With the help of a database in which nearly 800 compounds with their m/z and CCS values are listed, an identification of the compounds in the database is possible and, therefore, a suspected target analysis of complex samples can be easily realized. If this database can be increased to several hundreds of thousands compounds, a suspected target analysis will get closer to a non-target analysis.
Materials and Methods
Chemicals
All solvents and mobile phases were of LC–MS grade. Methanol and Acetonitrile were purchased from VWR (Leuven, Belgium) and formic acid was from Merck (Darmstadt, Germany). Ultrapure water was generated with a water purification system from Sartorius Stedim (Goettingen, Germany).
Sample Preparation
Plant Extract
Hedyotis diffusa and Scutellaria barbata were acquired by a pharmacy in Wuppertal (Germany) and stored at −80° C. 40 mL of water was given to 0.5 g of each, Hedyotis diffusa and Scutellaria barbata, and allowed to swell up for 30 min, then cooked for 1 h and finally filtrated. 30 mL of fresh water was added again to the herb and cooked for another 30 min. Both extracts are combined and centrifuged at 4000 rpm for 15 min and finally filtered through a 0.2 µm syringe filter. 900 µL of the eluate was diluted with 100 µL methanol and spiked with 50 µL of a 10 µg/mL solution of cyclosphosphamide and ifosfamide.
Waste Water Inflow
The waste water is an inflow sample of a hospital sewage treatment plant and the sample was stored at <8 °C for less than 7 days. At first, the sample was filtered through a 1 µm-filter and enriched with an SPE cartridge (Waters Oasis HLB, 200 mg/6 mL). 200 mL of the sample was enriched on the SPE and dried by an air flow, wrapped in aluminum foil, and shrink-wrapped in a foil. The cartridges are stored at −20 °C. To analyze a sample, the cartridges were thawed and eluted with 1 mL methanol. The eluate was diluted with 9 mL water and spiked with 500 µL of a 10 µg/mL solution of cyclosphosphamide and ifosfamide.
Biocoal
Biocoal is a coal which is similar to fossil coal. Difference stems from the fact that biocoal is produced artificially from biomass by means of hydrothermal carbonisation (HTC) technology. 1 g of the resulting biocoal was extracted by accelerated solvent extraction with the following parameter (pre-heat 5 min, heat 5 min, static 15 min, flush % 10 vol, purge 150 s, cycles 1, pressure 100 bar, temperature 100 °C, solvent B 100% methanol). The extract was evaporated and re-solved in 1 mL methanol and diluted with 9 mL water and finally spiked with 500 µL of a 10 µg/mL solution of cyclosphosphamide and ifosphamide. Storage at −20 °C.
Instrumentation
An Agilent 1290 Infinity two-dimensional liquid chromatography system (Agilent Technologies, Waldbronn, Germany) was used, consisting of a 1290 Infinity binary pump (G4220A) using a Jet Weaver V35 mixer for both the first and the second dimension, a 1290 Infinity Flexible cube solvent management module (G4227A), a 1290 Infinity HiP sampler (G4226A), and a 1290 Infinity Thermostatted Column compartment (G1316C) with a 2D-LC-Quick Change Valve (2 Pos/4 Port duo valve, G4236A).
This system was used without the 2D-LC-Quick Change Valve and the second pump for the 1D-LC measurements of all samples, which were carried out on an Eclipse Plus C18 Narrow Bore RR column, 50 × 2.1 mm, 3.5 µm Particle from Agilent (Waldbronn, Germany) with water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B) as mobile phase, using a flow of 500 µL/min. A linear gradient was applied starting with 5% B, increasing to 95% B in 30 min at 40 °C. 5 µL of each sample was injected.
For the 2D-LC measurements (LC + LC), a 150 × 2.0 mm Luna CN column packed with 3.0 µm particles (Phenomenex, Aschaffenburg, Germany) was used in the first dimension with water containing 0.1% formic acid and acetonitrile as mobile phase (gradient from 3 to 80% ACN) and a flow rate of 100 µL/min. As second dimension column, an Agilent Poroshell 120 EC-C18 (50 × 3.0 mm; 2.7 µm particle) was used. The mobile phase consisted of water and methanol, both containing 0.1% formic acid. In the second dimension with a flow of 500 µL/min, a shifted gradient [19] was used, starting with a gradient from 10 to 60% methanol in the first sampling cycle and reaching a gradient from 50 to 90% methanol in the last sampling cycle (after 120 min). The injection volume of the prepared sample to the first dimension was 20 µL.
The interface between first and second dimension in the LC + LC method was set up with a dilution and flow splitting system using an additional 1290 Infinity binary pump. For the two-dimensional setup (Fig. 1), pump head B of the additional binary pump is used for diluting the effluent (100 µL/min) of the first dimension column with 0.1% formic acid in water (300 µL/min) to avoid high amounts of organic solvent being injected to the second dimension, resulting in broad peaks and bad resolution [20]. The flow was directed into a Jet Weaver V35 mixer to get a proper mixing of the eluate and the water. After this, a T-piece and Pump Head A of the additional binary pump are used for an active flow splitting, resulting in a total flow of 20 µL/min transferred to the loops.
According to the sampling time of 4 min and a loop volume of 100 µL, the loops are filled up to 80% with the diluted fractions eluting from the first dimension column before injection of the complete loop volume onto the second dimension. For the second dimension, a further binary pump was used. For more details about LC + LC see the recently published works from Stephan et al. [17, 18].
IM-qTOF measurements were performed with an Agilent 6560 IM-qTOF System, equipped with a Dual Agilent Jet Stream electrospray ionization (AJS ESI) Source. Nitrogen was used as buffer gas in the drift tube at a pressure of about 3.95 torr. For mass calibration of the MS as well as for direct CCS calibration, a low concentration tuning mix (G1969-85000, Agilent Technologies, Santa Clara, US) was used.
The instrument parameters for the CCS measurements of standards and for the LC- or LC + LC-IM-qTOF–MS measurements are listed in Table 1.
Results and Discussion
To demonstrate the separation power of the developed LC + LC-ESI-IM-qTOF-MS system, an extract of a Chinese drug (Hediotys diffusa), a waste water inflow sample (collected for 24 h), and a biocoal sample, all spiked with 476 ng/mL cyclophosphamide and ifosfamide, were analyzed with the same analytical method. Figure 2 shows the contour plots of the LC + LC analyses of all three samples. The x- and y-axis demonstrate the separation in the first (120 min) and second dimension (240 s), respectively. Every 4 min, a fraction from the first dimension is injected for separation on the second dimension column. This leads to the contour plots, as shown in Fig. 2, where compounds, presented as lines, are separated in the second dimension (y-axis) each 4 min. For example, Fig. 2 shows that cyclophosphamide and ifosfamide were eluted from the first dimension between 16 and 20 min and separated between 20 and 24 min in the second dimension (Fig. 2, top, middle, and bottom).
Each line of the contour plot is also separated in the third dimension (ion mobility spectrometry), which is an electrophoretical separation in the gas phase and, therefore, a real orthogonal separation in comparison to chromatography. Figure 3 shows the separation of the biocoal sample in the ion mobility drift tube of the fraction, which was separated between 16 and 20 min in the second dimension (black rectangle). The drift time is given in ms (x-axis) and the retention time in the second dimension in min (y-axis).
The red-marked part of the heat map (middle) was shown in more detail (bottom, right).
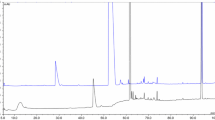
In addition to the separation shown in Fig. 3, for each spot in the heat map, a further separation according to the mass-to-charge ratio of the analytes is realized with the following TOF-MS. Figure 4 shows the mass spectra of some spots from the section of the heat map shown in Fig. 3 (right).
Figure 4 shows the separation of only 6 s (between 18.40 and 18.50 min) of a 60 min analysis of biocoal (there are no signals observed later than 60 min in the 120 min analysis). In these 6 s, more than 12 signals are separated in the drift tube and as an example five resulting pure mass spectra of these compounds are shown in Fig. 4. The pure spectra lead to an easier identification of the analyte and simplify the automated feature search.
Each spiked sample was analyzed three times and in Table 2, the average retention time and peak areas together with the relative standard deviation (RSD) in % are listed. The final retention time given in Table 2 is the sum of the first dimension retention time (4 min modulation time multiplicated with the fraction number) and the retention time in the second dimension. The retention times and also areas are highly reproducible for several injections and from day to day, which was also shown in a former work [17].
Because of the 2D-LC separation before ionization in the ion source, the ion suppression should be reduced and could be a further advantage of the LC + LC method. To verify this, the peak areas of both reference substances in all three samples are compared with the areas of the references in pure water (each 500 ng/mL). To determine the ion suppression, the area of the references in the complex sample was divided by the area in pure water. All values lower than 1 show an ion suppression. In addition, 30 min analyses with a one-dimensional LC and the same detector and ion source as used in LC + LC experiments were done to analyze all samples and determine the ion suppression (Table 3).
Table 3 clearly shows that the ion suppression is drastically reduced with a 2D-LC method such as LC + LC. In addition, it can be observed that the biocoal sample leads to the highest degree of ion suppression but for the plant and waste water sample the LC + LC results demonstrate that an external calibration would be possible due to the higher separation power of LC + LC, compared to 1D-LC. But of course, it has to be considered that the analysis time with LC + LC was also higher in comparison with 1D-LC.
Conclusion
The results demonstrate that the outstanding separation power of LC + LC-IM-qTOF-MS allows to analyze totally different complex samples, such as plant extract, waste water or biocoal, without any method optimization. The same column combination and gradient was used for all samples and the repeatability of the analysis are convincing. Furthermore, it was shown that the ion suppression of the plant and waste water analysis are strongly reduced for the two-spiked references, which would allow an external calibration for these compounds. This was not the case for a 30 min analysis with a one-dimensional approach.
Acknowledgements
We are thankful to Agilent Technologies for the third Infinity pump system and Phenomenex for the HPLC-column. Furthermore, we thank IUTA (Duisburg) for providing samples and standards. The research project no. 18861 of the research association Institut für Energie- und Umwelttechnik e. V. (IUTA) has been funded via AiF within the agenda for the promotion of industrial cooperative research and development (IGF) by the German Federal Ministry of Economics and Technology based on a decision of the German Bundestag.
References
Mukhopadhyay R. IMS/MS: its time has come. Anal Chem. 2008;80:7918–20. doi:10.1021/ac8018608.
Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: separating and assigning structures to ions. Mass Spectrom Rev. 2013;32:43–71. doi:10.1002/mas.21349.
Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, et al. An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument. Int J Mass Spectrom. 2007;261:1–12. doi:10.1016/j.ijms.2006.07.021.
May JC, McLean JA. Ion mobility-mass spectrometry: time-dispersive instrumentation. Anal Chem. 2015;87:1422–36. doi:10.1021/ac504720m.
Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI. Structural characterization of drug-like compounds by ion mobility mass spectrometry: comparison of theoretical and experimentally derived nitrogen collision cross sections. Anal Chem. 2012;84:1026–33. doi:10.1021/ac202625t.
Hofmann J, Struwe WB, Scarff CA, Scrivens JH, Harvey DJ, Pagel K. Estimating collision cross sections of negatively charged N-glycans using traveling wave ion mobility-mass spectrometry. Anal Chem. 2014;86:10789–95. doi:10.1021/ac5028353.
Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH. Ion mobility-mass spectrometry. J Mass Spectrom. 2008;43:1–22. doi:10.1002/jms.1383.
Tao L, McLean JR, McLean JA, Russell DH. A collision cross-section database of singly-charged peptide ions. J Am Soc Mass Spectrom. 2007;18:1232–8. doi:10.1016/j.jasms.2007.04.003.
Kurulugama RT, Darland E, Kuhlmann F, Stafford G, Fjeldsted J. Evaluation of drift gas selection in complex sample analyses using a high performance drift tube ion mobility-QTOF mass spectrometer. Analyst. 2015;140:6834–44. doi:10.1039/c5an00991j.
Counterman AE, Valentine SJ, Srebalus CA, Henderson SC, Hoaglund CS, Clemmer DE. High-order structure and dissociation of gaseous peptide aggregates that are hidden in mass spectra. J Am Soc Mass Spectrom. 1998;9:743–59. doi:10.1016/S1044-0305(98)00052-X.
Valentine SJ, Counterman AE, Clemmer DE. A database of 660 peptide ion cross sections: use of intrinsic size parameters for bona fide predictions of cross sections. J Am Soc Mass Spectrom. 1999;10:1188–211. doi:10.1016/S1044-0305(99)00079-3.
Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal Chem. 2010;82:9557–65. doi:10.1021/ac1022953.
Paglia G, Williams JP, Menikarachchi L, Thompson JW, Tyldesley-Worster R, Halldorsson S, et al. Ion mobility derived collision cross sections to support metabolomics applications. Anal Chem. 2014;86:3985–93. doi:10.1021/ac500405x.
Paglia G, Angel P, Williams JP, Richardson K, Olivos HJ, Thompson JW, et al. Ion mobility-derived collision cross section as an additional measure for lipid fingerprinting and identification. Anal Chem. 2015;87:1137–44. doi:10.1021/ac503715v.
May JC, Goodwin CR, Lareau NM, Leaptrot KL, Morris CB, Kurulugama RT, et al. Conformational ordering of biomolecules in the gas phase: nitrogen collision cross sections measured on a prototype high resolution drift tube ion mobility-mass spectrometer. Anal Chem. 2014;86:2107–16. doi:10.1021/ac4038448.
Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, Clemmer DE. Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am Soc Mass Spectrom. 2007;18:1249–64. doi:10.1016/j.jasms.2007.04.012.
Stephan S, Jakob C, Hippler J, Schmitz OJ. A novel four-dimensional analytical approach for analysis of complex samples. Anal Bioanal Chem. 2016;408:3751–9. doi:10.1007/s00216-016-9460-9.
Stephan S, Hippler J, Kohler T, Deeb AA, Schmidt TC, Schmitz OJ. Contaminant screening of wastewater with HPLC-IM-qTOF-MS and LC + LC-IM-qTOF-MS using a CCS database. Anal Bioanal Chem. 2016;408:6545–55. doi:10.1007/s00216-016-9820-5.
Li D, Schmitz OJ. Use of shift gradient in the second dimension to improve the separation space in comprehensive two-dimensional liquid chromatography. Anal Bioanal Chem. 2013;405:6511–7. doi:10.1007/s00216-013-7089-5.
Stoll DR, Talus ES, Harmes DC, Zhang K. Evaluation of detection sensitivity in comprehensive two-dimensional liquid chromatography separations of an active pharmaceutical ingredient and its degradants. Anal Bioanal Chem. 2015;407:265–77. doi:10.1007/s00216-014-8036-9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Stephan, S., Hippler, J., Köhler, T. et al. A Powerful Four-Dimensional Separation Method for Complex Samples. J. Anal. Test. 1, 1 (2017). https://doi.org/10.1007/s41664-017-0004-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41664-017-0004-x