Abstract
Comprehensive two-dimensional liquid chromatography (LC × LC) has received much attention because it offers much higher peak capacities than separation in a single dimension. The advantageous peak capacity makes it attractive for the separation of complex samples. Various gradient methods have been used in LC × LC systems. The use of continuous shift gradient is advantageous because it combines the peak compression effect of full gradient mode and the tailed gradient program in parallel gradient mode. Here, a comparison of LC × LC analysis of Chinese herbal medicine with full gradient mode and shift gradient mode in the second dimension was performed. A correlation between the first and second dimensions was found in full gradient mode, and this was significantly reduced with shift gradient mode. The orthogonality increased by 43.7 %. The effective peak distribution area increased significantly, which produced better separation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comprehensive two-dimensional liquid chromatography (LC × LC) has shown its powerful ability in the separation of complex sample since the first implementation by Erni and Frei [1] in1978 and was optimized by many other researchers in the last decade with regard to configuration, theoretical, and application aspects [2–7]. The increased peak capacity, selectivity, and resolution of LC × LC is very advantageous in comparison with one-dimensional high-performance liquid chromatography. An increasing number of applications in proteomics [8–10], polymers [11, 12], and natural product analysis [2, 13–16] witness the growing research interest in this technique. In an LC × LC system, the eluents from the first-dimension column are collected via an interface and are subsequently transferred into the second-dimension separation column [7 17]. The elution in both dimensions can be optimized separately, in either an isocratic mode or a gradient mode. Gradient elution is mainly used in reversed-phase liquid chromatography [18]. With respect to isocratic conditions, gradient elution provides a significant improvement in peak capacity owing to the narrower and almost constant bandwidths, especially for strongly retained compounds [19]. Various types of second-dimension gradients have been used in LC × LC, as shown in Fig. 1 and discussed in more detail in several articles [7, 12, 20]:
-
(a)
Full gradient (Fig. 1a): the gradient covers a wide range of mobile-phase compositions in a very short time. The second-dimension gradient program is the same during the whole run. The very steep full gradients run in very short times. This offers high bandwidth suppression and is commonly used in LC × LC. The short run time, in which very steep gradients are used, increases the probability of wrap-around behavior of some strongly retained compounds, which do not have enough time to be eluted within a single fraction and may continue into the next fraction(s). Furthermore, the compounds are not distributed evenly over the available two-dimensional retention area and tend to cluster more or less around the diagonal connecting the lower-left corner with the upper-right corner when the separation mechanisms in the two dimensions are similar [7, 9].
-
(b)
Segment gradient (Fig. 1b): the gradient has a lower gradient concentration in the first segment covering the early fractions and a higher concentration in the second segment [7]. The segment gradient, although somewhat less steep than the full gradient, provides significant bandwidth suppression; the probability of wrap-around behavior is diminished because several segments with various organic solvent concentration ranges can be adjusted to suit the retention of the sample [7, 21].
-
(c)
Parallel gradient (Fig. 1c): the gradient uses a program independent of the second-dimension run. For a single second-dimension cycle, there is a quasi-isocratic elution, which, however, offers a much shallower gradient in the second dimension [2, 7]. Quasi-isocratic elution provides a longer second-dimension time, as post-gradient equilibration is not necessary within the individual fraction cycles. The gradient program can be tailored to the retention characteristic to improve the effective separation space. However, the shallow gradient conditions yield larger bandwidths and thus somewhat lower total two-dimensional peak capacity.
-
(d)
Shift gradient (Fig. 1d): the gradient has a narrower range of mobile-phase composition and continuously varies the concentration range according to the retention [12]. The shift gradient is a combination of the full gradient and the parallel gradient. This gradient suppresses the bandwidth and diminishes the probability of wrap-around behavior.
To achieve the full theoretical peak capacity of a two-dimensional system, the two separation mechanisms should be completely orthogonal. The selectivity of the phase system is of primary concern when designing a two-dimensional separation as it affects the orthogonality and consequently the peak capacity (the number of peaks that can be separated in the available two-dimensional retention space) [22]. In practice, full orthogonality will rarely be encountered [3]. Clearly, the peak capacity of an incompletely orthogonal two-dimensional system will be lower than the theoretical maximum [12]. An LC × LC system with reversed-phase columns in both dimensions is an example of a system without fully independent techniques [13, 14, 21, 23–34]. The use of reversed phase in both dimensions has gained popularity because the separation efficiency is much higher than that of other modes. However, the orthogonality is limited by the correlation between the two dimensions.
The problematic correlation between the two reversed phases could be solved if a proper strategy is used. Therefore, we adopted the shift gradient in this work. This mode was first proposed by Bedani et al. [12], and the theory of shift gradient was discussed in great detail. However, software limited them to transferring only 40 fractions to the second dimension. In the present work, new software designed for LC × LC applications has been used, and an online, full shift gradient application is described. We used a water extract from Hedyotis diffusa and Scutellaria barbata as a complex sample. This extract has been used in some folk remedies in the past, but came to prominence only during the twentieth century in China. The extract is extensively used in modern practice to treat liver, rectal, and lung cancer, as well as some other syndromes. More importantly, 15 % of 1,700 herbal formulae for various cancer treatments contain Hedyotis diffusa as one of the main ingredients. The anticancer activities of this herb have been demonstrated through many preclinical and clinical studies [35].
Experimental
Instrumentation
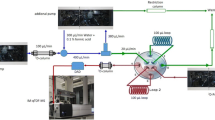
An Agilent 1290 Infinity two-dimensional liquid chromatography system was used, consisting of a 1290 Infinity Flexible Cube solvent management module, a 1290 Infinity sampler, a 1290 Infinity binary pump, a Jet Weaver V35 mixer, a 1290 Infinity diode-array detector, and a novel two-position eight-port valve. The 1290 Infinity system was controlled by the 1290 Infinity 2D-LC method and run-control software. The diode-array detector data in two-dimensional liquid chromatography runs were collected continuously and then imported into GC Image version 2.0 (GC Image, Lincoln, NE, USA) to generate a contour plot.
In the first dimension, a 150 mm × 2.0 mm Luna CN column packed with 3.0-μm particles (Phenomenex, Aschaffenburg, Germany) was used. The mobile phase was water and methanol. In the second dimension, a 50 mm × 3.0 mm Kinetex C18 column packed with 2.6-μm core–shell particles was used. The mobile phase in the second dimension was water and acetonitrile containing 0.1 % formic acid. The two dimensions were connected by a novel two-position eight-port valve (Agilent, Germany), with two 26-μL loops as shown in Fig. 2. In this design, all the flow paths are in the same direction, and no additional loops were needed for bridging between different ports. Here, the fill-in and analysis directions of the loop were the same, but this valve also allows analysis in the opposite direction (there are only minor differences between the two modes; data not shown).
Sample preparation
The two herbs of interest here are used medicinally in combination. Therefore, 0.5 g dried Hedyotis diffusa and 0.5 g dried Scutellaria barbata were soaked in 40 mL water for 30 min. Then, the solution was boiled for 1 h. The extract was collected, and 30 mL water was added to the plant material and boiled for 30 min. The two extracts were combined and centrifuged at 4,000 rpm for 15 min and then passed through a 0.2-μm PTFE filter before use.
Results and discussion
The peak capacity is a characteristic parameter for the potential quality of a chromatographic separation. The peak capacity of a single dimension can be approximated from the peak width at the base [36, 37]. The maximal potential peak capacity in LC × LC analysis should be the product of the peak capacities of the two dimensions [38].
This is suitable when the separations in the two dimensions are completely independent of each other and there is no loss of resolution. This is, however, rarely possible, and losses of resolving power are encountered in any implementation of two-dimensional chromatography as a result of correlation of the two dimensions, undersampling, and back-mixing. Another way to compare the separation power of an LC × LC system is to use the peak-distribution area. The definition of effective peak-distribution area and the vector method for determining the peak-distribution area in the contour plots of two-dimensional chromatograms were discussed in detail in our previous work [37]. The orthogonality O′ of the LC × LC separation can be estimated by Eq. 1 [13]:
where A eff is the effective peak-distribution area, and n 1grd and n 2grd are the theoretical peak capacities of an LC × LC system.
The selectivity of the phase system is of primary concern when designing an LC × LC separation. Several column combinations were investigated to optimize the LC × LC system for the sample used. The combinations of C18 with pentafluorophenyl (PFP) columns, PFP with C8 columns, PFP with C18 columns, and CN with C18 columns were investigated (results not shown). Figure 3 shows the LC × LC analysis of the Chinese herbal medicine with a CN column in the first dimension and a C18 column in the second dimension and optimized full gradient programs (as shown in Table 1). A clear correlation of the first and second dimensions and a small peak-distribution area were observed because the separation mechanisms in the two dimensions were similar (Fig. 3). The maximum peak-distribution area was 6,682. However, the effective peak-distribution area was only 3,320, which leads to an orthogonality of only 0.497. More than half of the separation space was not used.
Separation of herbal medicine by a full-gradient LC × LC system. The first-dimension column was a 150 mm × 2.0 mm Luna CN column packed with 3.0-μm particles; injection 20 μL; mobile phase was and methanol; flow rate 25 μL/min; the initial solvent was 100 % water and was kept for 10 min, then the gradient increased to 64 % methanol and 36 % water in 150 min; UV detection at 270 nm. The second-dimension column was a 50 mm × 3.0 mm Kinetex C18 column packed with 2.6-μm core–shell particles; mobile phase was with 0.1 % formic acid (solvent A) and acetonitrile with 0.1 % formic acid (solvent B); flow rate 2 mL/min. The gradient program is shown in Table 1
In an LC × LC system, with the two dimensions incompletely orthogonal, an LC × LC separation will most likely result in peaks concentrated around the main diagonal line of the separation area (see Fig. 3) [12, 22, 37]. The analytes eluted early in the first dimension will be only weakly retained in the second dimension; the analytes eluted in the middle of the first separation will be eluted in the middle of the second dimension; and the analytes eluted late in the first dimension will be strongly retained in the second dimension. Clearly, for systems using correlated separation mechanisms, two-dimensional gradients covering the full gradient will have a limited peak-distribution area. A narrower organic solvent span can be used then by changing the gradient program according to the elution properties. The tailored gradient program leads to a greater coverage of the separation space and can also reduce the total analysis time [12]. Therefore, a shift gradient was adopted in the present work to optimize the separation. The gradient program used is shown in Fig. 4. The red line is the program of the first-dimension run and the blue line is that of the second-dimension run. The second-dimension gradient covered a narrow organic solvent range, which varied continuously during the LC × LC run. The gradient program starts with 5 % acetonitrile and rises to 50 % ACN over 0.7 min (see Fig. 4b); at the end of the analysis, the gradient program in the second dimension starts at 20 % acetonitrile and rises to 75 % acetonitrile, as shown in Table 1. Because the compounds eluted at the beginning of the first-dimension run are weakly retained on the reversed-phase column, a low narrower gradient program was used in the second dimension. With the organic component ranging from 5 % acetonitrile to merely 50 % acetonitrile, the weakly retained compounds will be retained more strongly in the second column, which results in better separation. The compounds which eluted later in the first-dimension run are strongly retained on the reversed-phase column. Thus, the elution starts from 20 % acetonitrile and goes to 75 % acetonitrile. The higher percentage of organic solvent made possible the efficient elution of the strongly retained compounds.
The same sample as in the analysis shown in Fig. 3 was analyzed with LC × LC with a shift gradient in the second dimension. The resulting chromatogram (counter plot) shows a significant improvement in the retention space (Fig. 5). The use of a shift gradient (gradual increase of the proportion of organic solvent) gave better separation in the second dimension. There is no typical diagonal-line distribution, as was seen in Fig. 3. In addition, because of the narrower solvent range, the pressure turbulence was much smoother and steadier.
In Fig. 6b and c, the second-dimension separation of the 29th (Fig. 6b) and 80th (Fig. 6c) fractions from the first dimension (Fig. 6a) is shown. The smaller variation of the organic content in the gradient (from 7.7 to 54.5 % and from 2.5 to 62.5 % for the 29th and 80th fractions, respectively; Table 1) increases the separation efficiency over that of the full gradient mode (10 to 90 %).
Analysis of the herbal medicine. a First-dimension separation; the conditions (first dimension) were the same as for Fig. 3. b Comparison of the second-dimension separation of the 29th fraction. c Comparison of the second-dimension separation of the 80th fraction. Conditions of full gradient and shift gradient were the same as for Fig. 3 and 5 respectively
The above-mentioned method was used to calculate the peak-distribution area and orthogonality [37]. The theoretical peak-distribution area in shift gradient mode was a little less than with the full gradient because the bandwidth-compression effect was reduced in the shift gradient mode. However, the effective peak-distribution area was much greater. The smooth gradient program according to the retention characteristic in the first dimension effectively improved the separation in the second dimension. For a quantitative comparison of the separation power, the orthogonality can be used. In full gradient mode, O′ is 0.497, but it is 0.714 for the shift gradient mode. This was a significant improvement of LC x LC performance, in which the orthogonality increased by 43.7 %. And the effective peak-distribution area increased from 3,320 to 4,563.
Conclusion
A shift gradient method was used in this work to increase the effective peak-distribution area in LC × LC analysis of a Chinese herbal medicine extract. In the same operation time, the effective peak-distribution area and orthogonality are significantly increased. Therefore, the use of a shift gradient in an LC × LC system brings about a significant improvement in separation power and is a great advantage in the analysis of such complex samples.
References
Erni F, Frei RW (1978) J Chromatogr A 149:561–569
Stoll DR, Li X, Wang X, Carr PW, Porter SEG, Rutan SC (2007) J Chromatogr A 1168:3–43
Guiochon G, Marchetti N, Mriziq K, Shalliker RA (2008) J Chromatogr A 1189:109–168
Dugo P, Cacciola F, Kumm T, Dugo G, Mondello L (2008) J Chromatogr A 1184:353–368
Schoenmakers P, Marriott P, Beens J (2003) LCGC Eur 16:1–4
François I, de Villiers A, Sandra P (2006) J Sep Sci 29:492–498
Jandera P, Hájek T, Česla P (2010) J Sep Sci 33:1382–1397
Washburn MP, Wolters D, Yates JR 3rd (2001) Nat Biotechnol 19:242–247
Stoll DR, Carr PW (2005) J Am Chem Soc 127:5034–5035
Zhu S, Zhang X, Gao M, Yan G, Zhang X (2011) Se Pu 29:837–842
Berek D (2010) Anal Bioanal Chem 396:421–441
Bedani F, Kok WT, Janssen H-G (2009) Anal Chim Acta 654:77–84
Hu L, Chen X, Kong L, Su X, Ye M, Zou H (2005) J Chromatogr A 1092:191–198
Chen X, Kong L, Su X, Fu H, Ni J, Zhao R, Zou H (2004) J Chromatogr A 1040:169–178
Wang Y, Kong L, Lei X, Hu L, Zou H, Welbeck E, Bligh SWA, Wang Z (2009) J Chromatogr A 1216:2185–2191
Ma S, Liang Q, Jiang Z, Wang Y, Luo G (2012) Talanta 97:150–156
Guiochon G, Marchetti N, Mriziq K, Shalliker RA (2008) J Chromatogr A 2:1–2
Jandera P (2012) J Chromatogr A 14:112–129
Snyder LR, Kirkland JJ, Glajch JL (1997) Practical HPLC method development. Wiley, New York
Jandera P, Hájek T, Česla P (2011) J Chromatogr A 1218:1995–2006
Cacciola F, Jandera P, Hajdú Z, Česla P, Mondello L (2007) J Chromatogr A 1149:73–87
Jandera P (2006) J Sep Sci 29:1763–1783
Blahová E, Jandera P, Cacciola F, Mondello L (2006) J Sep Sci 29:555–566
Kivilompolo M, Hyötyläinen T (2007) J Chromatogr A 1145:155–164
Cacciola F, Jandera P, Mondello L (2007) J Sep Sci 30:462–474
Cacciola F, Jandera P, Blahová E, Mondello L (2006) J Sep Sci 29:2500–2513
Jandera P, Česla P, Hájek T, Vohralík G, Vyňuchalová K, Fischer J (2008) J Chromatogr A 1189:207–220
Murahashi T (2003) Analyst 128:611–615
Köhne AP, Dornberger U, Welsch T (1998) Chromatographia 48:9–16
Venkatramani CJ, Zelechonok Y (2003) Anal Chem 75:3484–3494
Gray MJ, Dennis GR, Slonecker PJ, Shalliker RA (2003) J Chromatogr A 1015:89–98
Mnatsakanyan M, Stevenson PG, Conlan XA, Francis PS, Goodie TA, McDermott GP, Barnett NW, Shalliker RA (2010) Talanta 82:1358–1363
Gilar M, Olivova P, Daly AE, Gebler JC (2005) Anal Chem 77:6426–6434
Gilar M, Olivova P, Daly AE, Gebler JC (2005) J Sep Sci 28:1694–1703
Cho WC (ed) (2011) Evidence-based anticancer materia medica. Springer, Heidelberg
Dolan JW, Snyder LR, Djordjevic NM, Hill DW, Waeghe TJ (1999) J Chromatogr A 857:1–20
Dück R, Sonderfeld H, Schmitz OJ (2012) J Chromatogr A 1246:69–75
Giddings JC (1984) Anal Chem 56:1258A–1270A
Acknowledgments
The authors thank the China Scholarship Council (CSC) and the German Academic Exchange Service (DAAD) for funding. We also thank Agilent Technologies and SIM for providing some equipment and software and Phenomenex for the columns.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Schmitz, O.J. Use of shift gradient in the second dimension to improve the separation space in comprehensive two-dimensional liquid chromatography. Anal Bioanal Chem 405, 6511–6517 (2013). https://doi.org/10.1007/s00216-013-7089-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7089-5