Abstract
Purpose
Photobiomodulation therapy has proven to be effective in accelerating cell proliferation, migration, and transcription. The study aimed to analyze the cell viability effects of different parameters of PBMT in a cultured cell line of human gingival fibroblasts after bacterial and ionizing radiation–induced stress.
Methods
Explant technique was used to produce a primary cell culture. Cells were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum until stressful condition induction with lipopolysaccharide of Escherichia coli, Porphyromonas gingivalis protein extract, and ionizing radiation. Laser irradiation was carried out in four sessions set with 660 nm wavelength, an output power of 30 mW and 40 mW and energy density of 2, 3, 4, and 5 J/cm2.
Results
After 24 h from the last laser irradiation session, the groups outputted in 30 mW of power maintained the cell viability while operating with 2, 4, and 5 J/cm2. However, 3 J/cm2 dose significantly decreased cell viability (p < 0.05). When the laser irradiation session was set in a higher power (40 mW), cell viability was reduced using 2, 3, and 5 J/cm2 doses, with statistical significance for 5 J/cm2 (p < 0.001). In addition, operating the same energy using lower power seems to be superior to a higher power, being statically significant for 5 J/cm2 dose (p < 0.001). This pattern followed with all different groups, except by 3 J/cm2.
Conclusions
The present study showed that delivering 2, 4, and 5 J/ cm2 of density of energy with 30 mW and more time of exposure presented better results on cell viability compared to the same density of energy with output power of 40 mW. Further studies comparing density energy should be conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobiomodulation therapy (PBMT) is a treatment modality that has proven to be effective in accelerating wound healing, pain relief, reducing dentin sensitivity and the severity of xerostomia, and herpes labialis frequency [1,2,3]. The application of PBMT in controlling adverse reactions of cancer therapies has shown promising results and reached significant attention. The Multinational Association for Supportive care in Cancer/International Society for Oral Oncology (MASCC/ISOO) guidelines recommend PBMT to patients undergoing head and neck radiotherapy, associated or not with chemotherapy, due to numerous data in which the incidence and severity of oral mucositis have been positively impacted by PBMT [1, 4, 5].
The therapy operates with photon emission from a low-level laser light that transfers low energy to tissues and does not generate heat [2]. The exposure of biological tissues to low-level laser light induces the modulation of cellular functions by activating several pathways involved in cell growth and survival, proliferation, migration, and transcription [2, 6]. At a cellular level, this therapy promotes local, regional, and systemic action, enhancing mitochondrial activity and increasing the production of adenosine triphosphate (ATP) and reactive oxygen species (ROS) [7, 8].
The mechanism of action of PBMT has been discussed and reported in multiple in vitro studies, showing that it can promote stimulation or inhibition, depending on light parameters [9]. A review has analyzed 32 in vitro studies and concluded that an energy density varying from 0.5 to 4.0 J/cm2 and a light wavelength from 600 to 700 nm for PBMT could enhance proliferation of different cell types [10]. These findings are in conformity with previous in vitro studies and literature reviews that show positive biostimulation effects on fibroblasts, keratinocytes, and osteoblasts [11,12,13,14].
Moreover, the primary challenge is applying the optimal laser parameters to deliver an ideal amount of energy to enhance the metabolism and improve clinical outcomes [2, 8, 15]. To test the hypothesis that the same density of energy with lower power could lead to an improvement on cell viability, stimulated gingival fibroblasts were exposed to different densities of energy and two different output powers. Thus, this pilot study aimed to analyze the effects of different parameters of PBMT on cell viability, using human gingival fibroblast cells stimulated with bacterial and ionizing radiation–induced stress.
Materials and methods
Cell isolation and primary culture
The explant technique was used to obtain a cell line of human gingival fibroblasts. Before the fragment’s collections, ethics registration and approval had been obtained from the Human Research Ethics Committee of the Health Sciences College of the University of Brasília (CAAE Nº 78,679,717.6.0000.0030), and all the donors signed the understanding and written consent. Then, the gingival fragments of young donors who underwent third molar extraction surgery were isolated and transported to the Laboratory of Oral Histopathology immersed in cold Dulbecco’s modified Eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO) supplemented with 20% fetal bovine serum (FBS) (Gibco®, Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). The fragments were washed twice with phosphate-buffered saline (PBS), explanted into small fragments, placed on 6-well plates, stabilized with a glass coverslip, covered by 2 mL of DMEM supplemented with 20% FBS and antibiotics, and maintained in a humidified incubator with ideal conditions (37 ºC and 5% CO2). The culture medium was replaced every 3 days and when 80–90% confluency was reached, cells were detached with trypsin (0.25%)/EDTA (1 mM) solution (Sigma-Aldrich, St. Louis, MO) and replaced in 100-mm dishes with DMEM plus 10% FBS and antibiotics to expand the culture or stored at − 80 ºC in a freezing solution containing FBS and 8% dimethyl sulfoxide (DMSO).
Bacterial and ionizing radiation–induced stress
For the stressful condition induction, cells were treated with three stimuli, as established in previous experiments of the Laboratory of Oral Histopathology of the University of Brasília (data not shown): lipopolysaccharide (LPS) of Escherichia coli 0111:B4 purchased from Sigma-Aldrich (St. Louis, Missouri, USA), Porphyromonas gingivalis protein extract (Pg), and ionizing radiation (IR). The protein extract of Pg was prepared in the University of Campinas, São Paulo, Brazil, as described by Albiero et al. [16] and donated to the Laboratory of Oral Histopathology of the University of Brasília. In order to achieve stress induction, before beginning the experiments, the cells were treated with LPS (1 µg/mL) and Pg (5 µg/mL), incubated for 1 h, and then irradiated with 8 Grays (Gy).
Photobiomodulation therapy parameters
The laser irradiation sessions were performed using a continuous-wave InGaAlP laser (Photon Lase III DMC, São Paulo, Brazil) in punctual and contact mode. The wavelength 660 nm laser was applied with output powers of 40 and 30 mW. The energy densities were 2, 3, 4, and 5 J/cm2 for each power. The complete treatment was performed in four sessions with 6-h intervals from each session according to Meneguzzo et al. [17] and Moreira et al. [18]. The overall parameters are presented in Table 1.
Experimental groups
The laser irradiation protocol presented in Table 1 was carried out in nine groups, considering a negative control group/vehicle (stimulated model without PBMT) and four energy densities (2, 3, 4, and 5 J/cm2) irradiated using two power doses (30 mW and 40 mW).
Cell viability
Gingival fibroblasts were seeded into 96-well plates at a density of 5 × 103 and incubated for 24 h. Then, the stressful stimuli protocol was applied in nine biological replicates for each group. The first photobiomodulation session was conducted right after ionizing radiation, according to experimental groups, and repeated three more times, every 6 h. After 24 h from the last session, the cells viability was assessed by the tetrazolium dye MTT (3-(4,5-dimethyl- thiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction assay. For this, 10 µL of MTT solution (Sigma-Aldrich, St. Louis, Missouri, USA) was added, and the cells were incubated and protected from light for 4 h. Then, the solution was removed, and 100 µL of acidified isopropanol (25 mL of isopropanol + 104 µL of HCl 100%) was added to each well. Cellular viability was analyzed after absorbance measurement using the spectrophotometer Thermo Plate TP Reader at 570 nm (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Statistical analysis
The Shapiro–Wilk test was applied to assess data normality. As data resulted in parametric distribution, the one-way ANOVA followed by Dunnett’s and Turkey’s post-tests were applied to compare groups. The level of statistical significance was 95% (p < 0.05). The tests were performed using GraphPad Prism 9.3.0 (GraphPad Software, CA, EUA).
Results
Combination of energy and power can differently modulate cell response
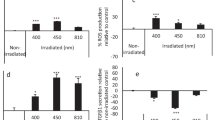
The PBMT effect was analyzed in cells stimulated with bacterial and ionizing radiation–induced stress. For cell viability analysis, all treated groups irradiated with different doses of low-level laser were compared to a control group (non-irradiated with low-level laser). After 24 h from the last laser irradiation session, the groups outputted in 30 mW of power presented cell viability when operated with 2, 4, and 5 J/cm2; however, 3 J/cm2 dose significantly decreased mitochondrial activity (p < 0.05). In contrast, when the laser irradiation session was set up in a higher power (40 mW), the cell viability was reduced using 2, 3, and 5 J/cm2 doses, with statistical significance for 5 J/cm2 (p < 0.001). Hence, the results indicated that the combination of energy and power can differently modulate cell response (Fig. 1).
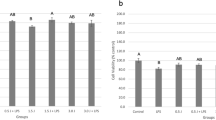
Cell viability after application of different photobiomodulation doses powered at 30 and 40 mW compared to a model of ionizing radiation and bacterial-induced stress. LPS lipopolysaccharide of Escherichia coli; Pg protein extract of Porphyromonas gingivalis; IR ionizing radiation. Analytical statistics: One-way ANOVA for parametric data followed by Dunnett’s post-test (*p < 0.05; *** < 0.001)
Inhibitory response could be dependent on laser parameters
The same density of energy can be delivered setting different parameters, while performing a PBMT protocol [9]. In this study, two power outputs (30 and 40 mW) were set, using different times of exposition to deliver and compare four energy densities (2, 3, 4, and 5 J/cm2). The results demonstrated that operating the same energy, using lower power, seems to be superior to a higher power, being statically significant for 5 J/cm2 dose (p < 0.001). This pattern followed with all different groups, except by 3 J/cm2. Thus, bacterial and ionizing radiation–induced cells exposed to four PBMT sessions of 2, 4, and 5 J/cm2 were more capable to keep mitochondrial activity, operating at 30 mW than 40 mW (Fig. 2). These results suggested that inhibitory response could be power-dependent.
Discussion
The effects of PBMT depend on laser parameters, including wavelength, power, energy, spot area, and time of exposure. Also, these combined aspects could differently influence cell activity, such as proliferation [15, 19,20,21]. Thus, the effects of different parameters in wound healing are in current interest, since it can enhance cell growth depending on the set output.
This pilot study investigated the effect of low-power InGaAlP laser irradiation comparing the output power of 30 and 40 mW and the corresponding energy densities of 2, 3, 4, and 5 J/cm2, while laser irradiation was performed in four sessions with an interval of 6 h between them. In accordance with published findings, lower power delivering the same density of energy was more capable to maintain cell viability than higher output power which suggests the influence of PBMT parameters in cell response [22,23,24,25,26,27].
Brueghel and Dop Bärr [9] suggested that power density and time of exposure seem to be more important than the total energy dose of PBMT on human fibroblasts. Azevedo et al. [20] found an inverse influence between power density and cell growth [9, 22, 28, 29]. Previous studies showed the same results, indicating that higher output power had inhibitory characteristics [6, 15, 24, 30].
Low doses of PBMT activate a proton gradient, releasing calcium from mitochondria into the cell’s cytoplasm. This process stimulates a cascade of cellular functions and protein secretion enhancing cell proliferation. In contrast, higher doses can release an excessive amount of calcium promoting hyperactivity of calcium-adenosine triphosphatase, inhibiting cell metabolism [30,31,32]. Our results indicated that the same energy density, outputted in the power of 30 mW, showed higher viable cells than 40 mW, suggesting that the power can determine the stimulatory or inhibitory effect of the laser irradiation on cellular responses.
Hawkins and Abrahams [30] demonstrated that the cumulative effect from the accumulated doses determines the biomodulation effect, multiple exposures at higher doses cause additional stress and significantly reduced cell viability, and lower doses and fewer exposures maintained cell viability.
The response of the tissue exposed to PBMT protocols is the combination of time of exposure, total energy delivered, and cell response. The study design was defined with a primary culture of gingival fibroblasts collected from patients. Even though the followed inclusion criteria considered healthy patients, the PBMT results may vary according to patients’ response, cell type, tissue condition, and explant methods used for the culture [24]. Also, it is possible that, in this preliminary study, the total energy delivered in 3 J/cm2 and 30 mW of output power associated with the time that the energy was delivered was unable to produce effect comparable with other parameters; this could be associated with different degrees of the effect produced by PBMT [6].
Considering that wound healing depends on cell proliferation, it is crucial to study and deeper understand the effect of power densities of PBMT in vitro, since this is the first step to understand the cascade process in a complex body [31]. There is not a well-defined standard of output power setting, although various studies have been performed to observe the effects of low-level laser in cellular response [2, 8, 10,11,12]. Comparing different protocols of application can contribute to determine the optimal combination of parameters according to different cell types and expected results. In addition, it is possible to obtain lower or higher doses to reach an energy density, controlling the output of power and the laser irradiation time which can facilitate the replication of protocols even when different equipment is available [6, 9].
There are some limitations in this study that should be addressed. First, this is a pilot study that only focused on cell viability. Considering the importance of laser parameters for different culture conditions, a single trial experiment assessing different PBMT outputs was conducted to allow future analysis since cell response depends on that. Second, the definition of output parameters was based on literature research, which contemplates different cell types and objectives. Thus, the future proposal is to continuously study cellular response after bacterial and ionizing radiation–induced stress, comparing output powers of 30 mW and lower with further experiments to analyze the effects of PBMT on cell morphology, proliferation, migration, gene and protein expression, and specific pathway signalization.
Conclusion
The present pilot study showed that PBMT effects can be influenced by the power outputted parameter. After analyzing PBMT set in 660 nm wavelength, output power of 30 and 40 mW, and energy densities of 2, 3, 4 and 5 J/cm2, it was possible to conclude the PBMT effect. The results using a protocol of PBMT, set with 660 nm of wavelength in four sessions of laser application with 6-h interval suggest that delivering 2, 4, and 5 J/cm2 of density of energy with output power of 30 mW, leading to more time of exposure, presented better results on cell viability compared to the same energy density with 40 mW. However, further studies comparing density energy should be conducted.
References
Sonis ST, Hashemi S, Epstein JB, Nair RG, Raber-Durlacher JE (2016) Could the biological robustness of low-level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol 54:7–14. https://doi.org/10.1016/j.oraloncology.2016.01.005
Pellicioli AC, Martins MD, Dillenburg CS, Marques MM, Squarize CH, Castilho RM (2014) Laser phototherapy accelerates oral keratinocyte migration through the modulation of the mammalian target of rapamycin signaling pathway. J Biomed Opt 19(2):028002. https://doi.org/10.1117/1.JBO.19.2.028002
Soares DM, Ginani F, Henriques ÁG, Barboza CA (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30(3):1171–1174. https://doi.org/10.1007/s10103-013-1436-9
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM et al (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Zadik Y, Arany PR, Fregnani ER, Bossi P, Antunes HS, Bensadoun RJ, Gueiros LA, Majorana A, Nair RG, Ranna V, Tissing WJE, Vaddi A, Lubart R, Migliorati CA, Lalla RV, Cheng KKF, Elad S (2019) Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27(10):3969–3983. https://doi.org/10.1007/s00520-019-04890-2
Marques MM, Diniz IM, de Cara SP, Pedroni AC, Abe GL, D’Almeida-Couto RS, Lima PL, Tedesco TK, Moreira MS (2016) Photobiomodulation of dental derived mesenchymal stem cells: a systematic review. Photomed Laser Surg 34(11):500–508. https://doi.org/10.1089/pho.2015.4038
Cronshaw M, Parker S, Anagnostaki E, Mylona V, Lynch E, Grootveld M (2020) Photobiomodulation and oral mucositis: a systematic review. Dent J (Basel) 8(3):87. https://doi.org/10.3390/dj8030087
Ferreira LS, Diniz IMA, Maranduba CMS, Miyagi SPH, Rodrigues MFSD, Moura-Netto C, Marques MM (2019) Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med Sci 34(4):659–666. https://doi.org/10.1007/s10103-018-2637-z
van Breugel HHFI, Dop Bärr PR (1992) Power density and exposure time of He–Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528–537
AlGhamdi KM, Kumar A, Moussa NA (2021) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 1:237–249. https://doi.org/10.1007/s10103-011-0885-2
Eduardo FP, Mehnert DU, Monezi TA, Zezell DM, Schubert MM, Eduardo CP, Marques MM (2007) Cultured epithelial cells response to phototherapy with low intensity laser. Lasers Surg Med 39:365–372
Almeida-Lopes L, Rigau J, Zangaro RA, Guidugli-Neto J, Jaeger MM (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Fujihara NA, Hiraki KR, Marques MM (2006) Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg Med 38:332–336
Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP (2004) Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34:260–265
Anders JJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 33:183–184
Albiero ML, Stipp RN, Saito MT, Casati MZ, Sallum EA, Nociti FH, Silvério KG (2017) Viability and osteogenic differentiation of human periodontal ligament progenitor cells are maintained after incubation with Porphyromonas gingivalis protein extract. J Periodontol 88(11):e188–e199. https://doi.org/10.1902/jop.2017.170116
Meneguzzo DT, Eduardo CP, Ribeiro MS, Marques MM (2008) Influence of the fractioned irradiation energy in the phototherapy with low intensity laser on the growth of human dental pulp fibroblasts. Mechanisms for Low-Light Therapy III 6846(68460A):1–9. https://doi.org/10.1117/12.761220
Moreira MS, Sarra G, Carvalho GL, Gonçalves F, Caballero-Flores HV, Pedroni ACF, Lascala CA, Catalani LH (2021) Marques MM (2021) Physical and biological properties of a chitosan hydrogel scaffold associated to photobiomodulation therapy for dental pulp regeneration: an in vitro and in vivo study. Biomed Res Int 25(2021):6684667. https://doi.org/10.1155/2021/6684667
Stein E, Koehn J, Sutter W, Wendtlandt G, Wanschitz F, Thurnher D, Baghestanian M, Turhani D (2008) Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr 120(3–4):112–117
Tuby H, Maltz L, Oron U (2007) Low-level laser irradiation pro- motes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med 39:373–378
Zaccara IM, Ginani F, Mota-Filho HG, Henriques ÁC, Barboza CA (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30(9):2259–2264. https://doi.org/10.1007/s10103-015-1803-9
Azevedo LH, de Paula EF, Moreira MS, de Paula EC, Marques MM (2006) Influence of different power densities of LILT on cultured human fibroblast growth: a pilot study. Lasers Med Sci 21(2):86–89. https://doi.org/10.1007/s10103-006-0379-9
Barboza CAG, Ginani F, Moura SD et al (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein 12(1):75–81
Eduardo Fde P, Bueno DF, de Freitas PM, Marques MM, Passos-Bueno MR, Eduardo Cde P, Zatz M (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40(6):433–438. https://doi.org/10.1002/lsm.20646
Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD (2005) Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med 36:8–12
Pereira AN, Eduardo CP, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Staffoli S, Romeo U, Amorim RNS, Migliau G, Palaia G, Resende L, Polimeni A (2017) The effects of low level laser irradiation on proliferation of human dental pulp: a narrative review. Clin Ter 168(5):e320–e326. https://doi.org/10.7417/T.2017.2028
Hawkins DH, Abrahamse H (2007) Time-dependent responses of wounded human skin fibroblasts following phototherapy. J Photochem Photobiol B 88:147–155
Matic M, Lazetic B, Poljacki M et al (2003) Low level laser irradiation and its effect on repair processes in the skin. Med Pregl 56:137–141
Hawkins D, Abrahamse H (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714
Friedmann H, Lubart R, Laulicht I et al (1991) A possible ex- planation of laser-induced stimulation and damage of cell cultures. J Photochem Photobiol 11:87–91
Schindl A, Schindl M, Pernerstorfer-Schon H et al (2000) Low-intensity laser therapy: a review. J Invest Med 48:312–326
Funding
This study received financial support from DGP – UnB (Graduate Dean of the University of Brasilia) no. 0004/2021 in the name of J. Amorim dos Santos. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication: M.M. Monteiro and J. Amorim dos Santos are supported by CAPES (Coordination for the Improvement of Higher Education Personnel), Brazil. V. Paiva Barbosa and E.N.S. Guerra are supported by the CNPq (National Council for Scientific and Technological Development), Ministry of Education, Brazil.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Mylene Martins Monteiro, Juliana Amorim dos Santos, and Victor Paiva Barbosa. The first draft of the manuscript was written by Mylene Martins Monteiro and Juliana Amorim dos Santos, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Human Research Ethics Committee of the Health Sciences College of the University of Brasilia (CAAE No. 78679717.6.0000.0030).
Consent to participate
Informed consent was obtained from all individual participants that donated gingival fragments in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monteiro, M.M., Amorim dos Santos, J., Paiva Barbosa, V. et al. Effects of different photobiomodulation therapy doses on cell viability after bacterial and ionizing radiation–induced stress: a pilot in vitro study. Laser Dent Sci 6, 205–210 (2022). https://doi.org/10.1007/s41547-022-00162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-022-00162-1