Abstract
Photobiomodulation (PBM) therapy is used to stimulate cell proliferation and metabolism, as well as reduce inflammatory cytokine synthesis, which plays a main role in the long-term stability of implants. This study assessed the response of gingival fibroblasts cultured on titanium (Ti) and zirconia (ZrO2), submitted to PBM and exposed to lipopolysaccharide (LPS). Cells seeded on Ti and ZrO2 were irradiated (InGaAsP; 780 nm, 25 mW) 3 times, using 0.5, 1.5, and 3.0 J/cm2 doses, and exposed to Escherichia coli LPS (1 μg/mL). After 24 h, cell viability (alamarBlue, n = 8), interleukin 6 (IL-6) and 8 (IL-8) synthesis (ELISA, n = 6), and IL-6 and vascular endothelial growth factor (VEGF) gene expression (qPCR, n = 5) were assessed and statistically analyzed (one-way ANOVA, α = 0.05). Cell morphology was evaluated by fluorescence microscopy. Increased cell viability occurred in all groups cultured on Ti compared with that of the control, except for cells exposed to LPS. Fibroblasts cultured on ZrO2 and LPS-exposed exhibited reduced viability. PBM at 3.0 J/cm2 and 1.5 J/cm2 downregulated the IL-6 synthesis by fibroblasts seeded on Ti and ZrO2, as well as IL-8 synthesis by cells seeded on ZrO2. Fibroblasts seeded on both surfaces and LPS-exposed showed increased IL-6 gene expression; however, this activity was downregulated when fibroblasts were irradiated at 3.0 J/cm2. Enhanced VEGF gene expression by cells seeded on Ti and laser-irradiated (3.0 J/cm2). Distinct patterns of cytoskeleton occurred in laser-irradiated cells exposed to LPS. Specific parameters of PBM can biomodulate the inflammatory response of fibroblasts seeded on Ti or ZrO2 and exposed to LPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental implant abutments are the main prosthetic components that promote the connection between the dental crown and the implant surgically installed in the bone. Since these components are in direct contact with the oral soft tissues, their biocompatibility and local biological sealing ensure the success of the treatment [1].
The biological sealing (BS) is defined as the intimate interaction between gingival epithelium, connective tissue, and the surface of implant components. The function of BS is to prevent local bacterial contamination at the implant-tissue interface, preventing soft tissue recession and underlying bone crest resorption, which are relevant factors for the long-term success of dental implants [2, 3]. Direct attachment of the connective tissue to the abutment surface is necessary to enable the occurrence of adequate BS [4]. It has already been demonstrated that some soft tissue parameters such as tissue phenotype, mucosal height with approximately 3 mm, and the amount of keratinized mucosa are very important to ensure the health of peri-implant tissues. Insufficient keratinized mucosa promotes higher accumulation of plaque, gingival index scores, epithelial migration, and attachment loss around the implants which may be the result of poor adhesion of the peri-implant tissue to the abutment surface [5].
However, when an infectious/inflammatory process is established in the connective tissue around the implant components, it is commonly known as peri-implant mucositis (PIM) [6]; the cells lose their adhesion to the abutment, triggering migration of the epithelium and compromising esthetics [7, 8]. Thus, the formation of an effective and biologically functional BS capable of resisting against bacterial infiltration is a challenge to maintaining the implant rehabilitation esthetics and function.
Previous studies showed that photobiomodulation (PBM) may enhance cell metabolism and proliferation as well as collagen and protein synthesis. This therapy also increases angiogenesis and causes over-expression of growth factors by the cells exposed to light irradiation [9,10,11,12,13]. PBM therapy using laser seems to stimulate bone and mucosal tissue healing around dental implants. Specific laser irradiation parameters improve cell adhesion to Ti and ZrO2 surfaces, stimulate cell proliferation and metabolism, and reduce inflammatory cytokine synthesis, which play a main role in the long-term stability of implants [14, 15]. Therefore, the use of PBM may be suggested to accelerate the cell adhesion process and stimulate synthesis of the extracellular matrix molecules and other types of proteins, in addition to cell proliferation, thereby supporting the formation an effective biological barrier after the insertion of dental implant abutments [14].
The composition, chemical and physical properties, and biocompatibility of materials used to produce abutments influence the quantity, type, conformation, and orientation of the membrane-bound proteins involved in adhesion between the oral mucosal tissue and abutment surfaces [16]. Although fibroblast adhesion to polished surfaces of titanium (Ti) and zirconia (ZrO2) occurs in a similar manner, it remains unclear whether PBM can improve cell adhesion by inducing more effective biological sealing (BS) on the two types of surfaces, in addition to modulating the synthesis of inflammatory cytokines produced by the cells when they are in the presence of inflammatory stimuli.
Therefore, the aim of this investigation was to evaluate whether specific parameters of PBM can upregulate the metabolism and influence the inflammatory response of gingival fibroblasts seeded on Ti and ZrO2 surfaces and then exposed to Escherichia coli LPS stimulus.
Materials and methods
Ti and ZrO2 disc preparation methods
Standardized titanium (Ti) and zirconia (ZrO2) discs (ICE ZIRKON transluzente plus, Zirkonzahn, Italy) measuring 8 mm in diameter and 2 mm thickness were polished with 400, 600, 1200 (Ti discs), and 2000 (ZrO2 discs) grit abrasive papers dampened with deionized water to eliminate grooves resulting from machining procedures [17]. After being polished, the Ti discs were cleaned according to a protocol defined by Park et al. [18]. The ZrO2 discs were submitted to the specific sintering furnace procedure.
Primary culture of gingival fibroblasts
A primary culture of gingival fibroblasts was obtained from gingival tissue donated by a healthy young patient (22 years old) during a surgical tooth extraction procedure (approved by the Research Ethics Committee of the Araraquara School of Dentistry - UNESP (CAEE: 55629215.7.0000.5416). Cells were isolated using an enzymatic cleavage method with collagenase type I (Worthington Biochemical Corp., Lakewood, NJ, USA) diluted in culture medium DMEM (Dulbecco’s Modified Eagle’s Medium, Gibco, Carlsbad, CA, USA) without fetal bovine serum (FBS, Gibco) [13, 19].
Cell culture
Gingival fibroblasts were cultured (5 × 104 cells/disc) on the Ti and ZrO2 discs placed in wells of 24-well plates (TPP) using DMEM containing 10% FBS. After this, the cells were irradiated using the LASERTable device (InGaAsP, 780 ± 3 nm, 0.025 W) [10, 20, 21]. This device features 12 laser diodes (InGaAsP) which are activated simultaneously enabling standardization for parallel irradiation. The laser parameters used in this study were low fluence (infrared at 780 ± 3 nm) and continuous mode operation at 0.025 W of power. Three irradiations were performed with 24-h intervals between them. The irradiation area was 2 cm2 per sample, at a distance of 2.5 cm between the laser diode and cells, with energy densities of 0.5, 1.5, and 3 J/cm2. Based on the following formula: energy density = power × exposure time × area−1 and the fixed parameters (power and area), it was determined the exposed times for each energy dose established.
After the third irradiation, the DMEM was replaced by a fresh culture medium without FBS, and the cells were exposed or not to the inflammatory stimulus using 1 μg/mL of Escherichia coli LPS (E. coli LPS), according to the groups presented in Table 1. Fibroblasts seeded on polished Ti and ZrO2 discs, without treatment (PBM and/or LPS exposition) were considered control groups. After 24 h, the experimental protocols were performed. The experimental design can be found in the Supplementary material (please see the “experimental_design” file and its documentation).
Cell viability (alamarBlue®, n = 8)
A solution of 450 μL of FBS-free DMEM containing 50 μL of alamarBlue® (Invitrogen, Eugene, Oregon, USA) was applied to the cells seeded on Ti and ZrO2 discs, individually placed in wells of 24-well plates. After 4-h incubation, two aliquots of 100 μL were obtained from each well and then transferred to wells of 96-well plates. Cell fluorescence was determined using a fluorimeter (Synergy - H1 - Biotek Winooski, VT, USA), at the wavelengths of 570 and 600 nm.
IL-6 and IL-8 synthesis (ELISA, n = 6)
The synthesis of inflammatory interleukins (IL-6 and IL-8) was assessed by enzyme-linked immunosorbent assay (ELISA), based on an antigen-antibody reaction, according to the manufacturer’s recommendations (R&D Systems, Minneapolis MN, USA).
IL-6 and VEGF gene expression (qPCR, n = 5)
For gene expression analysis, the 3.0 J/cm2 laser dose of PBM was selected to investigate the IL-6 and VEGF gene expression based on IL-6 and IL-8 synthesis results, which demonstrated better interleukin modulation using the highest dose of irradiation compared with other doses tested. RNA isolation was performed using RNAqueous Kit (Applied Biosystems, Foster City, CA, USA) filtration system. The RNA quantification of each sample was determined in a spectrophotometer (Take 3 System - Synergy H1). For gene expression analysis, the 3.0 J/cm2 laser dose of PBM was selected to investigate the IL-6 and VEGF gene expression based on IL-6 and IL-8 synthesis results, which demonstrated better interleukin modulation using the highest dose of irradiation compared with other doses tested. RNA isolation was performed using RNAqueous Kit (Applied Biosystems, Foster City, CA, USA) filtration system. The RNA quantification of each sample was determined in a spectrophotometer (Take 3 System - Synergy H1). For 1 μg of each RNA sample obtained, the cDNA was synthesized using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Grand Island, NY, USA). The cDNA amplification was performed according to the manufacturer’s instructions (Applied Biosystems) by a thermal cycling (Thermocycler, Bio-Rad, Hercules, CA, USA) at 25 °C (10 min), 37 °C (120 min), 85 °C (5 s), and 4 °C (∞).
After cDNA synthesis, the expression of the genes coding the IL-6 and VEGF was evaluated by quantitative PCR. Reactions were prepared according to the manufacturer’s recommendations using TaqMan Universal PCR Master Mix reagents (Applied Biosystems) as well as target-specific primers and probes (TaqMan assay). Fluorescence readings were performed using a Step One Plus Thermocycler equipment (Applied Biosystems) and analyzed by the Step One Software 2.1 (Applied Biosystems). All reactions were normalized according to the expression of the selected endogenous gene (βactin), and the results were represented as fold change.
Evaluation of phalloidin expression and cell spreading by fluorescence microscopy
Initially, the gingival fibroblasts were washed with 1× PBS and fixed in 4% paraformaldehyde for 10 min. After this, the cell membrane was permeabilized with 0.1% Triton X-100 for 15 min followed by incubation with the phalloidin fluorophore (Alexa Fluor®, Life Technologies, Eugene, OR, USA) for 20 min at concentrations indicated by the manufacturer for labeling of the cellular cytoskeleton. Nuclear counter tagging was carried out using the Hoechst fluorescent probe (Invitrogen) prepared at a concentration of 1: 5000 in buffered saline (1X PBS). Thus, the samples were analyzed under a fluorescence microscope (Leica, DMI4000B, Leica Microsystems, Wetzlar, Germany).
Statistical analysis
All experiments carried out in this laboratorial study, except qPCR, were performed at 2 different occasions for cell viability and protein synthesis. The final number of replicates was 8 for cell viability, 6 for IL-6 and IL-8 synthesis, and 5 for gene expression. The number of replicates by protocol/group was based on papers published in high-impact journals and previous experiments performed in our laboratory. Regarding blinding, only the researcher who performed the statistical analysis was blinded, that is, she was not aware of which data corresponded to each experimental group. Data from the alamarBlue, IL-6 and IL-8 synthesis (ELISA), and IL-6 and VEGF gene expression (qPCR) assays were subjected to Shapiro-Wilk normality test, Levene homoscedastic test, and the parametric ANOVA test complemented by the Tukey or Games-Howell test for pairwise group comparison. The significance level of 5% was established for statistical inferences. For qualitative analysis carried out in fluorescence microscopy, the results were descriptive.
Results
Increased cell viability was observed in all experimental groups in which the cells were seeded on the Ti surface compared with the respective control group, except for the group in which the fibroblasts were exposed to E. coli LPS (P < 0.05; Fig. 1a). Only the cells seeded on ZrO2 surfaces and exposed to LPS inflammatory stimulus exhibited reduction in cell viability (P < 0.05; Fig. 1b). In general, fibroblasts cultured on ZrO2 surfaces and submitted to laser irradiation showed approximately half of the viability observed for the cells seeded on Ti surfaces and laser-irradiated.
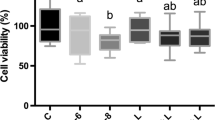
Enhanced IL-6 synthesis was observed in almost all groups in which gingival fibroblasts were seeded on Ti surfaces in comparison with the control group (P < 0.05; Fig. 2a). Cells irradiated with a dose of 3.0 J/cm2 and either exposed to E. coli LPS or not did not differ from the control group (P > 0.05; Fig. 2a). In general, cells seeded on Ti surfaces showed higher IL-6 synthesis values than those cells cultured on ZrO2 irrespective of the treatments (LLLT or LPS) performed (Fig. 2 a and b).
a Synthesis of IL-6 by gingival fibroblasts cultured on Ti discs. (% control group), n = 6; groups identified by different letters are statistically different from each other (Tukey, P < 0.05). b Synthesis of IL-6 by gingival fibroblasts cultured on ZrO2 discs. (% control group), n = 6; groups identified by different letters are statistically different from each other (Games-Howell, P < 0.05). c Synthesis of IL-8 by gingival fibroblasts cultured on Ti discs. (% control group), n = 6; groups identified by different letters are statistically different from each other (Tukey, P < 0.05). d Synthesis of IL-8 by gingival fibroblasts cultured on ZrO2 discs. (% control group), n = 6; groups identified by different letters are statistically different from each other (Tukey, P < 0.05)
Cells seeded on Ti surfaces had increased IL-8 synthesis, mainly in the groups in which the fibroblasts were exposed to E. coli LPS and then irradiated with laser at doses of 0.5 J/cm2 and 1.5 J/cm2 (Fig. 2c). Enhanced IL-8 synthesis was observed in the group in which cells seeded on ZrO2 were exposed to LPS. This interleukin synthesis was more effectively modulated by the laser irradiation of cells at 1.5 J/cm2 dose (Fig. 2d).
For fibroblasts seeded on Ti and ZrO2 surfaces, the IL-6 gene expression increases occurred when the cells were exposed to LPS (P < 0.05). However, the PBM of cells at a dose of 3.0 J/cm2 was capable of modulating this IL-6 gene expression (Fig. 3 a and b). In general, fibroblasts seeded on ZrO2 surfaces showed higher IL-6 gene expression in the control and LPS groups compared with cells seeded on Ti surfaces (Fig. 3b).
a Gene expression of IL-6 by gingival fibroblasts cultured on Ti discs. n = 5; groups identified by different letters are statistically different from each other (Tukey, P < 0.05). b Gene expression of IL-6 by gingival fibroblasts cultured on ZrO2 discs. n = 5; groups identified by different letters are statistically different from each other (Tukey, P < 0.05). c Gene expression of VEGF by gingival fibroblasts cultured on Ti discs. n = 5; groups identified by different letters are statistically different from each other (Tukey, P < 0.05). d Gene expression of VEGF by gingival fibroblasts cultured on ZrO2 discs. n = 5; groups identified by different letters are statistically different from each other (Tukey, P < 0.05)
Enhanced VEGF gene expression was observed in fibroblasts seeded on Ti surfaces and then submitted to PBM at a dose of 3.0 J/cm2 (Fig. 3c). However, cells seeded on ZrO2 surfaces and laser-irradiated exhibited no difference relative to VEGF gene expression when compared with the control group (Fig. 3d). In general, the expression of this growth factor was upregulated by the laser irradiation of cells seeded on Ti in comparison with ZrO2 surfaces.
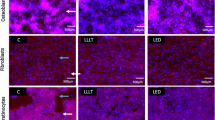
Fluorescence microscopy analysis demonstrated an increased number of fibroblasts and reduced cell cytoskeletal labeling in the groups in which the cells were seeded on Ti and ZrO2 surfaces and then exposed to E. coli LPS, indicating low cell spreading values for both substrates. However, the groups in which fibroblasts were either exposed to E. coli LPS or not, and submitted to PBM at a dose of 3.0 J/cm2, a larger number of cells were observed on Ti and ZrO2 surfaces. These cells exhibited higher cytoskeletal labeling values when compared with those of the control and LPS groups, demonstrating that PBM induced cell proliferation and spreading on the substrates (Fig. 4).
Discussion
Fibroblasts play an important and comprehensive role in tissue healing. These cells secrete a large amount of extracellular matrix (ECM) components, such as collagen and proteoglycans, which can fill wound defects creating conditions for complete tissue repair [22, 23]. Therefore, the main purpose of the present study was to assess and compare the metabolism of human gingival fibroblasts seeded on Ti and ZrO2 surfaces, submitted to laser irradiation and then exposed to inflammatory stimulus with LPS. Cell viability analysis showed a different behavior depending on the substrate on which the cells were cultured (Ti or ZrO2).
Fibroblasts seeded on Ti surfaces and irradiated with laser showed greater viability than that observed in the control group (Fig. 1a) irrespective of whether they were exposed to E. coli LPS stimulus or not. Cells seeded on ZrO2 discs, either submitted to PBM or not, and exposed to LPS had decreased viability (Fig. 1b). These different cellular behaviors could be attributed to the materials used as substrate. It has already been demonstrated that fibroblasts attach mainly to microgrooves present on the Ti surface, indicating that this type of material may be interesting to use for manufacturing implant abutments since it creates a better biological seal in the transmucosal area [24]. In addition, cells exhibited better adhesion to Ti placed in an aqueous environment since hydroxyl groups were formed on the Ti surface favoring the adsorption of proteins present in the cells [25]. The reduced viability observed in fibroblasts seeded on ZrO2 surfaces and exposed to LPS may be related to the difficulty of these cells adhering to this specific material surface due to the metabolic changes caused by mitogen-activated protein kinase (MAPK) signaling pathway, which mediates cell proliferation and cell survival [26].
Previous studies demonstrated that fibroblasts stimulated with LPS activate the synthesis of Toll-like receptor 4 (TLR4) and lipopolysaccharide-binding protein (LBP), which are responsible for producing inflammatory cytokines that play a role in the innate immune response [25, 27]. Fibroblasts seeded on Ti and ZrO2 surfaces and exposed to LPS showed higher levels of IL-6 and IL-8 synthesis compared with those of the control group. This cell function was downregulated in the groups in which the fibroblasts were previously irradiated with laser, especially at a dose of 3.0 J/cm2. Increased synthesis of pro-inflammatory mediators present in acute inflammation, such as IL-6 and IL-8, has been observed in other in vitro studies, in which cultured cells were exposed to E. coli LPS [28, 29]. A study has been reported that during gingival tissue inflammation, the increased expression of IL-6 and IL-8/CXCL8 by epithelium cells induced an intense local recruitment of polymorphonuclear cells, mainly neutrophils [30]. However, the exacerbated synthesis of these inflammatory mediators reduced the mitogenic and migratory activities of oral mucosa cells, as previously demonstrated by Basso et al. [29] and Pansani et al. [21]. In the present in vitro study, enhanced IL-6 gene expression occurred in the groups in which cells were seeded on Ti and ZrO2 surfaces and exposed to LPS. This result was corroborated by the data obtained using the ELISA immunoassay. Therefore, the authors determined that the IL-6 gene expression was increased in this investigation and that this interleukin was specifically synthetized by fibroblasts. In the groups in which the fibroblasts were irradiated with laser at a dose of 3.0 J/cm2 and exposed to LPS, modulation of IL-6 gene expression occurred, irrespective of the type of surface on which the cells were seeded. These data ratify the biomodulator potential of PBM, which can prevent, or at least, reduce the occurrence of local inflammation.
Changes in the cytoskeleton and increased number of cells were observed in this study when gingival fibroblasts were seeded on Ti and ZrO2 surfaces and exposed to LPS compared with their respective control groups. These results seem to indicate a discrete cytopathic effect of LPS, which is capable of interfering with cellular functions. However, when fibroblasts were seeded on both Ti and ZrO2 surfaces, then irradiated with laser at a dose of 3.0 J/cm2 and either exposed to LPS or not, higher cytoskeleton labeling and cell proliferation values were observed. Similar data have previously been shown by Garrido et al. [31], when laser irradiation at a dose of 3.0 J/cm2 delivered to pulp cells enhanced the fibronectin synthesis as well as increased the mitochondria and rough endoplasmic reticulum in the laser-irradiated cells. Based on these interesting findings, the authors considered the further clinical use of PBM to be promising and justifiable for improving cell adhesion and downregulating the local tissue inflammation.
Although most studies have described the intracellular effects of infrared laser on mitochondrial cytochrome c oxidase, Tang et al. [32] demonstrated that PBM acts in the signaling pathway of transforming growth factor beta 1 (TGF-β1) and induces the expression of human β-defensin 2 (HBD-2) which participates in wound healing and immunomodulation process [32, 33]. TGF-β promotes wound re-epithelialization, inducing keratinocyte migration, stimulating extracellular matrix deposition, and contracting the wound by myofibroblasts present in the tissue [34].
The increased VEGF gene expression is directly related to endothelial cell proliferation, an event that results in local neovascularization, inflammatory cell chemotaxis, and exudate extravasation [35]. In this study, enhanced VEGF gene expression was observed in cells seeded on Ti surfaces and submitted to PBM at a dose of 3.0 J/cm2, demonstrating that this specific therapy activates cellular and molecular events capable of contributing to tissue vascularization. This study obtained data similar to that of das Neves et al. [36]. The authors used the same irradiation parameters to assess the in vivo potential of this therapy to increase the expression of VEGF, by immunohistochemistry assay. In the present study, decreased VEGF gene expression was observed in the groups in which fibroblasts were seeded on ZrO2 surfaces, laser-irradiated, and either exposed to LPS or not, in comparison with the group exposed to LPS. However, no difference was found between all experimental groups and the control (Fig. 4d).
As the distance between the laser tip and the target area increases, the delivered energy is scattered over a larger area, intensely affecting power density at a cellular level [20]. Therefore, if the target area is larger than the laser tip diameter, it is recommended a sequence of punctual irradiations to cover the entire area. In the present study, the laser tip was kept 2 cm distant from the bottom of the well of 24-well plates. This is because between each laser diode of the LASERTable device and the bottom of a well, a pair of lenses is placed to promote the divergence of the laser beam. These lenses allow the irradiation of the entire bottom of the well, and therefore, all cells adhered to that area are irradiated with a single laser application. Preliminary tests proved that the irradiation delivered was the same at any point of the well bottom as reported previously [20].
Despite that limitation, it was observed that different cell responses occurred when fibroblasts were seeded on Ti and ZrO2 surfaces, demonstrating that biological sealing may be affected by the material used to produce the abutment. The presence of inflammatory stimulus, such as E. coli LPS, may affect signaling pathways that mediate cell proliferation and survival. Such as described by de Souza Costa et al. [37], data from in vitro studies cannot be directly extrapolated to clinical situations. However, the fact that specific laser irradiation parameters stimulated adhesion and metabolism of gingival fibroblasts seeded on different substrates indicated that this type of phototherapy may be further assessed in vivo with the purpose of determining its role in biological barrier formation after prosthetic rehabilitations.
Conclusion
According to the methodology used in this laboratory study, the authors could conclude that specific parameters of PBM upregulate the metabolism as well as biomodulate the inflammatory response of gingival fibroblasts seeded on Ti or ZrO2 surfaces and exposed to infectious agents. These exciting laboratorial data may drive the development of clinical protocols capable of stimulating the adhesion of mucous cells to the surface of abutments, improving the biological seal and preventing the installation of a persistent local inflammatory process.
References
Welander M, Abrahamsson I, Berglundh T (2008) The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res 19(7):635–641. https://doi.org/10.1111/j.1600-0501.2008.01543.x
Park JC, Kim HM, Ko J (1998) Effects of extracellular matrix constituents on the attachment of human oral epithelial cells at the titanium surface. Int J Oral Maxillofac Implants 13(6):826–836
An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A (2012) Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent Mater 28(12):1207–1214. https://doi.org/10.1016/j.dental.2012.08.007
Moon YH, Yoon MK, Moon JS, Kang JH, Kim SH, Yang HS, Kim MS (2013) Focal adhesion linker proteins expression of fibroblast related to adhesion in response to different transmucosal abutment surfaces. J Adv Prosthodont 5(3):341–350. https://doi.org/10.4047/jap.2013.5.3.341
Lin GH, Madi IM (2019) Soft-tissue conditions around dental implants: a literature review. Implant Dent 28(2):138–143. https://doi.org/10.1097/ID.0000000000000871
Lindhe J, Meyle J (2008) Group D of European workshop on periodontology. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol 35(8):282–285. https://doi.org/10.1111/j.1600-051X.2008.01283.x
Lindhe J, Berglundh T (1998) The interface between mucosa and implant. Periodontol 2000 17:47–54. https://doi.org/10.1111/j.1600-0757.1998.tb00122.x
Iglhaut G, Schwarz F, Winter RR, Mihatovic I, Stimmelmayr M, Schliephake H (2014) Epithelial attachment and downgrowth on dental implant abutments. J Esthet Restor Dent 26(5):324–331. https://doi.org/10.1111/jerd.12097
Hawkins DH, Abrahamse H (2006) The role of lasers fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon lasers irradiation. Lasers Surg Med 38(1):74–83. https://doi.org/10.1002/lsm.20271
Basso FG, Pansani TN, Turrioni APS, Bagnato VS, Hebling J, de Souza Costa CA (2012) In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent 2012:719452
Basso FG, Oliveira CF, Kurachi C, Hebling J, Costa CAS (2013) Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28:367–374. https://doi.org/10.1155/2012/719452
Basso FG, Soares DG, Pansani TN, Cardoso LM, Scheffel DL, de Souza Costa CA, Hebling J (2016) Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Laser Surg Med 48(10):1006–1014. https://doi.org/10.1002/lsm.22553
Pansani TN, Basso FG, Turrioni APS, Soares DG, Hebling J, de Souza Costa CA (2017) Effects of low-level laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals. Lasers Med Sci 32(1):45–52. https://doi.org/10.1007/s10103-016-2081-x
Khadra M, Kasem N, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Laser therapy accelerates initial attachment and subsequent behaviour of human oral fibroblasts cultured on titanium implant material: a scanning electron microscope and histomorphometric analysis. Clin Oral Implants Res 16(2):168–175. https://doi.org/10.1111/j.1600-0501.2004.01092.x
Lopes CB, Pinheiro AL, Sathaiah S, da Silva NS, Salgado MA (2007) Infrared laser photobiomodulation (lambda 830 nm) on bone tissue around dental implants: a Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg 25(2):96–101. https://doi.org/10.1089/pho.2006.2030
Thevenot P, Hu W, Tang L (2008) Surface chemistry influences implant biocompatibility. Curr Top Med Chem 8(4):270–280. https://doi.org/10.2174/156802608783790901
Pansani TN, Basso FG, Souza IDR, Hebling J, de Souza Costa CA (2019) Characterization of titanium surface coated with epidermal growth factor and its effect on human gingival fibroblasts. Arch Oral Biol 102:48–54. https://doi.org/10.1016/j.archoralbio.2019.03.025
Park JH, Olivares-Navarrete R, Baier RE, Meyer AE, Tannenbaum R, Boyan BD, Schwartz Z (2012) Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater 8(5):1966–1975. https://doi.org/10.1016/j.actbio.2011.11.026
Pansani TN, Basso FG, Soares DG, Hebling J, Costa CA (2016) Functional differences in gingival fibroblasts obtained from young and elderly individuals. Braz Dent J 27(5):485–491. https://doi.org/10.1590/0103-6440201600993
Lins EC, Oliveira CF, Guimarães OC, de Souza Costa CA, Kurachi C, Bagnato VSA (2013) A novel 785-nm laser diode-based system for standardization of cell culture irradiation. Photomed Laser Surg 31(10):466–473. https://doi.org/10.1089/pho.2012.3310
Pansani TN, Basso FG, Soares DG, Turrioni APDS, Hebling J, de Souza Costa CA (2018) Photobiomodulation in the metabolism of lipopolysaccharides-exposed epithelial cells and gingival fibroblasts. Photochem Photobiol 94(3):598–603. https://doi.org/10.1111/php.12877
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17(2):153–162. https://doi.org/10.1111/j.1524-475X.2009.00466.x
Cho JW, Kim SA, Lee KS (2012) Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med 29(1):32–36. https://doi.org/10.3892/ijmm.2011.803
Pae A, Lee H, Kim HS, Kwon YD, Woo YH (2009) Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed Mater 4(2):025005. https://doi.org/10.1088/1748-6041/4/2/025005
Benz K, Schöbel A, Dietz M, Maurer P, Jackowski J (2019) Adhesion behaviour of primary human osteoblasts and fibroblasts on polyether ether ketone compared with titanium under in vitro lipopolysaccharide incubation. Materials (Basel) 27: 12(17). https://doi.org/10.3390/ma12172739
Navolanic PM, Steelman LS, McCubrey JA (2003) EGFR family signaling and its association with breast cancer development and resistance to chemotherapy. Int J Oncol 22(2):237–252. https://doi.org/10.3892/ijo.22.2.237
Yuen CY, Wong SL, Lau CW, Tsang SY, Xu A, Zhu Z, Ng CF, Yao X, Kong SK, Lee HK, Huang Y (2012) From skeleton to cytoskeleton: osteocalcin transforms vascular fibroblasts to myofibroblasts via angiotensin II and Toll-like receptor 4. Circ Res 111(3):e55–e66. https://doi.org/10.1161/CIRCRESAHA.112.271361
Dale BA (2002) Periodontal epithelium: a newly recognized role in health and disease. Periodontol 30:70–78. https://doi.org/10.1034/j.1600-0757.2002.03007.x
Basso FG, Pansani TN, Soares DG, Scheffel DL, Bagnato VS, Costa CAS, Hebling J (2015) Biomodulation of inflammatory cytokines related to oral mucositis by low-level laser therapy. Photochem Photobiol 91(4):952–956. https://doi.org/10.1111/php.12445
Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ (2007) Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res 86(4):306–319. https://doi.org/10.1177/154405910708600403
Garrido PR, Pedroni ACF, Cury DP, Moreira MS, Rosin F, Sarra G, Marques MM (2019) Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J Photochem Photobiol B 194:149–157. https://doi.org/10.1016/j.jphotobiol.2019.03.017
Tang E, Khan I, Andreana S, Arany PR (2017) Laser-activated transforming growth factor-β1 induces human β-defensin 2: implications for laser therapies for periodontitis and peri-implantitis. Periodontal Res 52(3):360–367. https://doi.org/10.1111/jre.12399
Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, Mooney JD (2014) Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci Transl Med 28; 6(238): 238ra69. https://doi.org/10.1126/scitranslmed.3008234
Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT (2014) FOXO1, TGF-β regulation and wound healing. Int J Mol Sci 15;15(9): 16257-69. https://doi.org/10.3390/ijms150916257
Capp C, Zennig N, Wajner S, Maia AL (2009) The role of vascular endothelial growth factor in tumor. Revista HCPA 29:51–59
das Neves LM, Leite GP, Marcolino AM, Pinfildi CE, Garcia SB, de Araújo JE, Guirro EC (2017) Laser photobiomodulation (830 and 660 nm) in mast cells, VEGF, FGF, and CD34 of the musculocutaneous flap in rats submitted to nicotine. Lasers Med Sci 32(2):335–341. https://doi.org/10.1007/s10103-016-2118-1
de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784. https://doi.org/10.1016/j.dental.2014.04.010
Funding
The authors received financial support from the São Paulo Research Foundation - FAPESP (grant no. 2015/19364-8) and the National Council for Scientific and Technological Development - CNPq (grant no. 408721/2018-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in accordance with the local Ethics Committee (CAAE: 55629215.7.0000.5416).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Pansani, T.N., Basso, F.G. & de Souza Costa, C.A. In vitro effects of photobiomodulation applied to gingival fibroblasts cultured on titanium and zirconia surfaces and exposed to LPS from Escherichia coli. Lasers Med Sci 35, 2031–2038 (2020). https://doi.org/10.1007/s10103-020-03062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03062-7