Abstract
The ignition of flammable gas leaks can cause catastrophic accidents involving explosions and detonations. A detailed chemical kinetic reaction mechanism is used here to determine the influence of chemical inhibitors on the detonability of hydrogen-air mixtures. A series of halogenated compounds and halons are used in the present study to describe the inhibition of hydrogen oxidation under detonating conditions. The chemical effects of retardants were studied in the present work, and their efficacy in suppressing a given detonation wave was estimated. The inhibiting efficiency of halogen acids, halomethanes, haloethanes, haloethenes, and carbon compounds containing more than one halogen atom is studied in the current work. One-dimensional ZND computations were carried out for the H2-air mixtures in the presence of different series of retardants. The numerical results suggest that iodine-containing halogenated compounds are the most effective class of inhibitors, whereas fluorine-containing halogenated compounds are the least effective class of inhibitors for H2-air detonating mixtures. The ability of retardants to successfully suppress a detonation wave was evaluated by considering the effect they produce on the induction length and the detonation velocity. For H2-air mixtures, based on the induction length analysis, bromo-ethane (C2H5Br) was found to exhibit the highest inhibition efficiency among all the inhibitors studied in the present work. Similarly, CF3I showed the best inhibition effect for H2-air mixtures based on the CJ detonation velocity analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catastrophic hydrogen explosions or detonations could occur during accidents at nuclear power plants and in process industries. It is now known that explosions resulting from the buildup of pressurized hydrogen gas in such power plants and process industries could be devastating to both human life and property. One of the probable causes of hydrogen accidents is accidental leakage that results in the formation of a vapor cloud. Hydrogen vapor clouds can ignite with very low energy input, nearly 1/10th of that required for the gasoline-air mixture due to the high flammability and reactivity of hydrogen. Once ignited, deflagration to detonation transitions (DDT) can occur in such systems with minimal or even no congestion if the mixture is sufficiently reactive and turbulent. Such vapor clouds can quickly explode, generating very high overpressures that could be devastating to both life and property. Preventing detonation transmission and survivability during hydrogen fire accidents is essential to avoid substantial damage to life and property. Hence, the safety systems should be adequate and practical for containing detonations to reduce life and property damage. Inhibition and extinction of hydrogen oxidation in flames and detonations by chemical inhibitors is a practical and theoretical problem that has been studied experimentally for many years. Examination of how chemical inhibitors alter the flame and detonation structures and propagation rates can lead to more efficient methods of controlling combustion in potentially hazardous environments. Since most chemical inhibitors are potentially dangerous to use in the laboratory, modeling analyses can provide a safe alternative to some experimental studies where they can lead to valuable insights into the fundamental physics that govern all combustion processes.
The mechanism of detonation inhibition/suppression for hydrogen and hydrocarbon fuel–air detonation using chemical inhibitors is essential for safety and regulation and had been studied extensively. The addition of chemical inhibitors to the fuel-oxidizer mixtures could alter the structure of a detonation wave and its propagation rates, resulting in controlled combustion in a potentially hazardous environment (Oran and Gamezo 2007). The inhibition mechanism by which the chemical inhibitors suppress or inhibit a detonation wave can be broadly classified into two categories: the physical and chemical mechanisms (Babushok et al. 1997). When the effects of dilution and heat capacity dominate, the inhibition mechanisms are governed by the physical mechanisms. The chemical mechanism of inhibition is the result of the radical scavenging action and the chemical kinetic effects. Here, the chemical inhibitors or flame retardants interfere with the combustion chemistry and can help in scavenging the active radicals to bring about significant inhibition effects. Flame retardants or suppressants behave both chemically and physically. Halogen acids and hydrocarbon compounds containing one or more halogen atoms are an excellent class of inhibitors. Babushok et al. analyzed inhibiting characteristics of several kinds of halogenated compounds on the flame speed of C1-C2 hydrocarbon flames and used an extensive inhibition parameter to provide the relative efficiency of these additives (Babushok et al. 1996). Experimentally it is observed that iodine-containing species are more effective in reducing the flame speed than the bromine-containing species, followed by the chlorine and fluorine-containing species (Evariste et al. 1996). The inhibition effect of chemical inhibitors increases with increasing inhibitor concentration in the reacting fuel-oxidizer mixtures. Also, a linear relationship was found to exist between the inhibitor concentration and the burning velocity. The flame speed or burning velocity is an important parameter and can be used to quantify the inhibition effect of fire retardants in a laminar flame (Westbrook 1982a).
In the case of detonations, the detonation cell width is found to be important in determining the limiting parameters for detonation propagation (Gavrikov et al. 2000; Dahake and Singh 2022a; Kumar et al. 2022, Iyer et al. 2022a). The effect of inhibitors on the structure of a detonation wave can be evaluated by investigating the corresponding effect they produce on the detonation cell width or size. The induction and reaction zone length in a ZND detonation structure can be empirically correlated to the detonation cell size or width for many fuel–air-diluent mixtures over a wide range of initial and boundary conditions (Lee 1984, 2008; Crane et al. 2019; Knystautas et al. 1984; Stamps et al. 2006; Ciccarelli et al. 1994). Thus, the characteristics of a self-sustained detonation wave (cell size) can be correlated to the physicochemical characteristics of explosive mixtures (induction lengths/times, lengths of the heat release zone). A dramatic increase in induction or reaction zone length could decouple the reaction zone from the leading shock front and could transform a self-sustained detonation into a loosely coupled shock-flame complex. On a more fundamental level, the reaction zone in such cases increases in thickness and eventually decouples from the leading shock front. In such scenarios, the underlying detonation wave attenuates and degenerates into a deflagration wave. Thus, the inhibition efficiency of chemical inhibitors can be measured from the corresponding induction length analysis of a given fuel–air mixture. Also, inhibition mechanisms by chemical inhibitors could lead to the damping of transverse waves by altering the structure of the underlying detonation wave. Transverse waves that are critical for the formation of detonation cells play an important role in the self-sustained propagation of a detonation wave. Transverse waves are unsteady and continue to decay in time unless the periodic reignition of the reactive mixture is accomplished behind the leading shock front. Chemical inhibitors could inhibit the periodic reignition of the mixture behind the leading shock front by decreasing the detonation velocity to levels such that the post-shock temperatures fall below the autoignition temperatures of the reactive mixture. In such cases, transverse waves are damped, and the underlying detonation wave then transforms into a loosely coupled shock-flame complex and eventually degenerates into a deflagration wave. Thus, the reduction in detonation velocity could also be used as a parameter to determine the inhibition efficiency of chemical inhibitors.
The halogenated inhibitors have been widely studied for laminar flames using different test flow configurations for a wide range of fuel-oxidizer mixtures (Hamins et al. 1994; Pagliaro et al. 2016; Xu et al. 2017; Takahashi et al. 2015). However, the inhibition effect of a particular inhibitor greatly depends on the combustion environment. It has been observed that the same inhibitors could have different inhibition effects in laminar flames and detonations. For example, Moen et al. (Moen et al. 1984) reported that CF3Br has a better inhibition efficiency than CO2 in suppressing laminar flames, but the efficiency gets reversed in the suppression of a detonation wave for the same fuel–oxidizer mixtures. Experimental studies have been carried out by Mathieu et al. (Mathieu et al. 2015) to study the effect of CF3I addition on auto ignition and flame speed for hydrocarbon fuels. However, their study was limited to laminar flames. Santosh et al. studied the effect of the addition of CO2, H2O, and CF3I on H2-O2/air detonations, where they reported the promotion and suppression effects of CF3I addition at smaller and larger concentrations, respectively (Kumar and Singh 2021; Kumar et al. 2021). Experimental and computational studies were carried out by Evariste et al. to determine the inhibition efficiency of halogenated compounds in suppressing the underlying detonation wave (Evariste et al. 1996). Halogenated compounds generally increase the induction length and time scales of a fuel-oxidizer mixture by slowing down the rate of chain-branching reactions. A larger induction length and time scale for a given fuel-oxidizer mixture are indicative of a less detonable mixture and vice versa (Lee 2008; Dahake et al. 2022a; Iyer et al. 2022b; Kumar and Singh 2023; Ivin and Singh 2023). Thus, an understanding of the inhibition mechanisms of different sets of halogenated compounds for suppressing a detonation wave is important for improving risk management in the oil and gas industry and will help in creating a framework on which worker safety regulations can be based. The present work is offered as a contribution towards this end.
In the past, different halogenated compounds were ranked according to their ability to reduce the laminar burning velocity of a given flame. However, their effectiveness in inhibiting more violent forms of combustion, such as those involving detonations, has not been clarified. The current study aims to bridge the gap in our fundamental understanding of the inhibition mechanisms of chemical inhibitors for suppressing a detonation wave. In the present work, different series of chemical inhibitors are ranked based on their ability to suppress a detonation wave. Different classes of halogenated inhibitors such as halogenated acids, halomethanes, haloethanes, haloethenes, and inhibitors containing more than one halogen atom are studied in the present work. The effect of the addition of these halogenated compounds on hydrogen-air detonation was studied using the one-dimensional ZND model. The inhibition efficiency of chemical inhibitors is ranked based on their ability to increase the induction length of a ZND detonation structure in stoichiometric H2-air explosive mixtures. The inhibition efficiency of the halogenated compounds is also ranked based on their ability to decrease the CJ detonation velocity.
Numerical Methodology
One-dimensional ZND detonation model is adopted in the present study to study the inhibition efficiency of a series of halogenated compounds. A detailed chemical kinetic reaction mechanism is used here to describe the inhibition of hydrogen oxidation by halogenated species. The ZND computations are carried out using the modified version of the CalTech Shock and Detonation Toolbox (Kao and Shepherd 2008; Browne et al. 2008). Cantera (Goodwin et al. 2009) integrated with MATLAB is used to compute the species concentration profiles and the chemical reaction rates. USC Mech II was used to model the oxidation chemistry of hydrogen-air mixtures (Wang et al. 2007). The detailed chemical kinetic reaction mechanism for several retardants was obtained from Westbrook (1982b) and Manion et al. (2015). The chemical reactions of hydroxyl radicals (OH) and oxygen atoms (O) with halogenated species that were not considered by Westbrook (1982b) have also been included in the reaction model to get a clear understanding of the inhibition mechanisms of halogenated compounds. The chemical kinetics model was validated by computing the laminar burning velocity for hydrogen-air mixtures in the presence of inhibitors and comparing it with the available experimental data. The computational results obtained were found to be in close agreement with the experimental data of Noto et al. (Noto et al. 1998). The methodology employed for the computation of the detonation length/time scales and the critical detonation parameters can be found in the literature elsewhere (Noto et al. 1998; Dahake et al. 2021, 2022b, c; Iyer et al. 2022b; Dahake and Singh 2022b). After the estimation of the ZND detonation structure, the induction length can be obtained as the location of the maximum temperature gradient. Similarly, the induction delay time is defined as the period from the shock to the maximum temperature gradient point (Lee 2008; Iyer et al. 2021; Dahake and Singh 2022d; Dahake et al. 2022c).
Results and Discussions
The induction length and time scales of a ZND detonation structure were computed for H2-air-retardant mixtures at P0 = 1 atm and T0 = 298 K. The induction length and CJ velocity were computed for hydrogen-air-retardant mixtures to ascertain the effects of halogenated compounds on the given parameters. The retarding efficiency of different series of inhibitors is studied. The chemical inhibitors considered include the halogen acids HF, HCl, HBr, and HI, species with one F, Cl, Br, or I atom substituted for an H atom in methane, ethane, and ethylene. More complex halogenated hydrocarbons with more than one halogen species like CF4, CF3Cl, CF3Br, and CF3I are also considered. These complex halogenated hydrocarbons are frequently used as fire and flame suppressants. The inhibition mechanism of these series of inhibitors involves different reaction pathways by which active radicals like H, OH, and O are abstracted from the radical pool. These active radicals play a vital role in propagating the chain-branching reactions and thereby improve the detonability of a given fuel-oxidizer mixture. Thus, in the presence of halogenated species, the available radical pool is reduced, which lowers the overall rate of chain-branching reactions. This results in the suppression or inhibition of an underlying detonation wave. The important reaction pathways by which H radicals are extracted from the radical pool by different series of retardants are given below.
For halogen acids:
where X = F, Cl, Br, or I atom. Reactions (R1) to (R4) have been shown to describe inhibition by halogen acids in hydrocarbon-air and hydrogen-air flames. The reactions (R1), (R2), and (R4) above constitute a catalyzed recombination of H atoms into relatively non-reactive H2 or HX molecules, which are then unavailable for chain branching through reaction with O2 molecules (see reaction R14) or reaction with fuel molecules in the pre-flame pyrolysis region. This reduces the available radical pool and thus lowers the overall rate of chain branching.
For halogenated hydrocarbons, it is observed that C\(-\)X bond energies are much lower than the C-H bond energies. For instance, CH3\(-\)H, CH3\(-\)Cl, CH3\(-\)Br, and CH3\(-\)I have bond energies of 104, 83.5, 70, and 56 kcal/mole, respectively. Therefore, the abstraction of the halogen atom has a larger rate than the abstraction of the H atom for the halogenated hydrocarbons. It must be noted that initial halogen atom abstraction quickly reduces the kinetic mechanism to that for the halogen acid HX. The following inhibition reactions become important for the halogenated hydrocarbons.
For halogenated hydrocarbons:
where X represents the halogen atoms, while R represents the methyl, ethyl, or vinyl radicals in the above reactions. Like the reactions R1–R4, the net result of the cycle of reactions R5–R8 is H + H = H2, a catalyzed recombination of H atoms into relatively non-reactive H2 molecules, which decreases the overall rate of chain branching.
For inhibition by halons like CF3X, where X = Cl, Br, I, the simple mechanism of halogen abstraction followed by hydrocarbon fragment oxidation does not apply. Because of the differences between the bond energies for the C\(-\)F and C\(-\)X bonds in CF3X, the X atom is removed first. CF3 radicals are consumed along two parallel paths, one leading through CF2O and the other through radical recombination to form C2 species, particularly CH2CF2. The final state for the F atoms is HF which is not further oxidized. The following inhibition reactions become important for halons.
For halons:
where X represents the halogen atoms, while R represents the trifluoromethyl group (CF3) in the above reactions. The above reactions result in the binding of active radicals like H, O, and OH and their substitution with less reactive radical R. Further, the abstraction of O and OH radicals are accomplished by the reactions:
Thus, halogenated species act by catalyzing the recombination of H, O, and OH radicals into relatively non-reactive molecules. Due to the scavenging of active radicals like H, O, and OH, the overall rate of chain-branching reactions is reduced. The reactions R1-R14 compete with the chain-branching reactions R15-R16 and inhibit flame propagation.
Chemical Efficiency of Inhibitors at 20,000 ppmv of Retardant
The inhibition efficiency of halogenated compounds was computed based on the increase in the induction length. The mitigating ability of the retardants on H2-air detonating mixtures can be evaluated by the induction length analysis since the induction length or time is a critical factor in determining the detonability of fuel-oxidizer mixtures. In the given analysis, the induction length of the retardant case (H2-air-retardant mixture) is compared with the induction length of the no-retardant case (H2-air mixture). The calculations were performed for stoichiometric H2-air mixtures at an initial pressure and temperature of 1 atm and 298 K, respectively. The results are tabulated in Table 1.
The last column of the table represents the factor of increase (FOI) in the induction length. It is defined as the ratio of the induction length of the H2-air-retardant case to the induction length of the H2-air case without retardants. The stoichiometric H2-air mixtures were doped with 20,000 ppmv (molar concentration) of the flame retardants.
The results in Table 1 show the inhibition efficiency of different retardants in decreasing order based on the increase in induction length. The addition of 20,000 ppmv of bromo-ethane increases the induction length by a factor of 39.10 compared to the induction length of H2-air detonation without inhibitors. The computed results also suggest that for a particular class of inhibitors, the compounds containing bromine atoms are more efficient suppressants, followed by the iodine, chlorine, and fluorine atom. However, in the halomethane series of inhibitors, iodomethane has a better inhibition tendency than bromomethane, followed by fluoromethane and then chloromethane.
It was observed that most of the vinyl halide compounds, when added to the H2-air mixtures in small amounts (20,000 ppmv), promote detonation. This is because the addition of vinyl halides provides more fuel in the form of the C2H3 radical which tends to increase the energy release in the reaction zone and promotes faster kinetics. The halogen atom on the other hand will tend to inhibit the chain-branching reactions through the catalytic recombination of H atoms as discussed earlier (see reactions R1–R4). However, vinyl iodide and vinyl bromide are the exceptions in this series which offer a very good inhibition effect on stoichiometric H2-air detonations. This is because the concentration of I and Br atoms is much higher ahead of the flame due to lower bond energies that result in large production rates of I2 and Br2 molecules and relatively effective detonation inhibition by reactions R1–R4. When the level of halogen atom (X) is low, the production rate of halogen molecule (X2) is also small and therefore catalytic recombination reactions are relatively ineffective in removing the H atoms from the radical pool. It must be noted that in the case of halogenated hydrocarbons, RX, where R represents the methyl, ethyl, or vinyl radicals the inhibition efficiency of iodides (R-I) and bromides (R-Br) are comparable and are considerably larger than chlorides (R-Cl) and fluorides (R-F). It is observed that complex halogenated hydrocarbons like CF3I, CF3Br, and CF3Cl exhibit good inhibition efficiency except for CF4, which acts as a promoter rather than an inhibitor for hydrogen-air explosive mixtures (see Table 1). It must be noted that the hydrocarbon part of a halogenated hydrocarbon molecule acts as a fuel and that the halogen part of the molecule is the effective inhibitor. Therefore, the inhibiting action of halogenated hydrocarbons can be explained in terms of the addition of both inhibitors and extra fuel to the system. It’s the relative competition of the two effects that determine the inhibition efficiency of a given inhibitor. The additional fuel content of the halogenated hydrocarbons makes their inhibition efficiency vary with the initial conditions and therefore the fuel content of the halogenated hydrocarbon must be included in calculating the inhibition efficiency of a given inhibitor. Since many halogenated hydrocarbons are combustible in the air themselves, a detailed reaction mechanism was employed in the present study to describe the complex interactions between hydrocarbon oxidation and inhibition kinetics.
The increase in the induction length is similar for CF3Br and CF3I at concentrations of 20,000 ppmv. The chemical kinetics of the reactant mixture change with the addition of retardants, which leads to a change in fuel consumption and the temperature rise of the compressed reactant gases in the reaction zone. To get a better understanding of the phenomena, species concentration profiles of active radicals, thermicity, and the temperature variation in the reaction zone are presented in Fig. 1. The solid lines in Fig. 1 represent the no-retardant case, whereas the dashed lines represent the case with the addition of 20,000 ppmv of C2H5Br to the stoichiometric H2-air mixture at P0 = 1 atm and T0 = 298 K. It can be observed that the oxidation of hydrogen gets delayed with the addition of C2H5Br. Also, the ignition delay time increases due to the addition of the retardant C2H5Br. Behind the leading shock front, hydrogen gets oxidized very fast if there is no retardant in the reactant mixture. The concentration of active radicals such as H and OH that promote chain-branching reactions is also high in the absence of flame retardants. On the other hand, the addition of C2H5Br reduces the concentration of these active radicals and slows down their production rate (see Fig. 1). The temperature rise in the reaction zone is also reduced considerably with the addition of C2H5Br, as shown in Fig. 1. Thus, the post-shock environment in the case of inhibitor C2H5Br is chemically weaker to achieve periodic reignition of the reactive mixture. Lower post-shock temperatures also result in slower kinetics and result in larger induction lengths for hydrogen-air-retardant mixtures. Due to the reduction in the available radical pool with the addition of C2H5Br, the overall rate of chain-branching reactions is reduced, and ignition is delayed. Thus, the inhibition effects of C2H5Br can be readily seen in Fig. 1.
The concentration profiles of key species for stoichiometric H2-air, H2-air-HI, and H2-air-CF3Br detonations at initial conditions of 1 atm and 298 K are shown in Fig. 2. The retardants HI and CF3Br are chosen to represent the different classes of halogenated inhibitors. The production of active radical species like H and OH is delayed with the addition of HI and CF3Br retardants to H2-air mixtures. The oxidation of hydrogen is also delayed in the presence of chemical inhibitors (see Fig. 2). The concentration of radicals and their production rate after the addition of retardant can be compared with the no-retardant case. It is observed that the maximum H and OH radical concentration is almost the same for HI and no-retardant cases (refer to Fig. 2a and b). However, the maximum H radical concentration reduces by more than an order of magnitude after the addition of CF3Br at 20,000 ppmv. It can also be seen from Fig. 2b and c that the location of the peak radical concentrations is also increased with the addition of the retardants. This indicates that the addition of retardants not only reduces the maximum radical concentration but also slows down its production rate.
The concentration of I and I2 increase with the addition of HI due to the reaction R1 and R3, respectively (refer to Fig. 2b). Further, I2 consumes H radicals by reaction R2. The retardant CF3Br removes the H atom from the radical pool by reaction R9. An increase in the species mole fractions of CF3 and HBr can be seen in Fig. 2c, which suggests a catalyzed recombination of H atoms into HBr. Thus, CF3Br acts as a flame inhibitor and delays ignition.
Effect of Varying the Inhibitor Concentration on Induction Length
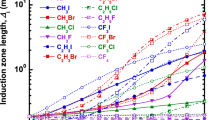
The detonability of a given fuel–air mixture decreases with increasing retardant concentration. Figure 3 illustrates the effect of increasing the inhibitor concentration of several inhibitors on the induction length for stoichiometric H2-air mixtures at P0 = 1 atm and T0 = 298 K. In Fig. 3, the variation of induction length with the molar concentration of retardants of each series is plotted to compare the inhibition effects of various retardants. The induction length increases as the molar concentration of the retardants increases in the reactant mixture.
At low concentrations (up to 8000 ppmv), it can be observed that the addition of HI resulted in the largest increase in the induction length, as shown in Fig. 3. HI seems to exhibit a higher inhibition effect than other series of inhibitors at lower concentrations up to 8000 ppmv. However, after that, a significant increase in induction length can be observed for the case of inhibition with C2H5Br and C2H5I additives. Figure 3 shows that the induction length increases more for CF3Br when compared to CH3Br in the range of molar concentrations of 17,000 ppmv to 20,000 ppmv. It indicates the inhibition effect due to additional fluorine atoms in CF3Br. A significant difference in the induction length can be observed for the series of inhibitors containing iodine and chlorine atoms (see Table 1). The C–I bond energies are much less than the C–Cl bond energies (for instance, the bond energy for C–I and C–Cl is 56 and 83.5 kcal/mole, respectively), and hence the abstraction of the I atom has a much larger rate than the Cl atoms. Since the bond energy of carbon with iodine (C\(-\)I) is much less than that of bromine and chlorine (C\(-\)Cl), the I atoms are more readily available when compared to Cl atoms, where they reduce the rate of chain-branching reactions via consumption of H radicals (see reaction R4). Thus, the inhibition effectiveness of halogenated compounds containing I atoms is more when compared to the halogenated compounds containing Cl atoms. It was observed that the induction length first decreases for smaller concentrations of inhibitors such as CF3Cl, CF3I, C2H3I, and C2H5I (refer to Fig. 3), where a local minimum in the induction length was observed for such inhibitors at lower concentrations. It suggests that the addition of these retardants to H2-air explosive mixtures at lower concentrations has a promotion effect on the resulting detonation structure as it reduces the induction length.
It is observed that the induction length varies significantly beyond 10,000 ppmv of molar inhibitor concentration. The factor by which the induction length is increased with the addition of HI, HBr, C2H5Br, C2H5I, C2H3I, CF3Br, CF3I, CH3Br, and CH3I at 10,000 ppmv is 2.96, 4.63, 2.31, 2.31, 0.89, 1.53, 1.68, 1.58, and 1.00, respectively. However, the induction length is increased by a factor of 12.38, 33.33, 38.89, 31.35, 4.29, 20.79, 20.79, 9.74, and 10.21, respectively, at larger concentrations of 20,000 ppmv. Thus, a significant difference in the FOI can be observed for C2H5Br, C2H5I, and C2H3I when their concentration is varied from 10,000 ppmv to 20,000 ppmv. A higher concentration of inhibitors leads to the large availability of halogen atoms or molecules, and these halogen atoms or molecules consume a significant amount of H radicals available in the radical pool via reactions R2–R4. The halogen acids and halogenated hydrocarbons also consume the active radicals through the reaction R1 and R5, respectively. Furthermore, these species slow down the chain-branching reactions and promote the chain-terminating recombination reactions. In the current work, bromine-containing species were found to possess the best inhibition efficiency, with fluorine-containing inhibitors being the least efficient as evaluated by the induction length analysis.
Figure 4 shows the species concentration profiles for active radicals H and OH at various concentrations of HI, C2H5Br, and CH3Br retardants. It can be noticed from Fig. 4a that the maximum molar concentration of active radicals (H and OH) remains the same for no dopant case and at various concentration levels of HI. However, the location of peak radical concentration increases with the addition of HI. This shows that the production rate of active radicals like H and OH is slowed in the presence of HI, which manifests the inhibition effects of HI. However, in the case of C2H5Br and CH3Br, increasing the retardant concentration in the H2-air mixture reduces the peak concentration of the active radicals. The FOI for HI was found to be 120% greater than that of C2H5Br at 5000 ppmv of retardant concentration, and it is lower by 317% than C2H5Br at 20,000 ppmv (see Fig. 3). The reason behind this can be observed from Fig. 4a and b. At lower concentrations (less than 8000 ppmv), the production rate of active radicals like H and OH is greater in C2H5Br than in HI addition, and hence ignition delay will be larger in the case of HI. The maximum concentration of active radicals for the HI case does not vary much over the range of inhibitor concentrations considered in the present work. However, at higher concentrations (greater than 8000 ppmv), significant ignition delay and decrease in the concentration of active radicals like H and OH can be noticed for C2H5Br (refer to Fig. 4b).
Species concentration profiles of active radicals (H and OH) for stoichiometric H2-air detonations in the presence of inhibitors at varying molar concentrations (0 ppmv, 5000 ppmv, 10,000 ppmv, 15,000 ppmv, and 20,000 ppmv). a HI, b C2H5Br, and c CH3Br. The computations were carried out at P0 = 1 atm and T0 = 298 K
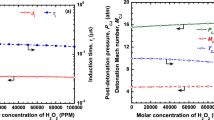
The post-shock (von Neumann, vN) and post-detonation (CJ plane) parameters, along with the induction length and time scales, were computed in the presence of various inhibitors at 10,000 ppmv and 20,000 ppmv, and are tabulated in Table 2. It can be observed that the post-shock and post-detonation parameters (vN and CJ) do not vary much with the addition of retardants. The post-shock pressure and temperature (PVN and TVN) for the stoichiometric H2-air mixture without any retardants were computed to be ~ 28 atm and 1537 K, respectively. Similarly, the post-detonation pressure and temperature (PCJ and TCJ) were computed to be ~ 16 atm and ~ 2960 K, respectively. A small increase in PVN, TVN, PCJ, and TCJ can be observed for all the retardants except in the case of halogen acids (HBr, HI, HCl, HF), where a small decrease can be seen (refer to Table 2). Although the post-shock (vN) and post-detonation (CJ) properties change with the addition of the retardants, the change is very minimal. The maximum change in the post-shock temperature (TVN) and pressure (PVN) was evaluated to be ~ 5% and ~ 7%, respectively, for the case of the addition of 20,000 ppmv of C2H5Cl (when compared to other inhibitors) in the fuel-oxidizer mixture. The maximum change in the CJ temperature of ~ 4% was observed for C2H5I at 20,000 ppmv (for the other retardants, it was found to be less than 4%), and the maximum change in post-detonation pressure of ~ 6% was observed for 20,000 ppmv of C2H3F (for the other retardants it was found to be less than 6%). Thus, the addition of retardants minimally affects the post-shock and post-detonation parameters. The detonation velocity (VCJ) generally decreases with the addition of retardants except for CH3Cl, CH3F, C2H3Cl, C2H3F, C2H5Cl, and C2H5F. The reason for such behavior is discussed in the subsequent section. However, it is observed from the computational results that the speed of sound in the initial mixture (a1) also decreases with the addition of retardants. Thus, the overall change in the detonation Mach number (MCJ) is found to be minimal with the addition of chemical inhibitors/retardants. Thus, the inhibition effects of various retardants are due to the scavenging of active radicals from the radical pool since they do not affect the post-shock and post-detonation parameters much. Scavenging of active radicals decreases the overall rate of chain-branching reactions and produces dramatic inhibition effects by increasing the ignition delay time.
Effect of Inhibitor Concentration on Detonation Velocity
The effect of various inhibitors on detonation velocity was also investigated at various inhibitor concentrations. The detonation velocity (VCJ) is the property of the energetic gas (fuel-oxidizer mixture). The detonation velocity is independent of the initial and boundary conditions, and it solely depends on the energy content of the reacting fuel–air mixture. Figure 5 represents the variation of the detonation velocity VCJ, with the retardant concentration for different series of retardants in stoichiometric H2-air mixtures at P0 = 1 atm and T0 = 298 K.
As discussed earlier, periodic reignition of reactive mixture behind the leading shock front is essential to prevent the damping of transverse waves. Transverse waves govern the propagation of self-sustained detonations. However, they are unsteady and continue to decay in time. They stay alive as long as the periodic reignition of the reactive mixture is accomplished behind the leading shock wave. This, in turn, depends on the shock strength and hence the detonation velocity.
If the shock strength or detonation velocity drops below some critical value, the periodic reignition of the reactive mixture may not occur (since post-shock temperatures drop below the autoignition temperatures due to reduced shock strength), and the shock front may get decoupled from the reaction zone. In such scenarios, detonations are inhibited. The addition of inhibitors affects this coupling by reducing the detonation velocity and the leading shock strength, and if added in sufficient amounts, may inhibit the underlying detonation wave due to the decoupling of the reaction zone from the leading shock front. The detonation velocity reduces with the increase in the concentration of retardants in the reactant mixture (see Fig. 5).
At a lower concentration of C2H5Br, the detonation velocity reaches a maximum and then decreases with an increase in its concentration. A peak in VCJ is observed at 4000 ppm of C2H5Br (see Fig. 5). The detonation velocity is a property of the energetic fuel–air mixture and depends on the normalized energy release. It was observed that with the addition of C2H5Br to H2-air mixtures, the normalized energy release first increases up to 4000 ppmv and then decreases (> 4000 ppmv), while in the case of other inhibitors, the energy release was found to decrease continuously for all concentration levels. As the VCJ is the function of normalized energy release for a given fuel-oxidizer mixture, similar trends are observed for the variation of VCJ. However, this peak in detonation velocity does not represent the promotion effects of C2H5Br. It must be noted that no promotion effect was observed for C2H5Br at concentrations less than 10,000 ppmv as Δi and τi (induction delay time) were found to increase continuously with the retardant concentration. The detonation velocity reduces by 5.2% if the concentration of CF3I is increased from 0 ppmv to 20,000 ppmv, while it is reduced by 5% for HI. The lowest reduction in detonation velocity is observed for the addition of 20,000 ppmv of HF. The retarding effect of CF3I, based on the CJ velocity analysis, is the highest among all the halogenated inhibitors studied in the current work, followed by that of HI and CF3Br.
It was observed that the addition of several retardants increased the CJ detonation velocity of stoichiometric H2-air explosive mixtures. The CJ detonation velocity of H2-air mixtures was found to increase in the presence of inhibitors such as CH3F, C2H3F, C2H5F, CH3Cl, C2H3Cl, and C2H5Cl. The primary reason for the enhancement of the detonation velocity with the addition of the retardants is the presence of the methyl, vinyl, and ethyl groups in such inhibitors. These hydrocarbon groups act as fuel, thereby increasing the energy content of the reacting mixture. Thus, the normalized energy release increases in the presence of such compounds. The inhibition effect of a halogenated hydrocarbon R-X (R is a methyl, vinyl, or ethyl group, and X is a halogen atom) is primarily due to the presence of a halogen atom, X (Westbrook 1982a). Simmons and Wolfhard, in their work, emphasized that the hydrocarbon part of an R-X inhibitor acts as a fuel and the halogen part of the given molecule act as an effective inhibitor (Simmons and Wolfhard 1955). It is interesting to note that the CJ detonation velocity increase can be observed only for halogenated hydrocarbons that contain fluorine and chlorine as halogen atoms. Since the inhibition efficiency of halogen atoms follows the order of I > Br > Cl > F, iodides and bromides are the most effective inhibitors, whereas chlorides and fluorides are the least effective inhibitors. Therefore, the CJ detonation velocity increase is only observed for the inhibitors that contain F and Cl as halogen atoms. In the case of a halogenated hydrocarbon, an increase in the detonation velocity is a result of competition between the hydrocarbon part and the halogen part of the given molecule. It’s the relative competition of the two effects that determine the inhibition efficiency of a given inhibitor. Based on normalized energy release calculations, it is found that the halogenated hydrocarbons that contain fluorides and chlorides act as a fuel, whereas they act as an inhibitor in the case of bromides and iodides.
It would also be interesting to see the effect of these velocity-enhancing retardants on the induction length of H2-air-retardant mixtures. Based on the induction length analysis, it has been observed that out of the six retardants that increase the CJ detonation velocity, only two retardants namely C2H3Cl and C2H3F, decreased the induction length, which suggests a promotion effect on the resulting detonation structure. C2H5Cl and C2H5F showed a dual behavior, where it first reduces the induction length and then increases it. Thus, an increase in the CJ detonation velocity does not necessarily mean that a given retardant is an ignition promoter as the induction length and induction delay time can still increase with the addition of such retardants. Thus, to distinguish between an ignition promoter and an inhibitor and to evaluate the inhibition efficiency, the induction length is the best-suited parameter.
The detonation velocity was found to linearly decrease with the concentration of inhibitors for most of the halogenated inhibitors studied. The ranking of the inhibitors based on their ability to reduce the detonation velocity when added to stoichiometric H2-air mixtures at 20,000 ppmv is given as follows:
The ranking shows that the net effect of retarding characteristics of iodine-containing retardants is more significant in their respective series, and fluorine-containing species have the least.
It must be noted that the impact of the retardants on the CJ speed is measurable but minimal (5% decrease, 2% increase maximum). Since retardants minimally affect the detonation velocity which may lead to minimal change in the post-shock environment, the detonation velocity is not an effective parameter to judge the inhibition efficiency of chemical inhibitors. It is found that the induction zone length and induction delay time is the most suited parameter for determining the inhibition effect of the retardants and is potentially related to detonation initiation and detonation propagation sensitivities. A larger induction zone length and induction delay time are indicative of a loose coupling between the leading shock front and the reaction zone and quantitatively represent mixtures that are less detonable.
Conclusions
The inhibition of hydrogen-air gaseous detonations was studied in the current work using a series of halogenated inhibitors. The halogenated inhibitors investigated in the current work include halogen acids (HI, HBr, HCl, HF), halomethanes (CH3I, CH3Br, CH3Cl, CH3F), haloethanes (C2H5I, C2H5Br, C2H5Cl, C2H5F), haloethenes (C2H3I, C2H3Br, C2H3Cl, C2H3F) and compounds with more than one halogen atoms (CF3I, CF3Br, CF3Cl, CF4). The inhibition effectiveness of these halogenated inhibitors was evaluated based on two parameters, the induction zone length (Δi) and the CJ detonation velocity (VCJ). While the former is potentially related to detonation initiation and detonation propagation sensitivities, the latter only marginally affects the detonation pressure and temperature. The inhibition effects of various retardants are due to the scavenging of active radicals from the radical pool since they do not affect the post-shock and post-detonation parameters much. It has been observed that the location of the peak radical concentrations is also increased with the addition of the retardants. This indicates that the addition of retardants not only reduces the maximum radical concentration but also slows down its production rate. Scavenging of active radicals decreases the overall rate of chain-branching reactions and produces dramatic inhibition effects by increasing the ignition delay time. The induction length and ignition delay time of the ZND detonation structure were found to increase with an increase in the concentration of the retardants. However, the post-shock and post-detonation properties were minimally affected by the addition of halogenated inhibitors to hydrogen-air mixtures. In the current work, bromine and iodine-containing species were found to possess the best inhibition efficiency, with chlorine and fluorine-containing inhibitors being the least efficient as evaluated by the induction length analysis. The addition of some inhibitors showed a dual effect, with the induction length decreasing first and then increasing with the inhibitor concentration. A local minimum in the induction length was observed for inhibitors such as CF3Cl, CF3I, C2H3I, and C2H5I when added to stoichiometric hydrogen-air mixtures. The complex halogenated hydrocarbons like CF3I, CF3Br, and CF3Cl exhibited good inhibition efficiency except for CF4, which acts as a promoter rather than an inhibitor for hydrogen-air explosive mixtures. In the case of hydrocarbon inhibitors, the hydrocarbon part of a halogenated hydrocarbon molecule acts as a fuel, and the halogen part of the molecule is the effective inhibitor. Therefore, the inhibiting action of halogenated hydrocarbons can be explained in terms of the addition of both inhibitors and extra fuel to the system. It’s the relative competition of the two effects that determine the inhibition efficiency of a given inhibitor. For H2-air mixtures, based on the induction length analysis, bromo-ethane (C2H5Br) was found to exhibit the highest inhibition efficiency among all the inhibitors studied in the present work at 20000 ppmv of the inhibitor concentration, followed by HBr, C2H5I, CF3I, and CF3Br.
The ranking of the inhibitors based on their ability to reduce the detonation velocity, when added to stoichiometric H2-air mixtures at 20,000 ppmv, shows that the net effect of retarding characteristics of iodine-containing retardants is more significant in their respective series, while the fluorine-containing species exhibit the least inhibition efficiency. The ranking of inhibitors also shows that haloethanes have better inhibition efficiency than halomethanes, followed by vinyl compounds. The CF3 series of inhibitors has a good inhibition tendency due to extra halogen atoms. In the case of a halogenated hydrocarbon, an increase in the detonation velocity is a result of competition between the hydrocarbon part and the halogen part of the given molecule. It’s the relative competition of the two effects that determine the inhibition efficiency of a given inhibitor. Based on the CJ detonation velocity analysis CF3I showed the best inhibition effect for H2-air mixtures followed by HI and CF3Br.
For H2-air mixtures, based on the induction length analysis, bromo-ethane (C2H5Br) was found to exhibit the highest inhibition efficiency among all the inhibitors studied in the present work. Similarly, CF3I showed the best inhibition effect for H2-air mixtures based on the CJ detonation velocity analysis.
In the present work, the inhibition or promotion effect of a given retardant was evaluated based on two parameters, the induction zone length (Δi) and the CJ detonation velocity (VCJ). In the present work, it is found that Δi is the most suited parameter for determining the inhibition effect of a given retardant. The detonation velocity is not an effective parameter to judge the inhibition efficiency of chemical inhibitors since retardants minimally affect the detonation velocity (maximum 5% decrease or 2% increase), which may lead to minimal change in the post-shock environment. A larger induction zone length is indicative of a loose coupling between the leading shock front and the reaction zone and quantitatively represents mixtures that are less detonable. Thus, the induction zone length was found to be a better parameter to evaluate the inhibition effect of a given retardant for H2-air mixtures.
References
Babushok V, Noto T, Burgess DR, Hamins A, Tsang W (1996) The influence of halogenated fire suppressants on the combustion of C1–C2 hydrocarbons. Proc Combust Inst 39:271–282
Babushok V, Hamins A, Tsang W, Noto T (1997) Chemical and physical influences of halogenated fire suppressants, NIST. https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=909906. Accessed 22 Nov 2021
Browne S, Ziegler J, Shepherd JE (2008) Numerical solutions methods for shock and detonation jump conditions. GALCIT report FM2006 6: 90. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.181.1150&rep=rep1&type=pdf. Accessed 25 Dec 2021
Ciccarelli G, Ginsberg T, Boccio J, Economos C, Sato K, Kinoshita M (1994) Detonation cell size measurements and predictions in hydrogen-air-steam mixtures at elevated temperatures. Combust Flame 99:212–220. https://doi.org/10.1016/0010-2180(94)90124-4
Crane J, Shi X, Singh AV, Tao Y, Wang H (2019) Isolating the effect of induction length on detonation structure: hydrogen–oxygen detonation promoted by ozone. Combust Flame 200:44–52. https://doi.org/10.1016/j.combustflame.2018.11.008
Dahake A, Singh AV (2021) Numerical study on NOx emissions from a synthetic biofuel for applications in detonation-based combustors. AIAA 2021–3678. AIAA Propuls Energy Forum Virtual Event. https://doi.org/10.2514/6.2021-3678
Dahake A, Singh AV (2022a) A comparative study of the detonation chemistry and critical detonation parameters for Jet A and a bio-derived jet fuel. Trans Indian Natl Acad Eng. https://doi.org/10.1007/s41403-022-00353-z
Dahake A, Singh AV (2022b) A comparative study of critical detonation parameters for jet A and an alcohol-to-jet synthetic biofuel. AIAA Scitech Forum and Exposition, San Diego. https://doi.org/10.2514/6.2022-0819
Dahake A, Singh AV (2022bc) Nitrogen oxides emissions from fuel-sensitized detonations for a synthetic biofuel. Trans Indian Natl Acad Eng. https://doi.org/10.1007/s41403-022-00354-y
Dahake A, Singh AV (2022d) Effect of fuel sensitization on NOx emissions from a synthetic biofuel under detonating conditions. AIAA SciTech Forum and Exposition, San Diego. https://doi.org/10.2514/6.2022-0518
Dahake A, Kumar DS, Singh AV (2022a) Using ozone and hydrogen peroxide for improving the velocity deficits of gaseous detonations. Trans Indian Natl Acad Eng 7:1033–1042. https://doi.org/10.1007/s41403-022-00345-z
Dahake A, Singh RK, Singh AV (2022b) Nitrogen oxides emissions from a bio-derived jet fuel under detonating conditions. Trans Indian Natl. Acad. Eng. (In press)
Dahake A, Singh RK, Singh AV (2022c) NOx mitigation and ignition promotion effects of hydrogen peroxide addition to H2-air mixtures, Trans Indian Natl. Acad. Eng. (In press)
Evariste F, Lefebre MH, Tiggelen JPV (1996) Inhibition of detonation wave with halogenated compounds. Shock Waves 6(4):233–239. https://doi.org/10.1007/BF02511380
Gavrikov AI, Efimenko AA, Dorofeev SB (2000) A model for detonation cell size prediction from chemical kinetics. Combust Flame 120:19–33. https://doi.org/10.1016/S0010-2180(99)00076-0
Goodwin DG, Moffat HK, Speth RL (2009) Cantera: an object-oriented software toolkit for chemical kinetics. Caltech, Pasadena. https://www.cantera.org. Accessed 20 July 2021
Hamins A, Trees D, Seshadri K, Chelliah HK (1994) Extinction of non-premixed flames with halogenated fire suppressants. Combust Flame 99(2):221–230. https://doi.org/10.1016/0010-2180(94)90125-2
Ivin K, Singh AV (2023) JP-10 propellant powered rotating detonation waves for enhancing the performance of hypersonic and supersonic missiles, In: Sivaramakrishna G, Kishore Kumar S, Raghunandan BN (eds) Proceedings of the National Aerospace Propulsion Conference, Lecture Notes in Mechanical Engineering. Springer, Singapore. pp. 387–413. https://doi.org/10.1007/978-981-19-2378-4_23
Iyer MSK, Singh AV (2021) NOx emissions from jet A-air detonations. AIAA Propuls Energy Forum Virtual Event. https://doi.org/10.2514/6.2021-3679
Iyer MSK, Singh AV (2022) Ignition kinetics of real distillate fuels under detonating conditions. AIAA SciTech Forum and Exposition, San Diego
Iyer MSK, Dahake A, Singh AV (2022a) Comparative studies on ignition kinetics and detonation chemistry of real distillate fuels. Trans Indian Natl Acad Eng 7:823–834. https://doi.org/10.1007/s41403-022-00331-5
Iyer, MSK, Dahake A, Singh RK, Singh AV (2022b) Numerical study on NOx emissions from Jet A-air detonations, Trans Indian Natl. Acad. Eng. (In press)
Kao S, Shepherd JE (2008) Numerical solution methods for control volume explosions and ZND detonation structure. Galcit report FM2006 7: 1–46. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.218.3756&rep=rep1&type=pdf. Accessed 20 July 2021
Knystautas R, Guirao C, Lee JHS (1984) A sulmistras, measurement of cell size in hydrocarbon-air mixtures and predictions of critical tube diameter, critical initiation energy, and detonability limits. Prog Astronaut Aeronaut 94:23–37. https://doi.org/10.1007/s00193-005-0260-y
Kumar DS, Singh AV (2021) Inhibition of hydrogen-oxygen/air gaseous detonations using CF3I, H2O, and CO2. Fire Saf J 124:103405. https://doi.org/10.1016/j.firesaf.2021.103405
Kumar DS, Ivin K, Singh AV (2021) Sensitizing gaseous detonations for hydrogen/ethylene-air mixtures using ozone and H2O2 as dopants for application in rotating detonation engines. Proc Combust Inst 38(03):3825–3834. https://doi.org/10.1016/j.proci.2020.08.061
Kumar DS, Dahake A, Singh AV (2022) Detonation chemistry of fuel-sensitized JetA1–air detonations. Trans Indian Natl Acad Eng 7:957–975. https://doi.org/10.1007/s41403-022-00339-x
Kumar DS, Singh AV (2023) Using ozone and hydrogen peroxide for manipulating the velocity deficits, detonabilility, and flammability limits of gaseous detonations. In: Sivaramakrishna G, Kishore Kumar S, Raghunandan BN (eds) Proceedings of the National Aerospace Propulsion Conference Lecture Notes in Mechanical Engineering. Springer, Singapore. pp. 493–508. https://doi.org/10.1007/978-981-19-2378-4_28
Lee JHS (1984) Dynamic parameters of gaseous detonations. Ann Rev Fluid Mech 16:311–336. https://doi.org/10.1146/annurev.fl.16.010184.001523
Lee JHS (2008) The detonation phenomenon. Cambridge University Press, Cambridge
Manion A, Huie RE, Levin RD, Burgess Jr DR, Orkin VL, Tsang W, McGivern WS, Hudgens JW, Knyazev VD, Atkinson DB, Chai E, Tereza AM, Lin CY, Allison TC, Mallard WG, Westley F, Herron JT, Hampson RF, Frizzell DH (2015) NIST Chemical Kinetics Database, NIST Standard Reference Database 17. https://kinetics.nist.gov/
Mathieu O, Goulier J, Gourmel F, Mannan MS, Chaumeix N, Petersen EL (2015) Experimental study of the effect of CF3I addition on the ignition delay time and laminar flame speed of methane, ethylene, and propane. Proc Combust Inst 35:2731–2739. https://doi.org/10.1016/j.proci.2014.05.096
Moen IO, Ward SA, Thibault PA, Lee JH, Knystautas R, Dean T, Westbrook CK (1984) The influence of diluents and inhibitors on detonations. Proc Combust Inst 20:1717–1725. https://doi.org/10.1016/S0082-0784(85)80668-8
Noto T, Babushok V, Hamins A, Tsang W (1998) Inhibition effectiveness of halogenated compounds. Combust Flame 112:147–160. https://doi.org/10.1016/S0010-2180(97)81763-4
Oran ES, Gamezo VN (2007) Origins of the deflagration-to-detonation transition in gas-phase combustion. Combust Flame 148:4–47. https://doi.org/10.1016/j.combustflame.2006.07.010
Pagliaro JL, Linteris GT, Babushok VI (2016) Premixed flame inhibition by C2HF3Cl2 and C2HF5. Combust Flame 163:54–65. https://doi.org/10.1016/j.combustflame.2015.08.015
Simmons RF, Wolfhard HG (1955) The influence of methyl bromide on flames. Part 1—Pre-mixed flames. Trans Faraday Soc 51(01):1211–1217
Stamps DW, Slezak SE, Tieszen SR (2006) Observations of the cellular structure of fuel-air detonations. Combust Flame 144(1–2):289–298. https://doi.org/10.1016/j.combustflame.2005.06.016
Takahashi F, Katta VR, Linteris GT, Babushok VI (2015) Combustion inhibition and enhancement of cup-burner flames by CF3Br, C2HF5, C2HF3Cl2, and C3H2F3Br. Proc Combust Inst 35(3):2741–2748. https://doi.org/10.1016/j.proci.2014.05.114
Wang H, You X, Joshi AV, Davis SG, Laskin A, Egolfopoulos F, Law CK (2007) USC Mech Version II. High- temperature combustion reaction model of H2/CO/C1-C4 compounds. http://ignis.usc.edu/USC_Mech_II.htm. Accessed 30 July 2021
Westbrook CK (1982a) Inhibition of hydrocarbon oxidation in laminar flames and detonations by halogenated compounds. Proc Combust Inst 19:127–141. https://doi.org/10.1016/S0082-0784(82)80185-9
Westbrook CK (1982b) Chemical kinetics of hydrocarbon oxidation in gaseous detonations. Combust Flame 46:191–210. https://doi.org/10.1016/0010-2180(82)90015-3
Xu W, Jiang Y, Qiu R, Ren X (2017) Influence of halon replacements on laminar flame speeds and extinction limits of hydrocarbon flames. Combust Flame 182:1–13. https://doi.org/10.1016/j.combustflame.2017.03.029
Acknowledgements
The authors acknowledge the financial support from Aeronautics Research and Development Board (ARDB) vide Sanction Letter # ARDB/01/1042000M/I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, R.K., Dahake, A. & Singh, A.V. Inhibition of H2-Air Detonations Using Halogenated Compounds. Trans Indian Natl. Acad. Eng. 8, 41–53 (2023). https://doi.org/10.1007/s41403-022-00376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-022-00376-6