Abstract
Self-sustained propagation of detonation waves near limits is essential for the successful operation of detonation-based combustors since they suffer from high-velocity deficits near limits due to geometric constraints. This can potentially lead to its failure or attenuation near limits. The failure or attenuation of a detonation wave under such circumstances could lead to the failure of a detonation-based engine altogether. Existing models like Fay’s Model reasonably predict detonation velocity deficits for only stable mixtures. The present work focuses on estimating velocity deficits for both stable and unstable mixtures. The proposed model is similar to Fay’s model, with the modified reaction zone thickness calculated using x = c*(∆i + ∆r). The value of c is found to be 33.2, 8.6, and 19.5 for H2–air, CH4–O2 (unstable mixture), and H2–O2–Ar mixtures (stable mixture) using existing experimental data. The proposed model predicts velocity deficits better than other existing models for both stable and unstable mixtures over a range of pressure ratios and tube diameters and also near the limits. The addition of O3 and H2O2 at modest concentrations was shown to reduce the velocity deficits near propagation limits. The present work shows that the use of ignition promoters in trace amounts could help in the widening of detonability limits for detonation-based combustors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widening of detonation propagation limits in gaseous detonations is one of the fundamental problems that need to be addressed for the successful operation of detonation-based engines, such as the rotating detonation engine (RDE). These detonation-based engines are expected to operate in a variety of conditions using fuels that currently range from energy-dense liquid hydrocarbon fuels to gaseous fuels. One of the significant problems associated with RDEs is the stabilization and sustainment of detonation waves in the narrow channel of the combustor, where they can destabilize around tight curves. This, in particular, is important for the development of a small-scale detonation device for propulsion applications. Generally, detonations within limits will propagate with a stable velocity close to the Chapman–Jouguet velocity (VCJ) with relatively small fluctuations. However, if the conditions approach the limits or are far from the limits, detonations propagate with significant velocity variations and deficits. The self-propagation of a detonation wave will depend on boundary conditions, particularly near the limits. The effect of the boundary condition is to reduce the propagation velocity below VCJ, resulting in a velocity deficit and causing the detonation to attenuate and fail. This velocity deficit can be due to the heat and momentum losses and can be attributed to the boundary layer effects, as proposed by Lee (2008). The finite thickness of the reaction zone is responsible for detonation vulnerable to boundary layer effects. The detonation wave velocity suffers high-velocity deficits in smaller passages, and in general, ∆V/VCJ is inversely proportional to the tube diameter, as proposed by Camargo et al. (2010), Chao and Ng (2009), Gao et al. (2015), Gao and Ng (2016), Ishii et al. (2002) and Ishii and Monwar (2011), and Jackson et al. (2016). The higher the tube diameter (1⁄d → 0), the closer the detonation wave would be to VCJ. The detonation velocity is greatly influenced by the boundary layer when the diameter of the tube or channel is comparable with the boundary layer thickness.
Zeldovich (1950) first investigated the effects of heat and momentum losses. Zeldovich (1950) proposed that since the total momentum associated with a detonation wave is a function of volume and because heat transfer and viscous drag are proportional to the wetted area of the tube, the velocity deficit in gaseous detonations should depend on the ratio of surface area to volume and thus on 1/d. However, such a simplified treatment couldn’t account for the two-dimensional effect of losses adequately. Manson and Guenoche (1957) proposed an alternate mechanism for the velocity deficit. They considered a layer of reactive mixture adjacent to the wall quenches as a result of heat losses. In such scenarios, the reaction rate decreases significantly, leading to a decrease in the total chemical energy that goes to support the detonation. Again, this mechanism produced a dependence on the surface area to volume ratio. A more definitive treatment of losses in 2-D was presented by Fay (1959), in which the boundary layer was assumed to cause a divergence in the reaction zone, thereby resulting in a velocity deficit. In Fay’s theory, the boundary layer causes the streamlines in the reaction zone to diverge and thus are responsible for a reduction in the detonation velocity. The flow divergence is due to the negative boundary layer thickness with respect to a reference coordinate system fixed to the shock wavefront. The boundary layer in the reaction zone responsible for the divergence of the streamlines will further result in a curved detonation front. Also, the detonation wave curvature is observed to be proportional to the rate of increase of the flow area away from the shock-wave front. For small curvatures, the detonation can be modeled using a quasi-one-dimensional ZND model.
Using Fay’s model, the velocity deficit for a given mixture and tube diameter can be calculated if the reaction zone thickness is known for estimating the boundary layer thickness. The reaction zone thickness can be determined from the ideal ZND detonation model. However, researchers in the past found it to be unsatisfactory while predicting the velocity deficits as the theoretical reaction zone thickness was found to differ from the experimental value by at least two orders of magnitude. Some researchers used the detonation cell size, λ, rather than the ZND reaction zone length for predicting the velocity deficits in real detonations. In real detonations, λ provides a more appropriate length scale to characterize the thickness of a cellular detonation. In a separate study, Lee (2008) proposed to use cell length Lc ≈ 1.5λ in place of reaction zone thickness for unstable detonations. Moen et al. (1982) later showed that critical tube diameter dc could also be chosen as a length scale to characterize a real detonation front. The critical tube diameter can be related to the cell size using the following correlation of dc ≈ 13λ, which is valid for most explosive mixtures. One of the primary reasons for using critical tube diameter as the length scale for calculations of velocity deficit is because it can be determined less unambiguously. The critical tube diameter may be defined as the minimum diameter through which a planar detonation wave could emerge into an open space and continue to propagate as a spherical detonation. Using the correlation of dc ≈ 13λ implies that λ ≈ dc/13 ≈ 0.077dc. Therefore, the cell length Lc ≈ 1.5λ ≈ 0.11dc. Using the experimental values of detonation cell size or the critical tube diameter, since they are readily known for a variety of explosive mixtures, the cell length can be determined for a given fuel-oxidizer mixture. The calculated cell length can be used as the reaction zone thickness for a real detonation, where it can be used to calculate the area of divergence ξ from the displacement thickness δ*. Therefore, the velocity deficit in various tubes of different diameters can be obtained.
Laberge et al. (1993) measured velocity deficits for stoichiometric acetylene-oxygen mixtures with high concentrations of argon dilution (stable mixtures), and the experimental results were found to agree with the Fays model. Since the transverse waves in stable mixtures are relatively weak when compared to unstable mixtures, stable mixtures exhibit a regular cell pattern. Fay’s model was found to predict the velocity deficits with reasonable accuracy in such mixtures. However, experimental results of unstable mixtures like C2H2 and C2H4 by Moen et al. (1982) with low percentages of argon showed a considerable discrepancy in velocity deficit when compared with Fay’s Model. This indicates that boundary layer effects don’t influence unstable detonations since their propagation mechanism is dominated by instability in the detonation structure. In unstable detonations, transverse waves are strong, and cell patterns are irregular as opposed to stable detonations. For unstable detonations, as in the case of fuel–air mixtures, the velocity deficits were found to deviate from Fay’s theory. Thus, it becomes essential to know whether the mixtures are stable or unstable for the application of Fay’s model. Fay’s model, in its present form, could only be applied for stable mixtures.
The initial studies on stability were made by Fickett et al. (1972) using a one-step reaction model. The importance of transverse waves on the stability of mixtures for self-sustained detonation was carried out by Dupre et al. (1988), Teodorczyk and Lee (1995) and Radulescu and Lee (2002). Later Ng et al. (2005) defined the stability parameter based on the ratio of the induction to the reaction zone length. They also included the temperature sensitivity of the induction reaction in the definition of the stability parameter. It is known that a long reaction time would tend to spread out the energy release and would reduce the effect of fluctuations in the induction time, which in turn could increase the stability of the mixtures. The numerical simulations by Radulescu et al. (2002) with varying concentrations of argon in acetylene-air mixtures indicate that the shock pressure oscillations changes from low-amplitude, high-frequency to low-frequency, high-amplitude mode with a decrease in argon dilution from 90 to 70%. The effect of the addition of argon was found to increase the stability of mixtures. The same phenomenon was observed by Ng et al. (2005), who characterized the mixtures using the stability parameter, χ. The results by Ng et al. (2005) indicate that the deviation of the stability parameter to higher values from the neutral stability boundary will increase the instability in mixtures. In contrast, the values below the neutral stability boundary would indicate stable mixtures. Various researchers mostly use the stability parameter χ used by Ng et al. (2005) for a quantitative description of the stability of detonation waves.
The velocity deficits are also greatly influenced by the type of boundary surfaces like smooth walls, rough walls, and porous walls. The maximum velocity deficits in smooth-walled tubes will be ~ 15% at detonation limits before it fails. However, a self-sustained detonation with rough walls can be observed with a velocity deficit of over 50%. The temperature behind the shock wave at very high-velocity deficits is very low for auto-ignition to occur, thus requiring new ignition and combustion mechanism for auto-ignition. Lee (2008) proposed that surface finish effects have to be taken into account for studying detonation phenomena, e.g., for smooth and rough walled tubes. In smooth-walled tubes, it was observed that with an increase in the concentration of nitrogen for C3H8–O2 mixtures, the detonation would transform from a multi-headed spin structure to a single-headed spin as the limit is approached. The detonation in a smooth tube fails with the decoupling of the leading shock front from the reaction zone. However, the rough-walled tube can maintain the detonation wave with higher velocity deficits. Hence, rough-walled tubes have a positive effect on maintaining a self-sustained detonation wave with large velocity deficits of ~ 50%. These detonations with velocity deficits as high as 50% are known as low-velocity detonations.
Teodorczyk and Knystautas (1989) carried out experiments to study the propagation of gaseous detonations in rough-walled tubes with obstacles to explain the ignition mechanisms where temperatures behind the shock front are well below the auto-ignition temperature. It is observed that the diffraction of detonation waves around an obstacle is responsible for the failure of detonation by decoupling of reaction zone from the shock front. However, the reflected shocks from obstacles merge with the leading shock front to form detonation again. Thus, failure is due to diffraction around the obstacles, and reinitiation is due to shock reflections. It is now known that the obstacles or barriers in the flow path play an active role in the generation of strong transverse waves in rough walls. Thus, it can be understood that detonation in rough tubes can be more robust and can maintain steady propagation, even for high velocity deficits where detonations in a smooth tube normally fail. The roughness of tubes can also be increased by inserting a coiled wire in tubes, in which the quasi-steady detonation wave speeds can be achieved with velocities as low as half of CJ velocity. The role of boundary conditions, nature of the surface, the stability of mixtures, and obstacles or barriers in the path of detonation wave play a significant role in the determination of velocity deficits, which is essential in the design of detonation-based engines like RDEs, as proposed by Randall et al. (2015), Bykovskii and Vedernikov (2009), Lu and Braun (2014) and Kailasanath (2000).
In real detonations, it is understood that detonation velocity will succumb to very high-velocity deficits of ~ 15% for stable mixtures and ~ 40% for unstable mixtures. In such scenarios, gaseous detonations may fail near their propagation limits. Since the velocity deficit is a function of reaction zone thickness, it is understood that lower reaction zone thickness will lead to lower velocity deficits. Hence, reducing the length and time scales of a detonation wave without changing the gas dynamics and the thermodynamic properties of the resulting mixture can be a promising solution for the reduction of velocity deficits near the limits. Ignition promoters such as ozone and hydrogen peroxide offer the opportunity to resolve the problem of velocity deficit in gaseous detonations. We propose to use ozone and hydrogen peroxide as fuel sensitizers to reduce the velocity deficits in gaseous detonations near their propagation limits. The methodology of sensitizing detonations with ignition promoters at low quantities can reduce the velocity deficits in gaseous detonations near their propagation limits. The effect of such a doping is to reduce the velocity deficits by changing the ignition kinetics tremendously without changing the gas dynamics and relevant thermodynamic properties of both unburned and burned mixtures. Recent results by Magzumov et al. (1998), Crane et al. (2019) and Kumar et al. (2020, 2022) for gaseous detonations support this notion of fuel-sensitization, and results by Liang et al. (2019), Kumar and Singh (2019), Ivin and Singh (2019) and Dahake and Singh (2022a, b, c, d) show that ozone can be used in enhancing the detonability limits of explosive mixtures.
The objectives of the present work are:
-
To formulate a modified theoretical model similar to Zhang and Liu (2019), which can predict the velocity deficits in both stable and unstable mixtures.
-
To investigate the effects of ignition promoters (ozone and hydrogen peroxide) on velocity deficits in gaseous detonations near their propagation limits.
-
To examine the impact of ignition promoters on the stability parameter.
Methodology
Fays Model
In Fay’s theory (1959), the boundary layer causes the streamlines in the reaction zone to diverge and are responsible for the reduction in detonation velocity. If the divergence area is small, the flow in the reaction zone can be approximated as quasi-1D flow, and the conservation equation can be written as:
where h′ includes the chemical energy Q. Integrating the above equations between the shock and the CJ plane gives Eqs. (4)–(6),
where \(\xi\) is the area divergence defined by,
Subscript 1 indicates the unburnt gaseous mixture upstream of the shock wave, and subscript 2 indicates the burned mixture downstream of the CJ plane, as shown in Fig. 1.
The velocity deficit can be expressed as
where VCJ is the theoretical CJ detonation velocity, V is the actual detonation velocity, and ΔV is the detonation velocity deficit.
Solving the above Eqs. (4–6) and applying boundary conditions will result in a velocity deficit described as,
where \(\gamma\) is the specific heat ratio, and \(\nu\) is defined as,
The area divergence, ξ can be reduced in terms of boundary layer thickness, \({\delta }^{*}\) for a round tube of radius R and diameter d, as
For smooth tubes, the boundary layer displacement thickness has been determined in shock tube experiments by Gooderum (1958) as,
where x is the distance from the shock front, μe is the viscosity of the gas in the reaction zone, and ρ1 and u1 are the density and the velocity upstream of the shock (in the shock-fixed coordinate system). The Eqs. (7–11) can be solved for a given mixture with initial conditions by computing the value of reaction zone thickness x. According to Fay’s Model, the x is calculated by an empirical formula. Lee et al. used cell length, Lc, in place of reaction zone thickness, x, for the calculation of velocity deficit.
Modified Theoretical Model
In the present study, a new modified version of Fay’s Model was used for calculating the velocity deficits in gaseous detonations. The recent work by Crane et al. (2019) suggested detonation cell length can be modeled in terms of induction length,\({\Delta }_{\mathrm{i}}\) and exothermic length, \({\Delta }_{\mathrm{r}}\). Zhang and Liu (2019) carried out velocity deficit calculations using the Fays model with modified reaction zone thickness as suggested by the work of Crane et al. (2019) as, \(x=c\left({\Delta }_{\mathrm{i}}+{\alpha \Delta }_{\mathrm{r}}\right)\), where c is a constant and \(\alpha\) is the proportionality factor between \({\Delta }_{\mathrm{i}}\) and \({\Delta }_{\mathrm{r}}\) i.e., \({\Delta }_{\mathrm{i}}\)/\({\Delta }_{\mathrm{r}}\). The value of c is calculated by carrying out velocity deficit experiments and solving equations from (7) to (11). The equation used by Zhang and Liu (2019) for calculating reaction zone thickness, x, ultimately simplifies to a function of \({\Delta }_{i}\), i.e., \(x=c\left({2 \Delta }_{\mathrm{i}}\right)\), after substituting ‘α’ in \(x=c\left({\Delta }_{\mathrm{i}}+{\alpha \Delta }_{\mathrm{r}}\right)\). In the modified theoretical model proposed in this work, the reaction zone thickness (\(x\)) is modeled as \(x=c\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\), where \({\Delta }_{\mathrm{i}}\) and \({\Delta }_{\mathrm{r}}\) represent the induction and exothermic zone lengths, respectively, and can be calculated using a 1-D ZND model. The value of c in the above expression depends on the mixture composition. The value of ‘\(c\)’ was evaluated from the velocity deficit experimental data of Zhang and Liu (2019), Dove et al. (1974) and Gao and Ng (2016).
ZND Numerical Calculations
ZND computations were carried out using a modified version of the Caltech Shock and Detonation Toolbox (Browne et al. 2008). Cantera (Goodwin et al. 2009), integrated with MATLAB and Python, was used for chemical kinetics simulation and to calculate the ZND length scales for H2–O2 and CH4–O2 detonations. The methodology used for the calculation of the relevant detonation parameters and the chemical length and time scales has been documented in detail in our previous works (Kumar and Singh 2021; Kumar et al. 2022; Dahake and Singh 2021, 2022a, b; Iyer and Singh 2021; Iyer and Singh 2022; Iyer et al. 2022). The Foundation Fuel Chemistry Model Ver 1.0 (FFCM-1) by Smith et al. (2016) is used in this study. The Princeton ozone sub-model by Zhao et al. (2016) was used to carry out calculations with ozone as a dopant. The uncertainties associated with the FFCM-1 model and the ozone sub-models can be found in the literature elsewhere by Crane et al. (2019). The complete FFCM-1 model, including the ozone chemistry sub-model, comprises 39 species and 301 reactions.
Results and Discussions
The present work focuses on studying velocity deficit experiments in three mixtures, 2H2–O2 (unstable mixtures), CH4–O2 (unstable mixtures), and 2H2–O2–3Ar (stable mixtures). The reaction zone thickness (\(x\)) in the present study is modeled as \(x=c\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\), where \({\Delta }_{\mathrm{i}}\) and \({\Delta }_{\mathrm{r}}\) represent the induction and exothermic zone lengths, respectively. The velocity deficit experimental data of Zhang and Liu (2019), Dove et al. (1974) and Gao and Ng (2016) for different tube diameters for the above three mixtures were used for the evaluation of c. The value of c is calculated by using velocity deficit values from experimental results and solving equations from (7) to (11) using a 1D ZND model. If the velocity deficit is calculated from the experiment for a particular tube diameter, initial pressure, temperature, and equivalence ratio, the only unknown in Eqs. (7) to (11) is ‘\(c\)’ value, and the rest parameters can be calculated from the 1D ZND model. The non-linear Eqs. (7) to (11) were solved simultaneously using MATLAB with velocity deficit experimental data of Zhang and Liu (2019), Dove et al. (1974) and Gao and Ng (2016), where ‘\(c\)’ values for different initial conditions were calculated for stable and unstable mixtures.
Unstable Mixtures
For unstable mixtures, the experimental data of Zhang and Liu (2019) and Dove et al. (1974) for 2H2 + O2 and CH4 + 2O2 mixtures at stoichiometric equivalence ratio is used to evaluate the value of c in \(x=c\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\), see Fig. 2a, b. It can be seen that the value of c varies over a wide range of pressure, and the average value was calculated based on statistical averaging. The value of c is found to be 33.2 and 8.6 for hydrogen and methane oxygen mixtures, respectively, as shown in Fig. 2a, b. The reaction zone thickness was modeled as \(x=33.2\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\) and \(x=8.6({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\)) for 2H2 + O2 and CH4 + 2O2 mixtures, respectively. The modeled reaction zone thickness was then used in the Fays model to calculate the velocity deficits in the respective mixtures. It can be seen from Fig. 3a, b that the proposed modified theoretical model predicts better when compared to Fay's model and the theoretical model proposed by Zhang and Liu. Similar is the case for methane oxygen detonations where the modified reaction zone thickness as \(x=8.6\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\) in the modified theoretical model will reasonably predict the velocity deficits (see Fig. 3c). Fay’s model does not predict velocity deficits accurately for unstable mixtures when reaction zone thickness is modeled with cell length Lc.
Calculation of value of c value in reaction zone thickness formula \({\varvec{x}}={\varvec{c}}\left({\Delta }_{\mathbf{i}}+{\Delta }_{\mathbf{r}}\right)\) for a 2H2 + O2 mixtures b CH4 + 2O2 mixtures. ZND calculations were carried out at a stoichiometric equivalence ratio and an initial temperature of 295 K
Comparison of the proposed modified model (FFCM1) for the prediction of velocity deficit with the experimental data and other theoretical models for a, b hydrogen–oxygen detonations and c methane-oxygen detonations. ZND calculations were carried out at a stoichiometric equivalence ratio and an initial temperature of 295 K
However, the same is not the case with the proposed theoretical model, which reasonably predicts the velocity deficit data for both 2H2 + O2 and CH4 + 2O2 mixtures. Thus, the proposed modified model is more robust in predicting the velocity deficits in unstable mixtures when compared to earlier models. The same can be seen in Fig. 3, where the modified theoretical model is observed to predict the experimental velocity deficit data trends quite accurately.
Stable Mixtures
In the case of stable mixtures, the experimental data of 2H2 + O2 + 3Ar by Gao and Ng (2016), as shown in Fig. 4a, is used to evaluate the value of c in \(x=c\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\). It can be seen that the value of c is reasonably constant over the range of initial pressures. The average c value for hydrogen–oxygen mixtures diluted with 50% Ar is found to be 19.5, see Fig. 4a. In the case of stable mixtures, Fay’s model reasonably predicts the velocity deficits. The experimental velocity deficit results of Gao and Ng (2016) for 2H2 + O2 + 3Ar mixtures agree well with Fay’s model, where the reaction zone thickness is modeled as a cell length (see Fig. 4b). In the present study, the reaction zone thickness is also modeled with \(x=19.5\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\), and is then used in Fay’s model to predict the velocity deficit. It is observed that the proposed modified model predicts the velocity deficit trends of Gao et al. more accurately when compared to Fay’s model. It can be seen from Fig. 4b that near detonation propagation limits, Fay’s model, in its current form, performs poorly in predicting the velocity deficits for different tube diameters.
a Calculation of c in the reaction zone thickness formula \({\varvec{x}}={\varvec{c}}\left({\Delta }_{{\varvec{i}}}+{\Delta }_{{\varvec{r}}}\right)\) for 2H2 + O2 + 3Ar mixtures diluted with 50% Argon. b Comparison of velocity deficit results for the proposed model with the experimental data of Gao et al. and Fay's model for 2H2 + O2 + 3Ar mixtures diluted with 50% Argon. ZND calculations were carried out at a stoichiometric equivalence ratio and an initial temperature of 295 K
However, using a modified theoretical model proposed in the present work predicts the velocity deficits more accurately when compared to Fay’s Model for stable mixtures near the propagation limits. This is a remarkable result since, to date, no theoretical model can predict the velocity deficit trends in both stable and unstable mixtures. The proposed model is capable of predicting the velocity deficit trends in both the stable and unstable mixtures and holds a lot of promise for the detonation scientific community.
Effect of O3 and H2O2 on Velocity Deficit
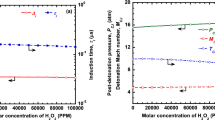
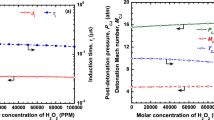
The effects of ignition promoters like ozone and hydrogen peroxide are studied for both stable and unstable mixtures. It can be seen from Fig. 5a–c that the addition of O3 and H2O2 will reduce the velocity deficits significantly near the propagation limits for H2–O2, CH4–O2, and H2–O2 mixtures diluted with 50% Argon for various tube diameters. The results by Crane et al. (2019) show the effects of the ignition promoter is to reduce the activation energy and increase the chain branching reactions leading to an increase in the generation of free radicals like H, O, and OH, resulting in faster kinetics. Thus, reaction zone thickness is reduced in the presence of ignition promoters, which significantly reduces the velocity deficits. From Fig. 5 and Table 1, it can be seen that the velocity deficits are significantly reduced near the propagation limits with the addition of ignition promoters from 0 to 20,000 PPM. It can be seen from Table 1 that velocity deficits can be improved by up to 20% by using O3 and H2O2 in trace amounts for methane-oxygen detonations, especially in narrow tubes. A similar improvement in the decrease of velocity deficit by ~ 13% can be seen for hydrogen–oxygen mixtures, with and without argon.
Effect of O3 and H2O2 on Stability Parameter
The stability of mixtures can be better explained with the stability parameter. The lower value of the stability parameter, χ, below the neutral stability boundary represents more stable mixtures, and higher values of χ represent mixtures that are unstable. It is desired to have stable mixtures for lower velocity and pressure fluctuations and uniform cell structures. The addition of ignition promoters like ozone and hydrogen peroxide can have a significant impact on the stability parameter by the reduction in activation energy and induction and reaction zone length ratios. The effects of the addition of ignition promoters like O3 and H2O2 on the stability of H2–O2, CH4–O2, and H2–O2 mixtures diluted with 50% argon were estimated at initial pressures of 15 kPa and 1 bar. The results are presented in Fig. 6a–c. It can now be understood that the stability parameter reduces significantly with the addition of ignition promoters for both the stable and unstable mixtures.
Hence, it can be inferred that the addition of ignition promoters can steer the mixtures from unstable regimes toward stable regimes. Thus, the presence of ignition promoters not only reduces the velocity deficits but also seems to have a stabilizing effect on the resulting detonation structure. The critical role of ignition promoters in detonating mixtures cannot be neglected where they not only prevent the attenuation of a detonation wave but also stabilizes them, thus making them more robust near their propagation limits. This methodology can be successfully implemented in detonation-based engines to reduce the velocity deficits for varied flow conditions and engine geometries. Similarly, detonability and flammability limits can be extended for various fuel-oxidizer mixtures in the presence of ignition promoters at modest concentrations.
Conclusions
The proposed theoretical model for the prediction of velocity deficits in gaseous detonations in tubes was developed by modeling the reaction zone thickness as \(x=c\left({\Delta }_{\mathrm{i}}+{\Delta }_{\mathrm{r}}\right)\) and using it as a length scale in Fay’s Model. The reaction zone thickness x was calculated using ZND chemical length scales like induction length, \({\Delta }_{\mathrm{i}}\) and exothermic length, \({\Delta }_{\mathrm{r}}\). The value of c was estimated to be 33.2, 8.6, and 19.5 for the H2–O2 (unstable mixtures), CH4–O2 (unstable mixtures), and H2–O2 mixtures diluted with 50% argon (stable mixtures), respectively by using experimental velocity deficit data from earlier works. The proposed theoretical model reasonably predicts velocity deficits for both unstable and unstable mixtures over a wide range of pressures, tube diameters, and also near detonation limits. The addition of ignition promoters like O3 and H2O2 in modest concentrations to fuel-oxidizer-diluent mixtures will have a significant impact on detonation structure, where it reduces the chemical length and time scales significantly. The overall effect of such doping would be to reduce the velocity deficits in gaseous detonations. This methodology of sensitizing a given fuel–oxidizer–diluent mixture with the help of ignition promoters like ozone and hydrogen peroxide can be used as a promising solution for reducing the velocity deficits, especially near the propagation limits. With this methodology, the detonation limits can be widened, and lower velocity deficits can be attained, which is essential for the sustenance of detonation waves for propulsion applications for a variety of engine geometries and varied flow conditions. This methodology could also prevent the failure of detonation waves near their propagation limits. Present results show that the addition of O3 and H2O2 at modest concentrations can significantly lower the stability parameter values (χ), even for unstable mixtures. Ozone and H2O2 seems to have a stabilizing effect on the resulting detonation structure and can be used to steer the mixtures from unstable regimes toward stable regimes.
Change history
13 July 2022
The original article has been corrected. Reference citation "Cantera (Goodwin et al. 2009)" is corrected in the article text.
Abbreviations
- \({\Delta }_{\mathrm{i}}\) :
-
Induction zone length (mm)
- \({\Delta }_{\mathrm{r}}\) :
-
Reaction zone length (mm)
- χ :
-
Stability parameter (–)
- \({\tau }_{\mathrm{i}}\) :
-
Induction delay time (\(\mathrm{\mu s})\)
- \({\tau }_{\mathrm{r}}\) :
-
Reaction time \((\mathrm{\mu s})\)
- VN:
-
Von Neuman state
- CJ:
-
CJ state
- i:
-
Induction zone
- r:
-
Reaction zone
References
Browne S, Ziegler J, Shepherd JE (2008) Numerical solution methods for shock and detonation jump conditions GALCIT Report FM2006 6 Pasadena, CA
Bykovskii FA, Vedernikov EF (2009) Heat fluxes to combustor walls during continuous spin detonation of fuel-air mixtures. Combust Explos Shock Waves 45:70–77
Camargo A, Ng HD, Chao J, Lee JHS (2010) Propagation of near-limit gaseous detonations in small diameter tubes. Shock Waves 20:499–508
Chao J, Ng HD (2009) Detonability limits in thin annular channels. Proc Combust Inst 32:2349–2354
Crane J, Shi X, Singh AV, Tao Y, Wang H (2019) Isolating the effect of induction length on detonation structure: hydrogen–oxygen detonation promoted by ozone. Combust Flame 200:44–52
Dahake A, Singh AV (2021) Numerical study on NOx emissions from a synthetic biofuel for applications in detonation-based combustors. In: AIAA propulsion and energy 2021 forum AIAA 2021-3678, p 3678
Dahake A, Singh AV (2022a) A comparative study of critical detonation parameters for jet A and an alcohol-to-jet synthetic biofuel. In: AIAA SCITECH 2022a forum AIAA 2022a-0819, p 0819
Dahake A, Singh AV (2022b) Effect of fuel sensitization on NOx emissions from a synthetic biofuel under detonating conditions. In: AIAA SCITECH 2022b forum AIAA 2022b-0518, p 0518
Dahake A, Singh AV (2022c) Nitrogen oxides emissions from fuel-sensitized detonations for a synthetic biofuel. Trans Indian Natl Acad Eng (in press)
Dahake A, Singh AV (2022d) A comparative study of the detonation chemistry and critical detonation parameters for jet A and a biofuel. Trans Indian Natl Acad Eng (in press)
Dove JE, Scroggie BJ, Senerjian H (1974) Velocity deficits and detonability limits of hydrogen-oxygen detonations. Acta Astronaut 1:345–359
Durpe G, Peraldi O, Lee JHS, Knystautas R (1988) Progress of detonation waves in an acoustic absorbing walled tube. Prog Astronaut Aeronaut 114:248–263
Fay J (1959) Two-dimensional gaseous detonations: velocity deficit. Phys Fluids 02:283–290
Fickett W, Jacobson J, Schott G (1972) Pulsating one-dimensional detonations with induction-zone kinetics. AIAA J 10:514–516
Gao Y, Ng HD (2016) An experimental investigation of detonation limits in hydrogen-oxygen-argon mixtures. Int J Hydrog Energy 41:6076–6083
Gao Y, Ng HD, Lee JHS (2015) Experimental characterization of galloping detonations in unstable mixtures. Combust Flame 162:2405–2413
Goodwin DG, Moffat HK, Speth RL (2009) Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. Caltech, Pasadena. https://www.cantera.org
Ishii K, Monwar M (2011) Detonation propagation with velocity deficits in narrow channels. Proc Combust Inst 33:2359–2366
Ishii K, Itoh K, Tsuboi T (2002) A study on velocity deficits of detonation waves in narrow gaps. Proc Combust Inst 29:2789–2794
Ivin K, Singh AV (2019) Sensitizing ethylene-air and ethylene-oxygen mixtures for optimal performance of detonation cycle engines. In: 33rd national convention of aerospace engineers and national conference on emerging technologies in aerospace structures, materials and propulsion systems, November 16–17, pp 14–19
Iyer MSK, Singh AV (2021) NOx emissions from jet A-air detonations. In: AIAA propulsion and energy 2021 forum AIAA 2021-3679, p 3679
Iyer MSK, Singh AV (2022) Ignition kinetics of real distillate fuels under detonating conditions. In: AIAA SCITECH 2022 forum AIAA 2022-0518, p 0518
Iyer MSK, Dahake A, Singh AV (2022) Comparative studies on ignition kinetics and detonation chemistry of real distillate fuels. Trans Indian Natl Acad Eng. https://doi.org/10.1007/s41403-022-00331-5
Jackson S, Lee J, Shepherd JE (2016) Detonation mode and frequency analysis under high loss conditions for stoichiometric propane-oxygen. Combust Flame 167:24–38
Kailasanath K (2000) Review of propulsion applications of detonation waves. AIAA J 38:1698–1708
Kumar DS, Singh AV (2019) Sensitizing gaseous mixtures for practical applications in rotating detonation engines. In: 33rd national convention of aerospace engineers and national conference on emerging technologies in aerospace structures, materials and propulsion systems, November 16–17, pp 14–19
Kumar DS, Singh AV (2021) Inhibition of hydrogen-oxygen/air gaseous detonations using CF3I, H2O, and CO2. Fire Saf J 124:103405
Kumar DS, Ivin K, Singh AV (2020) Sensitizing gaseous detonations for hydrogen/ethylene-air mixtures using ozone and H2O2 as dopants for application in rotating detonation engines. Proc Combust Inst 38(3):3825–3834
Kumar DS, Dahake A, Singh AV (2022) Detonation chemistry of fuel-sensitized JetA1-air detonations. Trans Indian Natl Acad Eng (in press)
Laberge S, Knystauts R, Lee JHS (1993) Propagation and extinction of detonation waves in tube bundles. AIAA Prog Astronaut Aeronaut 153:381–396
Lee JHS (2008) The detonation phenomenon. Cambridge University Press, Cambridge
Liang W, Wang Y, Law CK (2019) Role of ozone doping in the explosion limits of hydrogen-oxygen mixtures: multiplicity and catalyticity. Combust Flame 205:7–10
Lu FK, Braun EM (2014) Ting detonation wave propulsion: experimental challenges, modeling, and engine concepts. J Propuls Power 30:1125–1142
Magzumov AE, Kirillov I, Rusanov V (1998) Effect of small additives of ozone and hydrogen peroxide on the induction-zone length of hydrogen-air mixtures in a one-dimensional model of a detonation wave. Combust Explos Shock Waves 34:338–341
Manson N, Guenoche H (1957) Effect of the charge diameter on the velocity of detonation waves in gas mixtures. Proc Combust Inst 06:631–639
Moen IO, Murray SB, Bjerketvedt D, Rinnan A, Knystautas R, Lee JHS (1982) Diffraction of detonation from tubes into a large fuel–air explosive cloud. Proc Combust Inst 19:635–644
Ng HD, Higgins A, Kiyanda C, Radulescu M, Lee JHS, Bates K, Nikiforakis N (2005) Nonlinear dynamics and chaos analysis of one-dimensional pulsating detonations. Combust Theory Model 9(1):159–170
Radulescu M, Lee JHS (2002) The failure mechanism of gaseous detonations: experiments in porous wall tubes. Combust Flame 131:29–46
Radulescu M, Ng HD, Lee JHS, Varatharajan B (2002) The effect of argon dilution on the stability of acetylene-oxygen detonations. Proc Combust Inst 29:2825–2834
Randall S, Anand V, St George AC, Gutmark EJ (2015) Numerical and experimental study of heat transfer in a rotating detonation engine. In: 53rd AIAA aerospace sciences meeting, Florida, USA
Smith GP, Tao Y, Wang H (2016) Foundational fuel chemistry model version 1.0 (FFCM-1). http://web.stanford.edu/group/haiwanglab/FFCM-1/index.html
Teodorczyk A, Knystautas R (1989) Propagation mechanism of quasi-detonations. Proc Combust Inst 22:1723–1731
Teodorczyk A, Lee JHS (1995) Detonation attenuation by foams and wire meshes lining the walls. Shock Waves 4:225–236
Zeldovich YB (1950) Zho. Eksp. Teor. Fiz: 10:542 (1940) (Translated in NACA technical memorandum 1261)
Zhang B, Liu H (2019) Theoretical Prediction model and experimental investigation of detonation limits in the combustible gaseous mixture. Fuel 258:116132
Zhao H, Yang X, Ju Y (2016) Kinetic studies of ozone assisted low-temperature oxidation of dimethyl ether in a flow reactor using molecular beam mass spectrometry. Combust Flame 173:187–194
Acknowledgements
The authors acknowledge the financial support for this work from the Aeronautics R&D Board, Ministry of Defence, Govt. of India vide Sanction Letter # ARDB/01/1042000M/I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dahake, A., Kumar, D.S. & Singh, A.V. Using Ozone and Hydrogen Peroxide for Improving the Velocity Deficits of Gaseous Detonations. Trans Indian Natl. Acad. Eng. 7, 1033–1042 (2022). https://doi.org/10.1007/s41403-022-00345-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-022-00345-z