Abstract
Emerging detonation-based combustors like RDEs and PDEs motivate the studies related to the detonability limits for operating these combustors at lean mixtures. Maintaining a stable detonation wave is challenging, especially with real distillate fuels like JetA1, since the stable propagation of detonation wave in such cases is significantly affected over wide operating ranges, where chemical kinetics of JetA1 play a significant role. In the present work, we have used a detailed kinetics model to investigate the detonation chemistry of real distillate fuel. The computed results show that ignition promoters like ozone and hydrogen peroxide in smaller amounts could help in fastening the ignition kinetics of JetA1–air detonating mixtures and could give rise to more detonable mixtures with reduced detonation time and length scales. The results also suggest that sensitizing the fuel-oxidizer mixture with ozone and hydrogen peroxide can yield a robust detonation wave, which can be used as a plausible solution for broadening the detonability limits of conventional jet fuels. Such fuel-sensitization techniques could also help in widening the propagation and operating temperature limits of detonation-based combustors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The detonation-based combustors are known for higher efficiency and simple design for a given fuel-oxidizer mixture since they operate close to the constant volume cycle and generate minimum entropy during their operation (Lee 2008; Li et al. 2018). Emerging detonation-based engines motivate the study of stable detonation wave propagation and detonability limits in such engines. Rotating detonation engines (RDEs) are the most predominant ones among detonation cycle engines due to their simple design and high working frequency of several kHz.
Voitsckhovskii developed the first setup of RDE in the late 1950s (Li et al. 2018). Nicholls et al. succeeded in demonstrating the propagation of a rotating detonation wave in an annular combustor in the following years (1966). Because of their superior thermodynamic efficiency, simple design, and smaller combustion length and time scales, RDEs can be used for high-speed propulsion systems. The RDEs can be operated with both gaseous and liquid fuels. Over the past decade, single component gaseous fuels like hydrogen, acetylene, and ethylene have been widely researched for applications in RDEs due to their simplified fuel chemistry (Fotia et al. 2015; Anand et al. 2015, 2016, 2017; Bykovskii et al. 2003; Peng et al. 2018; Wang and Lee 2019; Xie et al. 2018; Rankin et al. 2017; Frolov et al. 2015). RDEs using kerosene as fuel was also studied experimentally (Kindracki 2014a, b; 2015; Zhong et al. 2019). Similarly, Zhong et al. (2019) researched RDEs using kerosene as fuel to study the reactivity and mixing of kerosene fuel vapors with a given oxidizer.
Modeling real distillate fuels like jet fuels and rocket fuels is difficult as they comprise thousands of components that can't be defined precisely. These fuels usually include hydrocarbons containing carbon atoms ranging from 4 to 20, indicating substantial compositional complexities. Till recently, the surrogate-fuel approach (Violi et al. 2002; Eddings et al. 2005; Zhang et al. 2007) was used to model the combustion chemistry of real distillate fuels. In this approach, a surrogate fuel is defined as several small, neat compounds with well-defined composition and structure, which can represent the chemical and physical functionality of real distillate fuels. The surrogate fuel approach helps to eradicate the difficulties related to the definition of fuel composition and transform it into a problem that can be easily tackled, at least in principle, from fundamental reaction mechanisms and their rates. This approach attempts to solve the issues related to real fuels' combustion modeling; however, it remains largely empirical at present and cannot be used to evaluate the combustion behavior of real fuels over a range of input conditions (Wang et al. 2018).
The recently proposed HyChem approach simplifies the kinetic modeling of real distillate fuels, which uses a physics-based model to evaluate the combustion chemistry of real distillate fuels, thereby eliminating the drawbacks of the traditional surrogate fuel approach (Wang et al. 2018). The present study uses the HyChem chemical kinetics model for computing the relevant detonation length and time scales of JetA1–air detonating mixtures using a simplified one-dimension model. The effect of chain-branching-termination mechanisms on the detonation structure can be adequately accounted for using the detailed reaction chemistry of real distillate fuels. It, therefore, can quantitatively describe many detonation responses of interest. The critical parameters of JetA1–air detonations were analyzed in the present study for applications in RDEs. A detailed reaction kinetics model in conjunction with a simplified ZND model is used in the present study to investigate the detonation chemistry and detonation length scales of JetA1–air-diluent mixtures with and without dopants. Though the model is simplified and cannot account for the three-dimensional structure of real detonation waves, it is widely used to probe the effects of fuel chemistry on the detonation length and time scales since it is computationally less intensive and tractable. The one-dimensional ZND model is a useful substructure where it gives the characteristic spatial and temporal scales for heat release based on chemical kinetics, which can be correlated empirically to the detonation cell size for a large number of fuels over a wide range of initial conditions (Stamps and Tieszen 1991). Therefore, a one-dimensional ZND model is employed in the present study since it allows us to investigate the detonation chemistry of real distillate fuels using a detailed kinetics model. It can also help us to understand the averaged detonation behavior, at least over one full cell cycle, though practically the conditions change within a detonation cell due to the large fluctuations of the reaction rates inside a single detonation cell (Garikov et al. 2000).
It is envisaged that in the near future, practical detonation-based combustors will be based on liquid hydrocarbon fuels like JetA1. One problem with real distillate fuels is their slow ignition kinetics, which could hamper their efficient combustion at supersonic speeds. The present study focuses on enhancing the ignition kinetics of JetA1–air detonations by adding ozone and hydrogen peroxide to the original mixture in trace amounts. These ignition promoters accelerate the ignition kinetics of the detonating mixture and can enhance the energy release rates considerably. They can also be utilized to broaden the detonability limits of practical fuels, which can improve the operating limits of detonation-based combustors. Another objective of the current study is to investigate the effect of the addition of ignition promoters on the ignition chemistry of real distillate fuels and the associated length and time scales under detonating conditions.
ZND results by Magzumov et al. (1998) show that ozone addition up to 4000 PPM to H2-air mixtures can shorten the induction zone length by 50%. Crane et al. (2019) carried out an experimental study on the effect of ozone on the reduction in the detonation cell sizes. The addition of ozone could also profoundly affect the explosion limits of a given mixture (Liang et al. 2019). The main question that lies ahead of the scientific community is whether fuel chemistry impacts a sustained detonation or not. It would be extremely beneficial if we could study the effects of chemistry in isolation without changing the bulk fluid thermodynamic properties of detonating mixtures. For example, induction or reaction length variation can be achieved through changes in equivalence ratio or inert diluents, which in turn can change the gas dynamics and relevant thermodynamic properties of both burned and unburned mixtures. It, therefore, becomes difficult to assess how much of these changes can be attributed to chemistry. This problem can be resolved using fuel sensitizers or promoters in trace amounts (at dopant levels). Using fuel sensitizers like ozone and hydrogen peroxide in trace amounts does not affect the gas dynamics and bulk thermodynamic properties of burned and unburned mixtures. Therefore, the effects of fuel chemistry on the underlying detonation wave structure can be studied in isolation using fuel sensitizers at dopant levels. It is observed that trace amounts of ozone and hydrogen peroxide can significantly reduce the induction time of certain mixtures without impacting the thermodynamic properties and gas dynamics of the resulting mixtures (Crane et al. 2019; Liang et al. 2019; Kumar et al. 2021). In the present work, the detonation chemistry of JetA1–air-diluent mixtures in the presence of ignition promoters is investigated to study the effect of fuel chemistry on detonation length and time scales.
Another problem associated with RDEs is the relatively high temperature of the burned products, which can be of the order of 4000 K. Such high temperatures of detonation products can grossly affect the operating temperature ranges of a detonation-based combustor and require complex cooling mechanisms to reduce the wall temperatures of such engines to desirable limits. The deployment of complex cooling mechanisms to reduce the wall temperatures of detonation-based engines result in a serious reduction of the power-to-weight ratio of such engines. This problem can be resolved by adding inert diluents to a given explosive mixture. The addition of chemically inactive inert diluents, like Ar or He, can reduce the CJ temperature of a given detonating mixture. However, in such scenarios, the stability of detonation waves may decrease due to an increase in the chemical length/time scales of an underlying detonation wave. The increased detonation length and time scales are indicative of a weak detonation wave and represent mixtures that are less detonable and can lead to inhibition or failure of a detonation wave near its propagation limits (Lee 2008). Fuel-sensitizers like O3 and H2O2, when added in small amounts, could help to resolve this issue by enhancing the ignition and chemical kinetics of a given fuel-oxidizer-diluent mixture. Also, using ignition promoters at dopant levels do not impact the gas dynamics and bulk thermodynamic properties of detonating mixtures. Thus, detonations can be successfully initiated and stabilized at large dilution levels by fuel sensitization of a given explosive mixture. Thus, the operating temperatures of detonation engines can be lowered in the combined presence of inert diluents and ignition promoters.
Similarly, operating RDE combustors under lean conditions is a challenging task due to higher instabilities under fuel-lean conditions. Also, the induction and reaction length scales at fuel-lean conditions are significantly high, which may render a detonation wave less robust at lower equivalence ratios. However, it is desirable to operate the detonation-based combustors at fuel-lean conditions since it could lower the operating temperatures drastically and can help in reducing NOx emissions for such devices. Operating detonation engines at fuel-lean conditions is essential as current detonation-based combustors can only operate for a few seconds due to excessive heating of such combustors since the associated temperatures are in the range of 3000–4000 K depending upon the type of explosive mixture. Fuel-sensitization can also help resolve such a problem where the operation of detonation engines at fuel-lean conditions can be readily achieved using ignition promoters in trace amounts. The problems associated with the operation of detonation-based combustors are systematically addressed in the present study using ozone and hydrogen peroxide in trace amounts for JetA1–air-diluent mixtures.
The purpose of this work is to investigate the utilization of JetA1–air-diluent mixtures for liquid-fueled detonation cycle engines while simultaneously exploring the effectiveness of ignition promoters on the detonation cell structure and the detonability limits through one-dimensional ZND computations. The JetA1–air detonations are also studied at stoichiometric and fuel-lean conditions in the presence of ignition promoters in trace amounts to explore the stability of JetA1–air detonations near their propagation limits. Operating RDEs under leaner mixture conditions (lower equivalence ratios) will ensure lower CJ temperatures (post-detonation temperatures) in such engines. Therefore, they could help to resolve the issues concerning NOx emissions and complex cooling mechanisms that are usually employed in such engines. The use of ignition promoters like ozone and hydrogen peroxide at dopant levels could accelerate the ignition chemistry significantly for JetA1–air detonating mixtures and could help in stabilizing the underlying detonation wave near its propagation limits. Such fuel-sensitization could also lower the constraints on the use of an oxidizer (for example, using air instead of pure O2) and can also help in increasing the power-to-weight ratio of such engines by eliminating complex cooling mechanisms. Fuel-sensitization of JetA1–air mixtures can also widen its detonability limits over a range of operating conditions. The present study suggests a plausible fuel-sensitization approach to achieve lower CJ temperatures for JetA1–air-diluent mixtures without sufficing the stability and robustness of an underlying detonation wave.
The use of conventional jet fuels in RDEs will aid in the resolution of payload and operational cost issues (Dahake and Singh 2021; Dahake and Singh 2022a, b; Iyer and Singh 2021; Iyer et al. 2022; Kumar et al. 2020). Therefore, the use of real distillate fuels like JetA1 is highly relevant to liquid-fueled detonation-based engines. To the authors' knowledge, the detonation chemistry of JetA1–air mixtures is numerically investigated for the very first time, where a detailed kinetics model for the thermal decomposition and oxidation of real distillate fuel is used to register the various detonation responses of interest.
Methodology
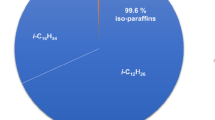
The CalTech SD Toolbox (Browne et al. 2008) is utilized for ZND computations in the present study. Cantera (Goodwin et al. 2009) integrated with MATLAB and Python is utilized for detailed chemical kinetic calculations for JetA1–air-diluent mixtures. ZND computations were performed for JetA1–air-diluent mixtures using the HyChem (Hybrid Chemistry) kinetics model (Wang et al. 2018), which consists of primary reaction pathways for the combustion of real distillate fuel. HyChem consists of an experimentally constrained fuel-pyrolysis model to model the fuel pyrolysis. In the HyChem approach, a detailed kinetics model, USC Mech II (Wang et al. 2007), is used to model the oxidation of pyrolysis products. The rate of the oxidation of the pyrolysis products is critical to radical growth and heat release, and for this reason, it is modeled with a detailed foundational fuel chemistry model (USC Mech II). In the HyChem model, the two sub-models (pyrolysis and oxidation models) are interconnected: the pyrolysis process provides the “reactants” for the oxidation process, while the oxidation process supplies heat and radical species to facilitate the endothermic, oxidative pyrolysis of the fuel. The Princeton O3 sub-model of Zhao et al. 2016 was integrated with the HyChem model to model the ozone chemistry. Overall, the full kinetics model, including the ozone chemistry, consists of 120 species and 851 reactions. The key properties of Jet A1 and its molecular composition are shown in Table 1. Due to code limitations, C10.8H21.6 was rounded off to C11H22 for modeling purposes.
The range of concentrations of ozone and hydrogen peroxide employed in the present work was based on the % change in the global detonation properties (exothermic and bulk thermodynamic properties such as the shape and amount of total heat release and the von Neumann and equilibrium states).
As the addition of ozone and hydrogen peroxide at higher levels brings out small changes in the global detonation properties, the concentrations were employed such that the % change in the global detonation properties (TVN, TCJ, PCJ, VCJ) is less than 2%.
The governing equations for a one-dimensional detonation model are as follows (Browne et al. 2008; Kumar and Singh 2021; Lu et al. 2003),
Continuity:
Species conservation:
Momentum conservation:
Energy conservation:
Equation (5) represents the enthalpy per unit mass of the mixture.
Where,
The perfect gas approximation can be expressed as,
Here, \(x\) represents the dimensional coordinate with the origin at the shock, \({Y}_{k}\) is the mass fraction of species \(k\), with \(\sum_{k}{Y}_{k}=1\). In Eqs. (1–7), K represents the total number of species, \(\omega\) is the molar production rate, and \(W\) represents the molecular weight. The universal gas constant is \({R}_{u}=\) 8.314 Jmol−1 K−1 and \({W}_{k}, {R}_{k}, {c}_{p,k},\) and \({h}_{f,k}^{0}\) represents the molecular weight, specific gas constant, specific heat, and the enthalpy of formation of the kth species. Also, \(p\), \(T\), \(\rho\), u, and h represent pressure, temperature, density, x-velocities, and enthalpy per unit mass of the mixture, respectively.
The governing equations for a one-dimensional detonation model are given above. The induction length (Δi) and time (τi) were calculated by first determining the detonation velocity, VCJ for the given initial conditions of T0, P0, and ϕ. In the present study, relevant VN and CJ conditions are calculated using well established Caltech Shock and Detonation Toolbox (Browne et al. 2008). Once the CJ detonation velocity is calculated, the normal shock relations can be used to calculate the post-shock temperature TVN, pressure PVN, density ρVN, and velocity uVN in the shock coordinate together with the ideal gas relation. The post-shock conditions can then be used as initial conditions for the chemical kinetics model. The post-shock gas mixture's structural evolution undergoing chemical reaction can then be traced through numerical integration of conservation equations (Kumar and Singh 2021). After the estimation of ZND structure, the induction length (Δi) was calculated as the distance measured from the leading shock front to the location of peak thermicity, where maximum temperature gradient [max(dT/dx)] occurs. The time scale corresponding to the peak thermicity or maximum rate of temperature rise during a chemical reaction [max(dT/dt)] defines the induction delay time (τi) in such a structure. It is the time taken to reach peak thermicity from the leading shock front.
The characteristic length for heat release based on chemical kinetics defines the total reaction length or reaction zone length. The total reaction length extends to the final phase of heat release where three-body reactions are important and includes the induction zone (Stamps and Tieszen 1991). Criteria for estimating the total reaction length are proposed by various researchers where reaction lengths based on a value of local Mach number of 0.9 and 0.75 are extensively used since they can be empirically correlated to experimental cell size over a wide range of input conditions (Stamps and Tieszen 1991; Shepherd 1986). For instance, Shepherd (1986) approximated it by the location where the local Mach number reaches 0.75 with respect to the wave frame of reference. The reaction zone length based on the local Mach number of 0.75 was shown to be the most effective in correlating earlier hydrogen-air-diluent cell size data over a broad range of operating conditions (Shepherd 1986); hence the same is used in the present study. Another justification for using a Mach number of 0.75 for calculating reaction zone lengths for JetA1–air detonations is based on the total heat release curve. It is observed that the heat release is almost complete up to M = 0.75 location. The reaction zone length based on M = 0.75 can thus be used to represent the characteristic length of heat release for JetA1–air detonations. In another study, Ng et al. (2010) suggested that the reaction zone length (main heat release zone length) can be defined based on maximum thermicity for one-dimensional pulsating detonations. Ng et al. (2010) defined the reaction zone length as \({\Delta }_{r}= {u}_{CJ}/{\dot{\sigma }}_{\mathrm{max}}\), where \({u}_{CJ}\) is the CJ particle velocity in the shock-attached frame of reference for a steady CJ detonation and \({\dot{\sigma }}_{\mathrm{max}}\) is the maximum thermicity. The reaction zone length in the present work is approximated by the location where the local Mach number reaches 0.75, as suggested by Shepherd (1986).
The induction length and the detonation cell size may be considered characteristic length scales used to characterize a particular detonable mixture. The detonation cell size can be strongly correlated to the induction and reaction zone length (Lee 2008; Crane et al. 2019; Shepherd 1986; Ng et al. 2005; Knystautas et al. 1984; Benedick et al. 1986; Lee 1984; Stamps et al. 2006; Westbrook et al. 1982; Mevel et al. 2008). The cell size is an intrinsic property of real detonations and is defined by detonations' multi-dimensional structure. A one-dimensional ZND model cannot physically describe a detonation wave's three-dimensional structure. Though the ZND approach is simple and neglects the effects of hydrodynamic-kinetic interactions, it has successfully predicted the detonation parameters as well have correlated data for many fuels over a wide range of initial conditions (Stamps and Tieszen 1991). For example, the detonation cell size can be strongly correlated to the induction and reaction zone length (Lee 2008; Crane et al. 2019; Shepherd 1986; Ng et al. 2010; Knystautas et al. 1984; Benedick et al. 1986; Lee 1984; Stamps et al. 2006; Westbrook et al. 1982; Westbrook et al. 1982; Mevel et al. 2008; Vasil'ev 1998; Westbrook 1982a, b; Moen et al. 1985). The estimation of detonation cell size based on detailed kinetic models has also been proposed for safety evaluations (Westbrook 1982a, b; Moen et al. 1985). Thus, the ZND structure serves as an essential model and a useful construct where the detailed chemical kinetics of the explosive reactions can be studied under the gas dynamic conditions that correspond to detonation processes (Westbrook 1982a, b; Moen et al. 1985). Thus, ZND model provides a characteristic length and time scale for a detonation process, which can be directly correlated to cell width or size.
Detonation Length and Time Scales
The length and time scales are defined in the present work with a frame of reference fixed to a shockwave. The induction zone length (\({\Delta }_{i}\)) is approximated by the location of the maximum temperature gradient where peak thermicity occurs. Similarly, the time taken by the postshock gas-particle to travel from shock front to peak thermicity defines induction time (\({\tau }_{i}\)). The reaction zone length (\({\Delta }_{r}\)) is approximated by the location where the local Mach number reaches 0.75 and is measured from the leading shock front. Similarly, the reaction time (\({\tau }_{r}\)) is defined as the time taken by the postshock gas particle to travel to the location where the local Mach number reaches 0.75. The location, where the local Mach number reaches 0.75 in the shock-attached frame of reference, is used for the calculation of reaction zone length and time scales since 97% of temperature increase occurs in this region, thereby suggesting that the heat release behind the leading shock front is almost complete at this point.
Results and Discussions
Pyrolysis and Oxidation of Jet A1–Air in Gaseous Detonations
In a ZND model, the initial premixed JetA1–air mixture will be compressed by the shock front, where the temperature and density will increase. Generally, the post-shock temperature rises to ~ 1500 K. Since the shockwave thickness is a few molecular mean free paths, there will be no decomposition or oxidation of fuel inside the leading shock. In the postshock induction zone, active radical species are generated by the thermal dissociation of the shock-heated molecules. The fuel-oxidizer mixture with large JetA1 molecules will undergo thermal decomposition first into several intermediates due to high post-shock temperature. Thus, the parent fuel molecule (JetA1) undergoes decomposition in the decomposition zone (zone immediately following the leading shock front), in which the temperature is high enough and small radical species (e.g., H and OH) are relatively abundant due to diffusion and thermal dissociation of the shock-heated molecules. These species interact with the fuel molecule and facilitate its dissociation in the decomposition zone of the ZND structure (shown in Fig. 2). The oxidation of decomposed intermediates will follow this. The thermal decomposition of JetA1 occurs regardless of the presence of molecular oxygen in the system. Thus, JetA1 fuel molecules undergo thermal or oxidative thermal decomposition first followed by the oxidation of decomposed products. The two processes can be separated in length and time scales. During thermal decomposition or pyrolysis of a real distillate fuel like JetA1, the number of significant products or intermediates is small (six to ten at most) (Wang et al. 2018). Further, the composition of these intermediates determines the global combustion properties of the original, multi-component real fuels (JetA1 in this case). Thus, the thermal decomposition or pyrolysis process provides the “reactants” for the oxidation process, while the oxidation process supplies heat and radical species to facilitate the endothermic, oxidative pyrolysis of the fuel molecule. It must be noted that the rate of the oxidation of pyrolysis products is critical to radical growth and heat release, and for this reason, it is modeled with a detailed foundational chemistry model (USC Mech II). Figure 1 shows the concentration of key intermediates during the thermal decomposition of JetA1 for stoichiometric JetA1–air detonations at an initial pressure and temperature of 1 atm and 295 K, respectively.
Figure 2a depicts the ZND detonation structure with species profile for stoichiometric JetA1–air mixtures at 1 atm, and 295 K. Figure 2b shows the magnified view of the fuel decomposition zone, in which the JetA1 decomposes into several small intermediates before the ignition location (denoted by the location of peak CH* concentration or thermicity peak and corresponds well with the maximum rate of temperature rise during a chemical reaction, max(dT/dt) or maximum temperature gradient, max(dT/dx)). The key intermediates of JetA1 are ethylene (C2H4), hydrogen (H2), methane (CH4), propene (C3H6), 1-butene (1-C4H8), iso-butene (i-C4H8), benzene (C6H6), and toluene (C7H8). Since these intermediates have larger molecular diffusivities relative to JetA1, they are quickly diffused into the reaction zone along with molecular oxygen. They are subsequently oxidized, resulting in the formation of detonation products; CO2, H2O, and CO, along with subsequent heat release. The molecular diffusion is responsible for the small decay in the concentration of molecular oxygen in the decomposition zone and is not due to its consumption via chemical reactions. The fundamental physics lead to the observed detonation structure. Since there is a large disparity in JetA1 and oxygen's molecular diffusivities, fragmentation of the JetA1 molecule is necessary; otherwise, the process would become unstable due to the large Lewis number of the resultant fuel–oxidizer mixture (Wang et al. 2018). For such a process to be stable, then the second reason must be the ease with which the parent fuel undergoes decomposition at high temperatures in the preheat zone (postshock zone), where small radical species like H and OH are relatively abundant due to radical generation and diffusion (Wang et al. 2018). These radical species interact with the parent fuel molecule and facilitate its dissociation in the preheat zone or decomposition zone (postshock zone).
The typical thermal decomposition zone in deflagration flames for large hydrocarbon fuel molecules has a convective residence time of ~ 100 μs (Wang et al. 2018). However, oxidation of thermally decomposed (pyrolysis) products requires a reaction time of 1000 μs (Wang et al. 2018). The ratio of oxidation to decomposition time is ~ 10, indicating that the thermal decomposition of the parent fuel molecule is very fast. Hence, the oxidation of thermally decomposed (pyrolysis) products will be the rate-limiting step for the entire course of the reaction. In stoichiometric JetA1–air detonations, the decomposition of JetA1 into pyrolysis products occurs within a time duration of 1.45 μs. However, pyrolysis products' oxidation takes approximately 5.1 μs and is rate-limiting for stoichiometric JetA1–air detonations (see Fig. 2). It is clear from Fig. 2 that the thermal decomposition of JetA1 is fast, and the oxidation of the thermally decomposed products will be rate-limiting before ignition. In gaseous detonations, due to the coupling of the leading shock front to the reaction zone, very short decomposition and oxidation times are observed, as seen in Fig. 2a, and the ratio of oxidation to decomposition time is ~ 3.3. Thus, in gaseous detonations, the decomposition of the parent fuel molecule (JetA1 in this case) and the decomposed products' oxidation occur ~ 100 and ~ 182 times faster than the corresponding conventional deflagration flames that we usually encounter in practical situations. It must be noted at this stage that the decomposition zone largely remains thermally neutral with negligible heat release.
The temperature and thermicity profile remain constant in the decomposition zone suggesting little or no heat release. The oxidation zone, where the oxidation of decomposed products takes place, is marked by a rise in temperature and thermicity, contributing significantly to heat release in the ZND structure. The oxidation of decomposed products with molecular oxygen and small radical species like OH and O is responsible for this heat release. As the decomposed products and molecular oxygen approaches ignition, a substantial increase in the heat release occurs, indicated by significant thermicity and temperature gradient changes. This heat release is primarily due to the oxidation of decomposed products, leading to CO, H2O, and CO2. The majority of the chemical heat release takes place in the recombination zone (the zone that follows the induction zone), where three-body recombination reactions are prevalent.
In a ZND detonation structure, the reaction zone comprises both the induction and the recombination zone. Thus, the reaction zone extends beyond the induction zone into the final phase of the heat release, where three-body reactions are prevalent. The dominant product of JetA1 decomposition is ethylene (C2H4), which reaches its maximum concentration at the end of the decomposition zone at approximately 1.45 μs and is shown in Fig. 2b. It must be noted that ethylene is a dominant intermediate of hydrocarbon pyrolysis, where the cause is purely thermodynamic and chemical kinetic. Beyond this decomposition time of 1.45 μs, the distribution of pyrolysis products approaches equilibrium conditions, and a temperature rise can be observed primarily due to the production of aromatics from ethylene and other intermediate species (Wang et al. 2018). This also explains a corresponding temperature and thermicity rise in the oxidation zone. Thus, the pyrolysis intermediates reach peak value in their yields in a very short duration of time, and their concentration levels will have little change for a substantially more extended period. However, there is a substantial decrease in their concentration as they approach ignition (peak thermicity) due to the oxidation of pyrolysis products. The oxidation zone defined in Fig. 2 represents the zone where oxidation of the pyrolysis intermediates occurs with molecular oxygen and small radical species like OH and O. Figure 2b is a magnified view of Fig. 2a for the decomposition/pyrolysis zone. We wish to clarify that the pyrolysis process for most hydrocarbon compounds found in real distillate fuels like JetA1 is purely endothermic.
Effect of O3 and H2O2 on JetA1–Air Detonations
The effects of the addition of O3 and H2O2 in trace amounts on the relevant detonation parameters are studied numerically for JetA1–air mixtures at stoichiometric conditions with varying dopant concentrations up to 20,000 PPM (by volume). Table 2 shows the effects of doping on various detonation parameters. The present calculations show that O3 and H2O2 addition in trace concentrations can substantially alter the chemical length/time scales. Thus, ignition promoters in small concentrations could affect the macroscopic detonation structure by shortening the detonation length/time scales. However, this is achieved with a negligible change in the exothermic and bulk thermodynamic properties such as the shape and amount of total heat release and the von Neumann and equilibrium states. Thus, the impact of the induction and reaction length on detonation structure can be studied independently of other properties. In Table 2, the calculations were carried out for stoichiometric JetA1–air mixtures at initial conditions of 295 K and 1 atm.
Ozone decomposes according to the following reaction,
During ignition, the O-atom accelerates the chain branching process and significantly shortens the chemical induction time, τi. Therefore, ozone alters the ignition kinetics by acting as an ignition promoter at various concentration levels. Similarly, H2O2 decomposes according to the reaction (9). The chain-branching process is accelerated by the production of OH radicals, which significantly shortens the induction delay time.
The computed results show that ignition promoters, when added in trace concentrations (16,000 PPM) to JetA1–air mixtures, will have a reduction in induction length by ~ 57% and ~ 34% with O3 and H2O2, respectively (see Fig. 3a and Table 2). Similarly, the reaction length can be reduced by ~ 47% and ~ 28% with O3 and H2O2, respectively. The reduction in reaction zone length is less when compared to the induction zone length, and the same is shown in Table 2 and Fig. 3.
This shows that ignition promoter has a more profound effect on the radical generation zone (where chain-branching reactions dominate), \({\Delta }_{i}\) compared to the radical recombination zone (where three-body recombination reactions dominate), \({(\Delta }_{r}- {\Delta }_{i})\). Thus, O3 and H2O2 addition can profoundly alter the induction length/time scales, thereby affecting the detonation structure. This catalytic potential of ignition promoters can be a promising method for initiating and maintaining a stable detonation wave in small detonation devices like rotating detonation engines. The present calculations suggest that ozone performs better than H2O2 as an ignition promoter for JetA1–air detonations (see Fig. 3 and Table 2).
In Table 2, the ignition promotion effects of ozone and H2O2 can be observed for stoichiometric JetA1–air detonations. Figures 4a, b show the shift of thermicity peak towards the leading shock front with O3 and H2O2 at 16,000 PPM and demonstrate the ignition promotion effects of ozone and hydrogen peroxide on detonation length/time scales.
The shift in the thermicity peak towards the leading shock front indicates a reduction in induction length and time scales. A more significant shift in peak thermicity is observed for O3 when compared to H2O2 for the same dopant concentration, suggesting that induction length and time scales in JetA1–air-O3 mixtures are shorter than in JetA1–air–H2O2 mixtures. Shorter induction length and time scales are indicative of tighter coupling between the shock wave and reaction zone. It also quantitatively represents the increased sensitivity of mixtures to detonate. Thus, ozone performs better than H2O2 as an ignition promoter. The thermodynamic and the gas-dynamic states of the detonating mixtures are negligibly affected by the presence of O3 and H2O2 in small amounts.
This can be seen in Table 2, where their addition in trace amounts seems to have a minimal effect on VVN, TCJ, VCJ MCJ, and PCJ. Since induction/reaction length and detonation cell width/size can be empirically correlated for practical detonations, the ZND calculations suggest that the addition of dopant levels of O3 and H2O2 could significantly alter the macroscopic detonation structure and cell size. This can be readily seen in Table 2, where induction and reaction lengths can be reduced by ~ 2.3 and ~ 1.9 times, respectively, when JetA1–air mixtures are doped with 16,000 PPM of ozone. Similarly, induction delay and reaction times can be reduced by ~ 2.6 and ~ 2.3 times, respectively, for JetA1–air mixtures doped with 16,000 PPM of ozone. The same observations were made for hydrogen peroxide, where induction and reactions lengths were reduced by ~ 1.52 and ~ 1.4 times, respectively, for H2O2-doped JetA1–air mixtures. Similarly, the induction delay and reaction times were reduced by ~ 1.65 and ~ 1.55 times, respectively, for H2O2-doped JetA1–air mixtures. Therefore, detonation length and time scales can be significantly reduced with the addition of O3 and H2O2, which can significantly alter the detonation structure.
Effect of O3 and H2O2 on Radical Generation
The species profile data can better explain the effect of O3 and H2O2 on the JetA1–air detonation structure. Figures 5 and 6 represent the species profiles for JetA1 mixtures with and without ozone and hydrogen peroxide addition (16,000 PPM), respectively. Generally, the reaction front or ignition location is marked by the peak CH* concentration. The maximum CH* concentration coincides with the peak thermicity value and serves as a reliable marker for induction delay time or ignition delay time. It must be noted that the induction zone includes both the fuel pyrolysis/decomposition zone and the pyrolysis products' oxidation zone. The majority of heat release occurs in the recombination zone (the zone that follows the induction zone). In a ZND detonation structure, the reaction (radical generation and recombination) zone comprises both the induction (radical generation) zone and the recombination (radical recombination) zone.
Thermal Decomposition or Pyrolysis Zone
The computed results of stoichiometric JetA1–air detonations reveal some basic features of the ZND detonation structure. In the absence of ignition promoters, the JetA1 fuel will undergo thermal decomposition due to high postshock temperatures of the order of ~ 1500 K. Thus, in the early stages of the induction zone, the JetA1 fuel molecules undergo thermal or oxidative thermal pyrolysis.
For conventional petroleum-derived real fuels like JetA1, the key pyrolysis products are few and comprise ethylene (C2H4), hydrogen (H2), methane (CH4), propene (C3H6), 1-butene (1-C4H8), iso-butene (i-C4H8), benzene (C6H6), and toluene (C7H8). The time scale associated with such a decomposition of parent fuel is typically small for gaseous detonations. It is observed that following the leading shock front, the thermal decomposition of jet fuel occurs at approximately 1.45 μs. The length scale associated with such a decomposition is also very small, which in this case, happens to be 0.38 mm. However, ozone addition in trace amounts of 16,000 PPM could dramatically reduce the pyrolysis (or thermal decomposition) time of the parent fuel molecule (JetA1 in this case), where the pyrolysis zone has a convective residence time of 0.16 μs. This signifies thermal decomposition of JetA1 is completed by 0.16 μs in the presence of trace amounts of ozone. This shows that the thermal decomposition time of the parent fuel molecule (JetA1) is significantly reduced by 89% with O3 addition (refer to Fig. 5b). Therefore, the pyrolysis or oxidative pyrolysis of JetA1 occurs faster in the presence of ozone, which shows the fuel-sensitization or promotion effects of ozone on the pyrolysis or oxidative pyrolysis chemistry of JetA1. Similarly, with the addition of hydrogen peroxide (16,000 PPM) to stoichiometric JetA1–air mixtures, the thermal decomposition/pyrolysis of JetA1 gets nearly completed by 0.44 μs. Thus, the thermal decomposition time of JetA1 reduces by 70% for mixtures doped with 16,000 PPM of H2O2 (refer to Fig. 6b). Thus, the thermal decomposition of a real distillate fuel like JetA1 occurs faster in the presence of ignition promoters like ozone and hydrogen peroxide.
The increased generation of O, H, OH, and CH3 free radicals due to ozone and hydrogen peroxide can also be observed in the pyrolysis zone that facilitates the chain-branching reactions and promotes the ignition kinetics of JetA1–air mixtures (refer to Figs. 5b and 6b). It can be observed that the concentration of major reactive radicals: O, OH, and H increases with the addition of both ozone (through reaction (8)) and hydrogen peroxide (through reaction (9)). The maximum increase in the concentration can be observed for the hydroxyl radical, where its concentration increases by an order of magnitude. It must be noted that although the concentration of small radical species increases in the presence of ignition promoters, the overall distribution of pyrolysis products is minimally affected (refer to Figs. 5 and 6). Thus, the presence of reactive radical species enhances the thermal decomposition of the parent fuel molecule. These species interact with the fuel molecule and facilitate its dissociation in the decomposition zone. The increase in radical concentration is higher for ozone when compared to H2O2. The increased concentration of reactive radical species like O, OH, and H facilitates the chain branching process and shortens the detonation length and time scales. Therefore, ignition in the presence of ignition promoters is practically achieved via radical proliferation instead of thermal feedback.
Figures 5 and 6 show the breakdown of O3 and H2O2 into O and OH free radicals and the thermal decomposition of JetA1 in the pyrolysis zone (before peak thermicity). In the induction zone, the shock wave will further cause the thermal dissociation of the molecules, resulting in the generation of active radical species. The increased generation of active radical species like O, H, OH, and CH3 facilitates the fuel decomposition in the thermally-neutral decomposition zone, where the high temperature (~ 1500 K) due to shock discontinuity is sufficient to facilitate such a decomposition. The presence of radicals like H, OH, and O will enhance the parent fuel molecule dissociation rate without any change in pyrolytic intermediates. Also, the thermal decomposition of JetA1 occurs well before the oxidation of its pyrolysis intermediates. Hence, these two processes can be treated separately. The pyrolysis or decomposition may become faster in the presence of molecular oxygen; however, the changes in the distribution of pyrolysis products and the consumption of O2 are both negligible. The key pyrolysis intermediates of JetA1 are C2H4, C3H6, i-C4H8, 1-C4H8, CH4, C6H6, C7H8, and H2, all of which have considerably higher molecular diffusivities than JetA1. They are diffused into the flame and are oxidized, leading to the production of CO, CO2, and H2O and heat release. The distribution of pyrolysis products is the most crucial to predicting the combustion behaviors of large hydrocarbon fuels and determines the global detonation properties of the fuel. It must be noted at this stage that the pyrolysis/thermal decomposition of JetA1 is not rate-limiting. The pyrolysis of JetA1 is relatively fast when compared to the oxidation of pyrolysis intermediates. Hence, the oxidation of pyrolysis products is rate-limiting during the entire course of the reaction. The oxidation of pyrolysis products is discussed next.
Oxidation Zone
The oxidation of the pyrolysis products occurs in the oxidation zone. The oxidation zone follows the decomposition zone and extends up to the peak thermicity location. The maximum thermicity coincides with the maximum rate of temperature rise [max(dT/dt)] and represents the induction delay time or ignition delay time on a time scale.
Since the time taken to complete the oxidation process is significantly higher than the time taken for the thermal decomposition or pyrolysis of JetA1, the oxidation process becomes rate-limiting. Hence, oxidation is treated by a detailed reaction model in the present study. During ignition, a significant amount of energy is released in the oxidation zone due to the oxidation of pyrolysis products. A sharp rise in gas-phase temperature and thermicity can be observed in the oxidation zone that manifests heat release due to the oxidation of pyrolysis products. The oxidation process also supplies heat and radical species to facilitate the endothermic, oxidative pyrolysis of the fuel. Also, the location of peak thermicity occurs at a shorter distance from the leading shock front in the presence of O3 and H2O2 (see Figs. 5a and Fig. 6a). This signifies a substantial reduction in the induction length, a change from ~ 2 mm without ignition promoters to ~ 0.9 mm and ~ 1.4 mm with the addition of O3 and H2O2, respectively. The pyrolysis products oxidation time is usually small ~ 5.1 μs. However, with ozone and hydrogen peroxide, the oxidation time drastically reduces to 2.3 μs and 3.5 μs, respectively. Thus, in the presence of ozone and hydrogen peroxide, pyrolysis products' convective residence time reduces by ~ 55% and ~ 31%, respectively. The induction length and time scales decrease significantly with the addition of O3 and H2O2, where they help in accelerating the ignition kinetics for a given fuel-oxidizer mixture. The proliferation of active radical species in the oxidation zone primarily affects the ignition kinetics and ZND length and time scales. It can also be observed that the concentration of radical species in the oxidation zone is more with the addition of ozone relative to H2O2, which indicates that ozone is a better ignition promoter than H2O2. It must be noted at this stage that the composition and distribution of pyrolysis products determine the global combustion and detonation properties of large hydrocarbon fuels, ranging from induction length and time scales to detonation propagation and extinction.
Combined Effect of Ignition Promoters and Inert Diluents
JetA1–air detonations at stoichiometric conditions have a CJ temperature of 2848 K; refer to Table 3 and Fig. 7. The addition of argon or helium up to 70% reduces the CJ temperatures from 2848 to 2036 K. Thus, for JetA1–air-diluent mixtures, inert diluents can lower the temperature of the detonation products. This particular aspect can be utilized to reduce the post-detonation temperatures of detonation-based combustors. However, increased dilution causes an increase in the induction and reaction length due to the reduced exothermicity of the mixture, which results in a lower temperature rise in the induction and reaction zone. This, in turn, reduces the detonation velocity VCJ, which in turn reduces the post-shock (TVN) and the post-detonation temperature (TCJ). The reaction rates of chain-branching and exothermic reactions are also reduced and cause a corresponding increase in the induction and reaction time scales. Such an increase in the induction length and time scales can destabilize a given detonation wave.
In the presence of inert diluents, the detonation length and time scales are significantly altered. For instance, the Δi and τi are increased by a factor of ~ 7 for the argon-diluted case (70% dilution), which significantly affects the detonation cell structure. Since the induction length scale is a measure of the strength of a detonation wave, a significant increase in the induction length and time scale is indicative of a loose coupling between the reaction zone and the leading shock front, which in turn indicates a less robust detonation wave. Thus, longer Δi and τi represent a loosely coupled shock-flame complex. It also quantitatively represents mixtures that are less detonable. Therefore, using inert diluents may render a detonation wave less robust near its propagation limits. This issue can be resolved by using O3 and H2O2 in modest concentrations. The addition of 16,000 PPM of O3 and H2O2 in the presence of large amounts of inert diluents can considerably decrease the Δi and τi (refer to Table 3 and Fig. 7).
Thus, using ignition promoters in conjunction with inert diluents can render a relatively strong detonation wave near its propagation limits and can prevent its attenuation or failure. At the same time, it can also help reduce the post-detonation temperatures (TCJ) of a given explosive mixture. Therefore, the operating temperature of detonation-based combustors can be reduced with the addition of inert diluents in the presence of dopants like O3 and H2O2.
The addition of inert diluents has a significant effect on the detonation wave structure and can help tailor its behavior for the desired results. For instance, the addition of inert diluents like Ar and He increases the chemical length and time scales of the ZND detonation wave structure. In experimental studies, it has been observed that the addition of inert diluents to the fuel-oxidizer mixtures results in the detonation cell regularity which indicates a stable detonation (Lee 2008; Zhang et al. 2014, 2018). Thus, the addition of inert diluents like argon and helium renders a detonation wave stable near its propagation limits (Lee 2008; Zhang et al. 2014, 2018). Also, based on the computational results from the current work, the detonation Mach number (MCJ) and the post-detonation pressure (PCJ) and temperature (TCJ) were found to decrease with the addition of argon and helium. Thus, the addition of inert diluents like argon and helium has a strictly thermal inhibiting effect on the detonation wave structure. The use of inert diluents can help reduce the post-detonation temperatures of a given explosive mixture. They also have stabilizing effect on the resulting detonation wave structure and can help stabilize a detonation wave near its propagation limits.
Effect of Ignition Promoters on JetA1–Air Detonations Under Fuel Lean Conditions
Fuel-sensitization can help operate detonation-based combustors like RDEs under fuel-lean conditions, thereby increasing the operational limits of such combustors. Operating under leaner equivalence ratios could also help reduce the CJ temperatures and hence the operating temperatures of such engines. Therefore, operating detonation-based combustors under leaner equivalence ratios could help eliminate complex cooling mechanisms associated with detonation-based engines.
The effect of ignition promoters was tested over a range of equivalence ratios in the present study. For a given explosive mixture, an exponential rise in τi and Δi can be observed as we approach leaner conditions from the stoichiometric conditions. This undesirable increase in the induction length and time under fuel-lean mixture conditions can be compensated by using ignition promoters in small concentrations (refer to Table 4 and Fig. 8). The computed results show that the lean-detonability limits of JetA1–air mixtures can be widened by doping them with O3 and H2O2. It is observed that smaller τi and Δi values can be maintained even under fuel-lean conditions for JetA1–air mixtures by adding ozone at 16,000 PPM. The promoters also help in extending the detonability limits of a given explosive mixture.
In the present study, the lean-detonability limit for JetA1–air mixtures was extended to 0.35 with the addition of ozone and H2O2 at 16,000 PPM, where the CJ temperature is ~ 1700 K. The lean-detonability limit of JetA1–air detonations without ignition promoters was computed to be 0.45. From Table 4, it is observed that the value of Δi with ozone and H2O2 in trace amounts of 16,000 PPM at ϕ = 0.5 and 0.6, respectively, are comparable to the undiluted case at the stoichiometric condition. Thus, a stronger detonation can be achieved even at lower equivalence ratios using ignition promoters. Also, ozone performs better than H2O2 at lower equivalence ratios, where a higher reduction in detonation length and time scales can be achieved when compared to H2O2.
It is possible to achieve CJ temperatures (post-detonation temperatures) below ~ 2100 K using ozone and hydrogen peroxide in small concentrations (up to 16,000 PPM) for JetA1–air mixtures under fuel-lean conditions (see Fig. 8a, b). The use of ignition promoters under fuel-lean conditions also helps in the stable propagation of a detonation wave near its limits.
Also, a wider operating range can be achieved for such mixtures where the post-detonation temperatures below 2400 K can be readily attained for practical detonation engines by fuel sensitization with dopants like ozone and hydrogen peroxide. Thus, sensitizing JetA1 mixtures with O3 and H2O2 in trace amounts can lower the post-detonation temperatures. The highlighted square boxes in Fig. 8 show the desired operating temperature range for detonation-based combustors, where the use of complex cooling systems can be avoided. The sensitization effects of O3 and H2O2 towards the widening of detonability limits are manifested in Fig. 8. Thus, the use of ozone and hydrogen peroxide can widen the operating limits of liquid-fueled detonation-based combustors.
Stabilizing Effect of O3 and H2O2 on JetA1–Air Detonations
The promotion and stabilizing effects of O3 and H2O2 in JetA1–air mixtures can also be explained by the energy release rates, stability and activation energy parameters. An effective activation energy parameter can be evaluated from constant volume explosion simulations. Effective activation energies indicate the sensitivity of the reaction zone subjected to thermodynamic perturbations. The effective activation energy parameter can be defined as,
where two constant-volume explosion simulations corresponding to (T1, τ1) and (T2, τ2) are run for each activation energy data point. Initial conditions for states one and two are generated by varying the shock velocity by \(\pm\) 1% VCJ. The jump conditions were solved with these perturbed velocities to obtain the post-shock conditions used as initial conditions in the constant-volume explosion simulations. The pressure variations in the detonation cellular structure increase with the activation energy parameter (Lee 2008). It was observed that as θ increases, the cellular pattern becomes sharper, and the regularity of cellular structure decreases (Lee 2008).
Considering the effect of chemistry on stability, it was found that the stability of the reactant mixture depends on factors that effectively dictate the ratio of the induction zone to the reaction zone length. Ng et al. (2010) defined the stability parameter as the ratio of the induction zone length and the reaction zone length. Ng et al. (2010) also incorporated the temperature sensitivity of the induction reaction into the definition of the stability parameter χ. According to Ng et al. (2010), the stability parameter can be defined as,
where εi denotes the normalized activation energy of the induction reaction with respect to the shock temperature. In Eq. (11), Δi and Δr denote the induction and reaction lengths, respectively. It is challenging to define the reaction zone length based on a particular post-shock temperature rise, heat release rate, or the local Mach number. Ng et al. (2010) defined reaction length as the main heat release zone length and used maximum thermicity and CJ particle velocity in the shock-attached frame to evaluate Δr. In the present work, the reaction length is calculated as the distance from the leading shock front to the location where MCJ = 0.75. Here MCJ is calculated in the wave frame of reference. The reason for such a selection has been discussed earlier.
The stability parameter is frequently used to predict cellular patterns' regularity in real detonations (Gavrikov 2000; Lee 2008). It is seen that a detonation wave’s stability is a result of the temperature sensitivity of the chemical kinetic reactions. Small fluctuations in the post-shock temperature can cause large perturbations in the induction time and the energy release rates in the recombination zone. An extended reaction zone has a stabilizing effect as it spreads out the energy release and reduces the impact of fluctuations in the induction time (Lee 2008; Ng et al. 2010). In the limit of a square wave model, it has been shown that when the reaction time is zero, which manifests instantaneous reaction after an induction time, detonations are always unstable (Lee 2008). Thus, a longer reaction time has a stabilizing effect on a reacting mixture, and this is taken into consideration explicitly by the stability parameter (Ng et al. 2010). Large values of the stability parameter χ manifest gas dynamic instabilities in the reaction zone and could lead to highly unstable pulsating detonations.
The energy release rate may be calculated by dividing the heat of reaction by the total time taken for the energy release, which is approximately equal to the sum of the induction and exothermic time and is given as:
where q, Δh0, τi, and τe are the energy release rate, the heat of reaction extrapolated to zero temperature, induction time, and exothermic time, respectively.
It can be seen from Fig. 9 that O3 and H2O2 addition to JetA1–air mixtures increase the energy release rates and reduce the stability and activation energy parameter. An increase in the energy release rate is due to a decrease in the relevant time scales in the presence of O3 and H2O2.
Thus, the promotion effects of O3 and H2O2 could be seen in Fig. 9, where they help in increasing the energy release rates of a given explosive mixture and could result in a more robust detonation wave. Higher energy release rates indicate tighter coupling between the shock front and the reaction zone, where the periodic reignition of the explosive mixture behind the leading shock now occurs at a shorter time scale. Lower values of stability and activation energy parameter signify higher stability of detonation wave for a given mixture. Thus, fuel-sensitization via dopants such as O3 and H2O2 has a stabilizing effect on the detonation wave structure. Such systems are characterized by lower pressure variations in detonation cells and can lead to more regular cell structures. One-dimensional detonation waves may fail if they become too unstable, where the amplitude of fluctuations during the low-velocity phase may result in a decreased strength of a shock below a critical value. In such cases, periodic reignition of explosive mixture behind the shock front could not be achieved and could lead to a decoupled shock-flame complex. Fuel-sensitization with O3 and H2O2 in small amounts could prevent the detonation wave failure near its propagation limits since they have a stabilizing effect on the resulting detonation wave structure.
Conclusions
The computed results of stoichiometric JetA1–air detonations reveal some basic features of the detonation structure. The JetA1 fuel molecule will decompose into a series of pyrolysis products first due to high post-shock temperatures, followed by the oxidation of pyrolysis products. The time scale associated with such a decomposition of parent fuel molecule is typically small when compared to oxidation of pyrolysis products, and hence oxidation becomes the rate-limiting step in such a process. Dramatic reduction in the pyrolysis and oxidation time can be observed in the presence of ignition promoters in trace amounts (up to 16,000 PPM). The production of free radical species like O, OH, CH3, and H is increased in the pyrolysis zone, and the ignition kinetics in the oxidation zone is accelerated via radical proliferation in the presence of ignition promoters or dopants. This decrease in chemical length and time scales is capable of altering the macroscopic structure of an underlying detonation wave. Ozone and hydrogen peroxide serves as excellent fuel sensitizers, where they lower the detonation length and time scales considerably for JetA1–air mixtures and can promote reaction kinetics and early ignition.
The addition of ignition promoters also increases the energy release rates, whereas it reduces the activation energy of the reactant mixture tremendously, even at fuel-lean conditions. The use of ozone and hydrogen peroxide can also broaden the detonability limits of a given JetA1–air mixture. Diluting JetA1–air mixtures with inert diluents like Ar and He can reduce the temperature of the detonation products up to ~ 2100 K. This could be used as a viable technique to decrease the operating temperatures of detonation devices and can thus eliminate the need for bulky and complex cooling mechanisms. However, larger induction length and time scales are observed at high dilution levels, which may hamper its propagation near its limits. The corresponding increase in the induction length and time can be mitigated by doping a given fuel-oxidizer-diluent mixture with ozone and hydrogen peroxide at modest concentrations. The ignition promotion effects of ozone and hydrogen peroxide are also observed under fuel-lean conditions, where they can help in achieving lower post-detonation temperatures without affecting the stability of a resulting detonation wave. The ignition promoters can have a profound effect in reducing the detonation length and time scales over a wide range of equivalence ratios. This could be used as a plausible solution for resolving the problems of detonation-based combustors that are supposed to operate over a range of initial conditions. It is observed that ozone acts as a better fuel-sensitizer than H2O2 for JetA1–air detonating mixtures. Also, fuel sensitizers like ozone and hydrogen peroxide have a stabilizing effect on the resulting detonation wave structure, where a decrease in the non-dimensional stability parameter (χ) signifies an increase in the stability of a given mixture.
Abbreviations
- P o :
-
Initial pressure (bar)
- T o :
-
Initial temperature (K)
- T CJ :
-
Post-detonation temperature (K)
- P CJ :
-
Post-detonation pressure (bar)
- V CJ :
-
CJ detonation velocity (m/s)
- MCJ :
-
CJ detonation Mach number (-)
- \({\Delta }_{i}\) :
-
Induction zone length (mm)
- \({\Delta }_{r}\) :
-
Reaction zone length (mm)
- \({(\Delta }_{r}- {\Delta }_{i}\)):
-
Recombination zone length (mm)
- \({\tau }_{i}\) :
-
Induction delay time (\(\mathrm{\mu s})\)
- \({\tau }_{r}\) :
-
Reaction time (\(\mathrm{\mu s})\)
- \(\dot{\sigma }\) :
-
Thermicity (\(\mathrm{\mu s^{-1}})\)
- VN :
-
Von Neuman conditions
- CJ :
-
Chapman–Jouguet conditions
- i :
-
Induction zone
- r :
-
Reaction zone
References
Anand V, George AS, Luzan CF, Gutmark E (2017) Rotating detonation wave mechanics through ethylene-air mixtures in hollow combustors, and implications to high-frequency combustion instabilities. Exp Therm Fluid Sci 92:314–325
Anand V, George AS, Driscoll R, Gutmark E (2016) Investigation of rotating detonation combustor operation with H2-air mixture. Int J Hydrog Energy 41:1281–1292
Anand V, George AS, Driscoll R, Gutmark E (2015) Characterization of instabilities in a rotating detonation combustor. Int J Hydrog Energy 40:16649–16659
Benedick WB, Guirao CM, Knystautas R, Lee JHS (1986) Critical charge for the direct initiation of detonation in gaseous fuel-air mixtures. Prog Astronaut Aeronaut 106:181–202
Browne S, Ziegler J, and Shepherd JE (2008) Numerical solution methods for shock and detonation jump conditions GALCIT Report FM2006 6 Pasadena, CA
Bykovskii FA, Zhdan SA, Vedernikov EF (2003) Continuous spin detonation in annular combustors. Combust Explos Shock Waves 39:323–334
Crane J, Shi X, Singh AV, Tao Y, Wang H (2019) Isolating the effect of induction length on detonation structure: Hydrogen–oxygen detonation promoted by ozone. Combust Flame 200:44–52
Dahake A, and Singh AV (2021) Numerical study on NOx emissions from a synthetic biofuel for applications in detonation-based combustors. In: AIAA Propulsion and Energy 2021 Forum AIAA 2021–3678: 3678
Dahake A, and Singh AV (2022a) A comparative study of critical detonation parameters for Jet A and an alcohol-to-jet synthetic biofuel. In: AIAA SCITECH 2022a Forum AIAA 2022–0819: 0819
Dahake A, and Singh AV (2022b) Effect of Fuel sensitization on NOx emissions from a synthetic biofuel under detonating conditions. In: AIAA SCITECH 2022b Forum AIAA 2022–0518: 0518
Eddings EG, Yan S, Ciro W, Sarofim AF (2005) Formulation of a surrogate for the simulation of jet fuel pool fires. Combust Sci Technol 177:715–739
Fotia ML, Schauer F, Kaemming T, Hoke J (2015) Experimental study of the performance of a rotating detonation engine with nozzle. J Propuls Power 32:674–681
Frolov SM, Aksenov VS, Ivanov VS, Shamshin IO (2015) Large-scale hydrogen-air continuous detonation combustor. Int J Hydrog Energy 40:1616–1623
Gavrikov AI, Efimenko AA, Dorofeev SB (2000) A model for detonation cell size prediction from chemical kinetics. Combust Flame 120(1–2):19–33
Goodwin DG, Moffat HK and Speth RL (2009) Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. https://www.cantera.org, Caltech, Pasadena
Iyer MSK, Singh AV (2021) NOx emissions from Jet A-air detonations. In: AIAA Propulsion and Energy 2021 Forum AIAA 2021–3679: 3679
Iyer MSK, Dahake A, Singh AV (2022) Comparative Studies on Ignition Kinetics and Detonation Chemistry of Real Distillate Fuels. Trans Indian Nat Acad of Eng. https://doi.org/10.1007/s41403-022-00331-5
Kindracki J (2014a) Study of detonation initiation in kerosene–oxidizer mixtures in short tubes. Shock Waves 24:603–618
Kindracki J (2014b) Experimental research on rotating detonation in liquid fuel–gaseous air mixtures. Aerosp Sci Technol 43:445–453
Kindracki J (2015) Experimental research on rotating detonationin liquid fuel–gaseous air mixtures. Aerosp Sci Technol 43(01):445–453. https://doi.org/10.1016/j.ast.2015.04.006
Knystautas R, Guirao CM, Lee JHS, Sulmistras A (1984) Measurement of cell size in hydrocarbon-air mixtures and predictions of critical tube diameter, critical initiation energy, and detonability limits. Prog Astronaut Aeronaut, 23–37
Kumar DS, Ivin K, Singh AV (2020) Sensitizing gaseous detonations for hydrogen/ethylene-air mixtures using ozone and H2O2 as dopants for application in rotating detonation engines. Proc Combust Inst 38(3):3825–3834
Kumar DS, Singh AV (2021) Inhibition of hydrogen-oxygen/air gaseous detonations using CF3I, H2O, and CO2. Fire Saf J 124:103405
Lee JHS (2008) The detonation phenomenon. Cambridge University Press, Cambridge
Lee JHS (1984) Dynamic parameters of gaseous detonations. Annu Rev Fluid Mech 16:311–336
Li JM, Teo CJ, Khoo BC, Wang JP, Wang C (2018) Detonation control for propulsion. Springer International Publishing, Cham
Liang W, Wang Y, Law CK (2019) Role of ozone doping in the explosion limits of hydrogen-oxygen mixtures: multiplicity and catalyticity. combust. Flame 205:7–10
Lu T, Law CK, Ju Y (2003) Some aspects of chemical kinetics in Chapman-Jouguet detonation: induction length analysis. J Propuls Power 19:901–907
Magzumov AE, Kirillov I, Rusanov V (1998) Effect of small additives of ozone and hydrogen peroxide on the induction-zone length of hydrogen-air mixtures in a one-dimensional model of a detonation wave. Combust Explos Shock Waves 34:338–341
Mevel R, Lafosse F, Catoire L, Chaumeix N, Dupre G, Paillaird CE (2008) Induction delay times and detonation cell size prediction of hydrogen-nitrous oxide-diluent mixtures. Combust Sci Technol 180:1858–1875
Moen IO et al (1985) The influence of diluents and inhibitors on detonations. Proc Combust Inst 20:1717–1725
Ng HD, Higgins AJ, Kiyanda CB, Radulescu MI, Lee JHS, Bates KR, Nikiforakis N (2005) Non-linear dynamics and chaos analysis of one-dimensional pulsating detonations. Combust Theory Model 09(01):159–170. https://doi.org/10.1080/13647830500098357
Ng HD, Higgins A, Kiyanda C, Radulescu M, Lee JHS, Bates K, Nikiforakis N (2010) Nonlinear dynamics and chaos analysis of one-dimensional pulsating detonations. Combust Theory Model 9(1):159–170
Peng H, Liu W, Liu S, Zhang H (2018) Experimental investigations on ethylene-air continuous rotating detonation wave in the hollow chamber with laval nozzle. Acta Astronaut 151:137–145
Rankin BA, Richardson DR, Caswell AW, Naples AG, Hoke JL, Schauer FR (2017) Chemiluminescence Imaging of an optically accessible non-premixed rotating detonation engine. combust. Flame 176:12–22
Shepherd JE (1986) Chemical kinetics of hydrogen-air-diluent detonations. Prog Astronaut Aeronaut 106:263–293
Stamps DW, Tieszen SR (1991) The influence of initial pressure and temperature on hydrogen-air diluent detonations. Combust Flame 83:353–364
Stamps DW, Slezak SE, Tieszen SR (2006) Observations of the cellular structure of fuel-air detonations. combust. Flame 144:289–298
Vasil’ev AA (1998) Effect of nitrogen on multifront detonation parameters. Combust Explos Shock Waves 34:72–76
Violi A, Yan S, Eddings E, Saroffin A, Granata S, Faravelli T, Ranzi E (2002) Experimental formulation and kinetic model for JP-8 surrogate mixtures. Combust Sci Technol 174:399–417
Wang H, You X, Joshi AV, Davis SG, Laskin A, Egolfopoulos F, Law CK (2007) USC mech version II. High-temperature combustion reaction model of H2/CO/C1-C4 compounds. http://ignis.usc.edu/Mechanisms/USCMech%20II/USC_Mech%20II.htm
Wang H et al (2018) A physics-based approach to modeling real-fuel combustion chemistry-I. Evidence from experiments and thermodynamic, chemical kinetics, and statistical consideration. Combust Flame 193:502–519
Wang Y, Le J (2019) A hollow combustor that intensifies rotating detonation. Aerosp Sci Technol 85:113–124
Westbrook CK (1982a) Inhibition of hydrocarbon oxidation in laminar flames and detonations by halogenated compounds. Proc Combust Inst 19:127–141
Westbrook CK, Urtiew PA (1982) Chemical kinetic prediction of critical parameters in gaseous detonations. Proc Combust Inst 19(1):615–623
Westbrook CK (1982b) Chemical kinetics of hydrocarbon oxidation in gaseous detonations. Combust Flame 46:191–210
Xie Q, Wen H, Li W, Li Z, Wang B, Wolanski P (2018) Analysis of operating diagram for H2/air rotating detonation combustors under lean fuel condition. Energy 151:408–419
Zhao H, Yang X, Ju Y (2016) Kinetic studies of ozone assisted low-temperature oxidation of dimethyl ether in a flow reactor using molecular beam mass spectrometry. Combust Flame 173:187–194
Zhang HR, Eddings EG, Sarofim AF (2007) Criteria for selection of components for surrogates of natural gas and transportation fuels. Proc Combust Inst 31:401–409
Zhong Y, Wu Y, Jin D, Chen X, Yang X, and Wang S (2019) Investigation of rotating detonation fueled by the pre-combustion cracked kerosene. Aerosp Sci Technol 95: Article 105480
Zhang B, Mehrjoo N, Ng HD, Lee JHS, Bai C (2014) On the dynamic detonation parameters in acetylene–oxygen mixtures with varying amounts of argon dilution. Combust Flame 161:1390–1397
Zhang B, Liu H, Wang C (2018) Detonation propagation limits in highly argon diluted acetylene-oxygen mixtures in channels. Exp Therm Fluid Sci 90:125–131
Acknowledgements
The authors acknowledge the financial support for this work from the Aeronautics R&D Board, Ministry of Defence, Govt. of India vide Sanction Letter # ARDB/01/1042000M/I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, D.S., Dahake, A. & Singh, A.V. Detonation Chemistry of Fuel-Sensitized JetA1–Air Detonations. Trans Indian Natl. Acad. Eng. 7, 957–975 (2022). https://doi.org/10.1007/s41403-022-00339-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-022-00339-x