Abstract
To study the influence of addition of MWCNT in vinyl-functionalized borosiloxane oligomer (BMV) synthesized from boric acid (BA), methyltriethoxysilane (MTEOS) and vinyltriethoxysilane (VTEOS) by solventless non-aqueous sol–gel process, ceramic conversion of BMV with varying amount of MWCNT (0.5, 1.0, 2.5 and, 5.0 phr) was carried out at 1500 °C and 1650 °C in an inert atmosphere. The samples were characterized by XRD, FTIR, Raman spectra and SEM. XRD data indicate that MWCNT promotes the conversion of amorphous SiBOC ceramics in to crystalline SiC and the effect is more pronounced at 1650 °C. At the heat treatment temperatures, BMV + MWCNT blend gets converted into a nanocomposite in which SiCNT formed from MWCNT is dispersed in SiC/SiBOC matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, researchers are showing considerable interest to synthesize new inorganic ceramic materials by preceramic route. The conversion of inorganic and organometallic compounds into ceramic materials by thermal treatment in a controlled atmosphere is fairly a new and simple process for developing new type of inorganic materials. The organometallic precursor molecules i.e., oligomers/polymers, are designed to retain structural units of the residual inorganic materials from which they are derived. The flowchart for the conversion of preceramic polymers to ceramics is shown in Scheme 1.
Since the first proposal of this concept by Popper (Chantrel et al. 1965), several researchers have been working in this field worldwide. The synthetic approaches adopted for the synthesis of preceramic polymers, their characterization, and controlled pyrolysis to ceramics are reported in the literature (Verbeek 1973, Desprec et al. 1995, Colombo et al. 2010, An et al. 2004, Katsuda 2005, Schiavon et al. 2004a, b, Sreejith 2011). The following aspects make the preceramic route the most attractive one for the development of new ceramic materials and components:
-
Amorphous materials can be produced from thermodynamically stable stoichiometric compounds with compositions not obtainable by conventional synthetic routes.
-
Amorphous stage formed is thermally stable at very high temperatures, before transforming into crystalline phases.
-
Because of the various fabrication capabilities of polymer process engineering, ceramic fibers, coatings and complex-shaped bulk parts can be produced in an easy manner.
The conversion of polymers to ceramics by pyrolysis is the most vital part of the preceramic route for the preparation of ceramic materials. There are only a few reports on the detailed ceramic conversion studies of borosiloxanes (Sreejith 2011; Young-Hag et al. 2001). Ceramic conversion of borosiloxanes, in general, follows the mechanism of formation of SiC from siloxanes with a few exceptions. Pyrolysis of borosiloxanes give siliconboronoxycarbide (SiBOC) ceramic, which is a boron-modified siliconoxycarbide (SiOC) phase. The incorporation of boron in the SiOC network has inhibited the formation of cristobalite (Liebau et al. 2004; Klonczynski et al. 2004) and enhanced the SiC crystallization (Schiavon et al. 2004a, b, Pena-Alonso et al. 2007, Sreejith et al. 2016, and Devapal et al. 2020). SiBOC ceramic phase gained importance because of its improved high temperature stability, thermo-oxidative stability, and creep resistance compared to SiOC ceramics (Liao et al. 2014; Xie et al. 2015). Devapal et al. (2006, 2010) studied the ceramic conversion of borosiloxanes synthesized using diglyme as solvent by non-aqueous sol–gel process. The synthetic approach used for the preparation of polyborosiloxane oligomers appears to influence the extent of incorporation of Si–O–B bonds in the oligomers. The elemental composition (boron, silicon, and carbon) of the polymer and the stability of SiBOC glass at higher temperature significantly affects the nature of ceramics and its crystallization behavior. Sreejith et al. (2011) observed that, by judicious choice of mixture of organotrialkoxysilane monomers and the monomer feed ratio, the stability of SiBOC ceramics could be remarkably improved by way of suppressing the carbothermal reduction even at 1650 °C. The crystallization behavior of poly (methylborosiloxane) was studied by adding pyrocarbon (PyC) as carbon source. An amorphous SiBOC ceramic was formed from the oligomer at 1500 °C, but when the sintering temperature was increased to 1650 °C, SiBOC matrix got converted to SiC ceramic. The conversion taking place between 1500 °C and 1650 °C is very critical, since the final ceramic obtained is getting converted from amorphous to crystalline in this temperature range. The borosiloxane oligomers are extensively investigated as precursors for SiBOC/SiC nanocomposites (Sreejith et al. 2016) and as matrix resins for carbon fiber and SiC fiber reinforced composites (Sreejith et al. 2018; Nair et al. 2018). Studies on the influence of carbon-based materials such as carbon black (Abraham 2019) and MWCNT (Devapal et al. 2017) on the crystallization of SiBOC are gaining importance. The ceramic conversion of CNT dispersed borosiloxane at 1500 °C shows sharp crystalline peaks in the XRD spectrum attributing to SiC formation compared to other carbon sources like carbon from pyrolysed carbon. Carbon fiber reinforced ceramic matrix composite prepared by the addition of 0.5 wt% of MWCNT in vinylborosiloxane (VBS) showed an improvement of 48% in flexural strength (Gopakumar et al. 2013). So, the present investigation is aimed at studying the influence of addition of varying quantities of MWCNT on the crystallization behaviour of SiBOC at 1500 °C and 1650 °C. The SiBOC is derived from vinyl-functionalized borosiloxane which is synthesized from boric acid and a mixture of methyltriethoxysilane and vinyltriethoxysilane by solventless non-aqueous sol–gel process.
Experimental
Preparation of BMV + MWCNT Blends
Vinyl-functionalized borosiloxane oligomer (BMV) was synthesized by reacting boric acid (BA), methyltriethoxysilane (MTEOS) and vinyltriethoxysilane (VTEOS) by solventless process following the procedure reported earlier (Sreejith 2011). MWCNT, procured from Nanolab, USA was used as received. BMV-MWCNT blends were prepared by the following procedure: 40 g of BMV resin was mixed with 0.2 g of MWCNT (0.5 phr) in 100 mL RB flask and sonicated for 30 min. BMV resin in which MWCNT was uniformly dispersed was taken in a petridish and dried in an air oven. The temperature of the oven was maintained at 75, 100, 125, and 150 °C for 30 min at each temperature and finally, the temperature was raised to 175 °C and maintained at this temperature for 2 h. The oven was allowed to cool to room temperature. The sample was weighed to estimate the weight loss during curing. BMV + MWCNT blend obtained is referred to as BMW (0.5). In the same manner, BMW (1.0), BMW (2.5), and BMW (5.0) samples were prepared using 1.0, 2.5, and 5.0 phr of MWCNT.
Ceramic Conversion Studies
Pyrolysis of the cured samples to convert them into ceramics was performed in a tubular furnace under the flow of argon. In a typical experiment, 5 g of the sample was taken in a graphite crucible and heated at a rate of 3 °C/min up to 1500 °C and maintained at this temperature for 2 h. The furnace was cooled to room temperature at a rate of 3 °C/min and the samples were weighed for determining the yield. The sample, thus obtained is referred to as BMV-1500. A known quantity of pyrolysed sample was taken in a graphite crucible and heated in a sintering furnace in argon atmosphere at the rate of 3 °C/min to 1650 °C and kept at this temperature for 3 h. The furnace was allowed to cool to 50 °C at a rate of 3 °C/min and switched off. The sample was taken out and weighed to estimate the weight loss during sintering. The sample, thus obtained is referred to as BMV-1650.
Results and Discussion
The repeating structural unit of vinyl-functionalized borosiloxane oligomer (BMV) used in the present study is shown in Fig. 1. Figure 2 shows the possible linkages formed during curing reaction of the oligomer at 175 °C.
Sreejith et al. studied in detail, the heat treatment of this oligomer at 1500 °C and 1650 °C. It was observed that this oligomer, unlike other borosiloxane oligomers synthesized from phenyltrialkoysilanes, show the presence of considerable amount of amorphous phase even at 1650 °C and this has been attributed to low carbon to oxygen ratio. Thus, blending with MWCNT is expected to influence the crystallization characteristics, as MWCNT would serve as a source of carbon. The reaction between BMV and MWCNT at 1500 °C and 1650 °C is of relevance, as this provides the scope for obtaining SiOBC/SiC in which SiC-nanotubes (SiC-NT) is formed presumably from MWCNT by reacting with SiBOC.
The photographs of BMV + MWCNT blends heat treated at 1500 °C and 1650 °C are shown in Fig. 3. The main observation is that when the heat treatment temperature is increased from 1500 °C to 1650 °C, the color of the ceramic is changed from black to grey and then to creamish yellow (with the increase in MWCNT concentration) which indicates the formation of different polytypes of SiC as elaborated in the later part of the paper. As the heat treatment temperature is increased from 1500 °C to 1650 °C, most of the β-SiC gets transformed to the alpha form and hence indicating the color change from black to grey and then to creamish yellow. The color of the as procured α-SiC and β-SiC is creamish yellow and ash respectively (Abraham et al. 2019).
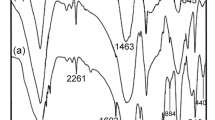
The IR spectra of BMV + MWCNT blends heat treated at 1500 °C are shown in Fig. 4a. The peaks at 815 cm−1, 1088 cm−1 and 1376 cm−1 correspond to the stretching vibrations of SiC, Si–O–Si and B–O, respectively. A peak at 3446 cm−1 corresponds to the O–H stretching vibration of Si–OH. On increasing the concentration of MWCNT, the absorption at 1376 cm−1 due to B–O stretching is reduced, which indicates the conversion of Si–O-B to SiC suggesting that addition of MWCNT promotes the formation of SiC.
IR spectra (Fig. 4b) of BMV + MWCNT blends sintered at 1650 °C show absorptions at 458 cm−1, 823 cm−1, 1081 cm−1 and 1382 cm−1 due to bending vibration of Si–O–Si and stretching vibration of SiC, Si–O–Si and B–O, respectively. With the increase in concentration of MWCNT, the intensity of the peak due to Si–O–Si stretching gradually decreases and finally disappears when MWCNT concentration is 5phr and this is attributed to increase in formation of SiC. The broad peaks at 888 cm−1 and 816 cm−1 for BMW (5)-1650 are due to the presence of a mixture of α-SiC and β-SiC respectively.
XRD patterns (Fig. 5a) of BMV + MWCNT blend heat treated at 1500 °C clearly suggest the presence of β-SiC as evidenced by the presence of diffraction lines at 2θ = 35.68, 60.04, 71.89° corresponding to (111), (220), (311) crystallographic planes (JCPDS No 29-1129). In addition, broad peaks due to amorphous SiBOC and SiO2 (JCPDS No 13-0026) are observed at 2θ = 21.98 and 26.44°, respectively. On increasing the amount of MWCNT, the extent of conversion of SiBOC to SiC increases. Comparison of XRD patterns (Fig. 5b) of BMV + MWCNT blends heat treated at 1650 °C with that of the corresponding samples heat treated at 1500 °C (Fig. 4a) suggests that SiBOC amorphous phase is converted to SiC to a larger extent even when MWCNT concentration is 1 phr. Unlike SiBOC amorphous phase, for this composition, SiO2 amorphous phase (broad peak at 26.44°) (JCPDS No 13-0026) is present even after heat treatment at 1650 °C. However, SiO2 amorphous phase is completely absent when MWCNT concentration is increased to 2.5 phr and 5.0 phr.
Raman spectra of the BMV + MWCNT blends sintered at 1500 °C (Fig. 6a) show peaks at ~ 1341 cm−1 (D-band) and ~ 1564 cm−1 (G-band) due to stretching mode of Csp3–Csp3 and Csp2–Csp2 bonds, respectively. The peak at ~ 2667 cm−1 is due to the second order D-band. Interestingly, peaks corresponding to β-SiC are not observed, though its presence is inferred by XRD and IR. The reason for this anomaly is not clearly understood at this stage. Raman spectra of BMV + MWCNT (Fig. 6b) differ remarkably from the corresponding ones (Fig. 6a) heat treated at 1500 °C. Raman spectra of all the samples heat treated at 1650 °C show peaks at 778 cm−1 and 948 cm−1 indicating the presence of both β-SiC and α-SiC. The peak observed at 1085 cm−1 is due to stretching vibration of Si–O–B bond. Stretching mode of terminal B–O bond is seen at 1477 cm−1. It is worth noting that samples with 0.5 phr and 1 phr of MWCNT heat treated at 1650 °C show peaks corresponding to D-band and G-band suggesting the presence of nanocarbon/graphitic carbon domains. Practically, vibration due to these bands disappears for the sample with 2.5 phr of MWCNT. For the sample with 5.0 phr MWCNT, vibration due to D band is present whereas that due to G band is absent. It is also seen that the sample with 5 phr of MWCNT show vibration at 482 cm−1 due to the stretching of Si–O–Si bond, probably due to the presence of amorphous SiO2. Interestingly, broad peak at ~ 26.4° due to amorphous SiO2 is practically absent in XRD (Fig. 5b). This suggests that amorphous SiO2 is present as discrete nanodomains which are difficult to be identified by XRD.
The SEM and EDS of BMV + MWCNT blends heat treated at 1500 °C are shown in Fig. 7. SEM results suggest that the morphology of all the five systems are almost same except for the fact that BMV with 5.0 phr of MWCNT is having a fluffy appearance compared to a rock like structure for BMV with 0.5 phr of MWCNT. From the EDS data, it is observed that the percentage of carbon content increases with increase in the addition of MWCNT and the oxygen content is least for the sample with 5.0 phr of MWCNT. Thus, it can be inferred that the conversion of SiBOC to SiC is occurring at 1500 °C.
SEM and EDS of BMV + MWCNT blend heat treated at 1650 °C are shown in Fig. 8. On increasing the concentration of MWCNT from 0.5 phr to 5.0 phr, the morphology of the sample changes from a rock-like morphology to a fluffy loosely bound structure due to the formation of SiC from SiBOC. This tendency increases with the increase in MWCNT concentration in the blend. The EDS data suggest that in general, the oxygen content decreases and carbon content increases with the increase in MWCNT concentration.
Comparison of the ceramic conversion of BMV oligomer carried out by Sreejith et al. [8] at 1500 °C and 1650 °C with the results presented in this paper suggest the following:
-
(1)
Blending BMV with MWCNT promotes the crystallization of amorphous SiBOC matrix at 1500 °C. The effect is more pronounced with the increase in concentration of MWCNT in the blend.
-
(2)
Though MWCNT promotes the crystallization at 1500 °C, appreciable amount of amorphous phase is present in the ceramic.
-
(3)
When the heat treatment temperature is increased to 1650 °C, the ceramic obtained is predominantly β-SiC with some amount of α-SiC. Such a complete crystallization of amorphous ceramic does not take place if MWCNT is not added to BMV oligomer.
-
(4)
The above observation suggests that MWCNT acts as a source of carbon promoting carbothermal reduction resulting in the formation of nano β-SiC crystallites which act as seed for promoting the crystallization of SiBOC ceramic. In the process, it is likely that MWCNT gets converted to SiCNT and thereby providing SiBOC/SiC/SiCNT nanocomposite at 1500 °C and SiC/SiCNT nanocomposite at 1650 °C.
As the matrix is dominated by SiC formed from SiBOC, the presence of SiCNT could not be identified by SEM. Further study of BMV+MWCNT systems in which MWCNT forms the major constituent is required to understand the influence of MWCNT on the crystallization process. The disintegration of MWCNT due to the reaction with SiBOC cannot be ruled out.
Conclusions
Effect of incorporation of 0.5, 1, 2.5, and 5.0 phr of MWCNT in vinyl-functionalized borosiloxane oligomer (BMV) on the ceramic conversion at 1500 °C and 1650 °C was investigated. FTIR, XRD, Raman spectra, and SEM studies suggest that MWCNT promotes the crystallization of SiBOC ceramic formed from BMV and the influence is more pronounced for the samples heat treated at 1650 °C. Carbothermal reaction of SiBOC with MWCNT is probably the route through which nano domains of SiC are formed which promote further crystallization. In effect, BMV + MWCNT blend gets converted into a nanocomposite in which SiCNT formed from MWCNT is dispersed in SiC/SiBOC matrix.
References
Abraham B, Sasikala TS, Gopakumar MP, Devapal D (2019) Tailoring the crystallinity of SiC ceramics derived from methylborosiloxane. Trans Indian Inst Met 72(6):1663–2167. https://doi.org/10.1007/s12666-019-01644-w
An L, Xu W, Rajagopalan S, Wang C, Wang H, Kapat J, Chow L, Fan Y, Zhang L, Jiang D, Guo B, Liang J, Vaidyanathan R (2004) Carbon nanotube reinforced polymer-derived ceramic composites. Adv Mater 16(22):2036–2040. https://doi.org/10.1002/adma.200306241
Chantrell PG, Popper P (1965) Special ceramics 1964: popper. P(Ed) academic press, London, pp 87–103
Colombo P, Mera G, Riedel R, Soraru GD (2010) Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J Am Ceram Soc 93:1805–1837. https://doi.org/10.1111/j.1551-2916.2010.03876.x
Desprec JF, Monthioux M (1995) Mechanical properties of C/SiC composites as explained from their interfacial features. J Eur Ceram Soc 15:209–224. https://doi.org/10.1016/0955-2219(95)93942-v
Devapal D (2006) Studies of borosiloxane oligomers and polysilahydrocarbons. PhD Dissertation, Mahatma Gandhi University
Devapal D, Packirisamy S, Sreejith KJ, Ravindran PV, George BK (2010) Synthesis, characterization and ceramic conversion studies of borosiloxane oligomers from phenyltrialkoxysilanes. J Inorg Organomet Polym 20:666–674. https://doi.org/10.1007/s10904-010-9380-7
Devapal D, Gopakumar MP, Prabhakaran PV, Packirisamy S (2017) A process for preparation of silicon carbide coated carbon nano-materials using polyborosiloxanes. Indian Patent No. 201741024214 dated 10.07.2017
Devapal D, Sreejith KJ, Swaminathan B, Chinthalapalli S, Bhuvaneswari S, Packirisamy S (2020) Influence of heat treatment temperature on the microstructure evolution of poly (vinylborosiloxane) derived ceramics. J Inorg Organomet Polym. https://doi.org/10.1007/s10904-020-01457-1
Gopakumar MP, Devapal D, Prabhakaran PV, Packirisamy S (2013) Development of carbon nanotube reinforced C-SiC ceramic matrix composites. National conference on Carbon materials, CCM-12 Mumbai 1–3 November 2012
Katsuda Y (2005) Reinforcement of precursor-derived Si-(B-)C-N ceramics with carbon nanotubes. Dissertation, Max-Planck-Institute for Metals Research, University of Stuttgart
Klonczynski A, Schneider G, Riedel R, Theissmann R (2004) Influence of boron on the microstructure of polymer derived SiCO ceramics. Adv Eng Mater 6:64–68. https://doi.org/10.1002/adem.200300525
Liao N, Xue W, Zhou H, Zhang M (2014) Molecular dynamics investigation of structure and high-temperature mechanical properties of SiBCO ceramics. J Alloy Compd 610:45–49. https://doi.org/10.1016/j.jallcom.2014.04.189
Liebau V, Hauser R, Riedel R (2004) Amorphous SiBCO ceramics derived from novel polymeric precursors. C R Chim 7:463–469. https://doi.org/10.1016/j.crci.2003.11.012
Nair SG, Sreejith KJ, Packirisamy S, Ganesh TB, Devasia R (2018) Polymer derived PyC interphase coating for C/SiBOC composites. Mater Chem Phys 204:179–186. https://doi.org/10.1016/j.matchemphys.2017.10.012
Pena-Alonso R, Mariotto G, Gervais C, Babonneau F, Soraru GD (2007) New insights on the high-temperature nanostructure evolution of SiOC and B-doped SiBOC polymer-derived glasses. Chem Mater 19:5694–5705. https://doi.org/10.1021/cm071203q
Schiavon MA, Gervais C, Babonneau F, Soraru GD (2004a) Crystallization behavior of novel silicon boron oxycarbide glasses. J Am Ceram Soc 87:203–208. https://doi.org/10.1111/j.1551-2916.2004.00203.x
Schiavon MA, Gervais C, Babonneau F, Soraru GD (2004b) Crystallization behavior of novel silicon boron oxycarbide glasses. J Am Ceram Soc 87:203–208. https://doi.org/10.1111/j.1551-2916.2004.00203.x
Sreejith KJ (2011) Polymer derived ceramics and their high temperature applications. PhD Dissertation, University of Kerala
Sreejith KJ, Prabhakaran PV, Laly KP, Dimple R, Packirisamy S (2016) Vinyl-functionalized poly (borosiloxane) as precursor for SiC/SiBOC nanocomposite. Ceram Int 42:15285–15293. https://doi.org/10.1016/j.ceramint.2016.06.166
Sreejith KJ, Rajasekhar BV, Vijay V et al (2018) Polymer-derived Cf/SiBOC ceramic matrix composites and a method of production thereof. Indian Patent Appl, No, p 201841020417
Tamayo A, Pena-Alonso R, Rubio F, Rubio J, Oteo JL (2012) Synthesis and characterization of boron silicon oxycarbide glass fibers. Journal of non-crystalline solids. J Noncry Sol 358:155–162. https://doi.org/10.1016/j.jnoncrysol.2011.09.002
Verbeek W (1973) Production of shaped articles of homogeneous mixtures of Silicon Carbide and Nitride. Ger. Pat. No.2218960
Xie S, Wang Y, Lei Y, Wang B, Wu N, Goua Y et al (2015) A simply prepared flexible SiBOC ultrafine fiber mat with enhanced high-temperature stability and chemical resistance. RSC Adv 5:64911–64917. https://doi.org/10.1039/C5RA03100A
Young-Hag K, Hae-Won K, Hyoun-Ee K (2001) Mechanical behavior of polymer and ceramic matrix nanocomposites. Script Mater 44(8–9):2061. https://doi.org/10.1016/S1359-6462(01)00892-2
Acknowledgements
The authors would like to thank the authorities of VSSC for granting the permission to present the work, Analytical and Spectroscopy Division (ASD) for spectral analysis and Materials Characterization Division (MCD) for SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jinitha, T.V., Gopakumar, M.P., Devapal, D. et al. Studies on the Effect of Addition of MWCNT on the Ceramic Conversion of Vinyl-Functionalized Polyborosiloxane. Trans Indian Natl. Acad. Eng. 6, 3–11 (2021). https://doi.org/10.1007/s41403-020-00158-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-020-00158-y