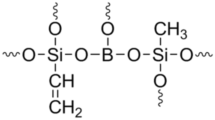
Abstract
Polycondensation of boric acid (BA) with mixtures of phenyltriethoxysilane (PTEOS) and vinyltriethoxysilane (VTEOS) and with mixtures of phenyltrimethoxysilane (PTMOS) and vinyltriethoxysilane (VTEOS) in diglyme at 83–87 °C using HCl as catalyst resulted in vinyl-functionalized borosiloxane oligomers. Effect of variation of PTEOS:VTEOS and PTMOS:VTEOS ratio (keeping BA:alkoxysilanes ratio constant) and the effect of variation of BA:alkoxysilane (BA:PTEOS:VTEOS and BA:PTMOS:VTEOS) ratio on the solubility, thermal stability and ceramic residue were studied. The oligomers obtained were characterized by FTIR, GPC, pyrolysis GC and TGA. For PTEOS + VTEOS system all the oligomers were soluble in the reaction medium, but after removal of alcohol (byproduct) and diglyme (solvent), the oligomers obtained in the solid form were insoluble in common organic solvents. Unlike the PTEOS-based system, for PTMOS-based system the oligomers synthesized from monomer feed ratios (BA:PTMOS:VTEOS) 1:1:1 and 1:1.67:0.33 even after the removal of ethanol and diglyme are soluble in tetrahydrofuran, dioxane and diglyme. These two soluble oligomers show bimodal molecular weight distribution with \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 3,650 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1860 for 1:1:1 mol ratio, and \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 2,540 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1700 for 1:1.67:0.33 mol ratio. 29Si-NMR spectra of the soluble oligomers show peaks at − 69 and − 78 ppm which are attributed to T2 and T3 structures respectively. Thermogravimetric analysis of vinyl-functionalized borosiloxane oligomers from PTEOS and PTMOS indicates that the ceramic residue of the oligomers in argon atmosphere at 900 °C varies from 68 to 89% depending on the monomer feed ratio of BA to organoalkoxysilane ratio and phenyltrialkoxysilane to VTEOS ratio. With the increase in concentration of VTEOS in the monomer feed the thermal stability as well as the ceramic residue of the oligomers increase and a reverse trend is observed for variation of BA concentration. Pyrolysis of the oligomers produce C2, C3 hydrocarbons and benzene as the main pyrolysis products. Ceramic conversion of a typical oligomer was carried out at 900 °C, 1500 °C and 1650 °C in argon atmosphere. The ceramics obtained at 900 °C and 1500 °C are amorphous SiBOC which transform to β-SiC at 1650 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Inorganic, organometallic and inorganic–organic hybrid polymers find wide range of applications such as high temperature protective coatings, thermal protective systems, matrix resins, precursors for ceramics, atomic oxygen resistant coatings, adhesives and NLO materials [1,2,3,4,5,6,7,8,9,10]. Of these applications, in recent years widespread importance is given towards utilizing these polymers as preceramic materials [5, 11,12,13,14,15]. Among the preceramic polymers, polysiloxanes [16,17,18,19,20,21,22] and poly(borosiloxane)s [23,24,25,26,27,28,29,30,31,32] have gained importance due to the ease of synthesis and their ability to form siliconoxycarbide (SiOC) and siliconboronoxycarbide (SiBOC) amorphous ceramics respectively in appreciable yield. SiBOC formed by pyrolysis of polyborosiloxane is considered to be a homogenous amorphous network of mixed siliconoxycarbide and boronoxycarbide. On heat treatment at temperatures above 1200 °C, SiBOC undergoes crystallization forming nano β-SiC and the crystallization is more pronounced with the increase in boron content. Crystallization of SiBOC takes place through the consumption of BC2O and BCO2 units and the formation of B(OSi)3 units [27]. Thus, the incorporation of boron in SiCO suppresses the devitrification of SiO2 and promotes the formation of nano β-SiC.
In our laboratory, over the years, we have focused our attention on the synthesis of poly(borosiloxane)s mainly by reacting organoalkoxysilanes with boric acid (BA-cheapest source of boron) by varying monomer feed ratios through non-aqueous sol–gel process and on the factors, which influence the processability, thermal stability, ceramic residue and the stability of SiBOC ceramics obtained from these precursors [25, 29,30,31, 33,34,35]. It has been observed that the borosiloxane oligomer synthesized from vinyltriethoxysilane (VTEOS) gives higher ceramic residue (~ 85%) compared to the oligomers synthesized from phenyltrimethoxysilane (72%) and phenyltriethoxysilane (65%) at 900 °C in argon atmosphere. The higher ceramic residue of vinyl-functionalized borosiloxane oligomer is attributed to the crosslinks that are formed due to the polymerization of vinyl groups [25, 31]. Though it is advantageous to synthesize borosiloxane oligomers from VTEOS in view of the higher ceramic residue, the major concern is that the VTEOS-based oligomers undergo premature gelation while attempting to remove diglyme (solvent) and ethanol (byproduct). On the other hand, PTMOS/PTEOS-based oligomers, exhibit solublility in organic solvents even after diglyme and ethanol are removed. It is desirable to synthesize a borosiloxane oligomer which combines both the attributes, viz., processability and high ceramic residue on pyrolysis so that such a precursor can serve as matrix resin for ceramic matrix composite and as precursors for ceramic coatings. Considering the above aspects, in the present investigation attention has been directed towards synthesizing borosiloxane oligomers from mixtures of VTEOS and PTMOS/PTEOS by non-aqueous sol–gel synthesis in an attempt to meet the above requirement. With a view to optimizing the monomer feed ratio, borosiloxane oligomers were synthesized by varying BA:Alkoxysilane ratio and also by varying VTEOS:PTEOS/PTMOS ratio for a chosen BA concentration. The effect of the monomer feed ratio on the processability, microstructure, thermal stability and ceramic residue of the oligomers has been studied. One of the borosiloxane oligomers which exhibits solubility and high ceramic residue has been chosen for heat treatment at different temperatures to obtain SiBOC and SiC ceramics.
2 Experimental
2.1 Materials
BA (Qualigens, Mumbai, India) VTEOS (Fluka, Buchs, Switzerland), PTEOS (Fluka, Buchs, Switzerland) and PTMOS (Fluka, Buchs, Switzerland) were used without further purification. Diglyme (Spectrochem, Mumbai, India) was distilled before use.
2.2 Synthesis of Borosiloxane Oligomers
A mixture of VTEOS, PTEOS and BA containing 28.55 g (0.15 mol) of VTEOS, 36.06 g (0.15 mol) of PTEOS and 9.27 g (0.15 mol) of BA were reacted in 50 ml of diglyme (solvent) using 1 ml of concentrated HCl as catalyst for 3 h at 83–87 °C following the procedure described elsewhere [29] to obtain borosiloxane oligomer. After the removal of ethanol by distillation, diglyme was distilled off under reduced pressure. The oligomer obtained as a light brown colored solid was dried under vacuum at 60 °C for 10 h and was designated as BSiPhVi-1. Similarly BSiPhVi-2 and BSiPhVi-3 were prepared by carrying out the reaction in two different mole ratios, namely BA:PTEOS:VTEOS in 1.5:1:1 and 2:1:1.
BSiPhVi-4 was prepared by carrying out the reaction using BA: PTMOS:VTEOS in 1:1:1 mol ratio by the same procedure followed for BSiPhVi-1.To study the effect of increase in concentration of BA in the system, BSiPhVi-5 and BSiPhVi-6 were prepared using the monomer feed ratios (BA:PTMOS:VTEOS) 1.5:1:1 and 2:1:1 respectively. To understand the effect of concentration of organoalkoxysilanes in the system, BSiPhVi-7 and BSiPhVi-8 were prepared using the monomer feed ratios (BA:PTMOS:VTEOS) 1:1.67:0.33 and 1:0.33:1.67 respectively where BA:alkoxysilane mole ratio is 1:2.
2.3 Conversion of Borosiloxane Oligomer to Ceramics
Pyrolysis of 10 g of BSiPhVi-4 was performed in an inconel furnace by heating the furnace at the rate of 2 °C/min, soaking at 900 °C for 2 h and then cooling to room temperature at the rate of 2 °C/min. under the flow of argon (~ 50 ml/min). In separate experiments, 2 g of the pyrolyzed sample was heat treated at 1500 °C and at 1650 °C following the above heating and cooling profile.
2.4 Characterization
FTIR spectra were recorded on a Perkin Elmer Spectrum GX spectrometer. Pyrolysis GC analysis of the oligomers was carried out at 700 °C using CDS 100 Pyroprobe interfaced with Fisons Mega 2 GC and SP-2100 column. The column temperature was programmed from 70 to 220 °C at a heating rate of 20 °C/min.
1H-, 13C- and 29Si-NMR spectra were recorded on Brucker Avance 300 spectrometer.1H-,13C- and 29Si-NMR spectra were recorded at 300, 75.5 and 59.6 MHz respectively. All chemical shifts were reported with respect to internal tetramethylsilane standard.
Thermogravimetric analysis (TGA) was performed on a TA Instrument SDT 2960 at a heating rate of 10 °C/min under nitrogen atmosphere over a temperature range from room temperature to 900 °C. Molecular weights (\(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) and \({\overline{\text{M}}}_{{\text{n}}}\)) were determined by GPC using Waters ‘Alliance’ instrument using HR1 and HR2 microstyragel columns, polystyrene standard and tetrahydrofuran as the eluent with a flow rate of 1 ml/min.
X-ray diffraction (XRD) patterns of powder samples were recorded on a Philips 1729 instrument using Cu-Kα radiation with nickel filter and PW 1710 diffractometer control unit.
3 Results and Discussion
3.1 Borosiloxane Oligomers from PTEOS and VTEOS
In order to study the effect of mole ratio of BA to organoalkoxysilanes on the processability and thermal stability of the product obtained, the reaction was carried out for the monomer feed ratios (BA: PTEOS:VTEOS) 1:1:1, 1.5:1:1 and 2:1:1 using diglyme as solvent at 83–87 °C in presence of hydrochloric acid catalyst for 3 h and the oligomers obtained are referred to as BSiPhVi-1, BSiPhVi-2 and BSiPhVi-3 respectively. The reaction scheme for the synthesis of borosiloxane oligomers from mixtures of PTEOS, VTEOS and BA is shown in Scheme 1. All the oligomers were soluble in the reaction medium, but after removal of ethyl alcohol (byproduct) and diglyme, the oligomers were obtained in the solid form and were insoluble in solvents like THF, diglyme, dioxane, chloroform, toluene and xylene. A typical IR spectrum of the oligomer (BSiPhVi-1) is shown in Fig. 1 and the peak assignments are given in Table 1.
The pyrograms of the three oligomers at 700 °C are shown in Fig. 2. All of them reveal that the oligomers produce C2, C3 hydrocarbons (retention time: ~ 0.72 min) and benzene (retention time: ~ 2.46 min) [36] as the main pyrolysis products along with small amounts of other high molecular weight silicon substituted products as indicated by the peaks after 3 min. The peak at retention time 1.88 min is attributed to ethyl alcohol formed due to the cleavage of residual Si-OEt linkages present in the oligomers. It is noticed that the intensity of this peak reduces when BA to alkoxysilane ratio is increased from 1:2 to 1.5:2 and with further increase in the ratio practically no peak is observed corresponding to ethanol. The above observation suggests that with increase in BA concentration, the concentration of residual Si-OEt groups reduces as extent of formation of Si-O-B linkages increases. As the lower hydrocarbons are produced from ViSiO moiety and benzene from PhSiO moiety, the relative concentration of C2, C3 hydrocarbons and benzene will depend on the concentration of ViSiO and PhSiO units in the borosiloxane oligomer. The ratio of the area due to C2, C3 hydrocarbons and benzene for BSiPhVi-1, BSiPhVi-2 and BSiPhVi-3 is almost the same (~ 0.83:1). This observation suggests that the concentration of PhSiO and ViSiO units does not change considerably with the change in BA concentration in the monomer feed. In the pyrogram of BSiPhVi-1, a small peak is observed at retention time 4.73 min and a prominent peak is observed at 5.09 min whereas in the pyrogram of BSiPhVi-2, the peak at 4.73 min is prominent and the peak at 5.09 min has merged with the peak at 4.73 min. It is worth noting that both these peaks are absent in the pyrogram of BSiPhVi-3. The peaks at 4.73 min and 5.09 min are probably due to some high molecular weight fractions containing silicon. Probably, with the increase in BA concentration, the formation of siloxane structural units due to self-condensation would be minimal as the possibility of formation of Si-O-B linkage increases.
The TG curves of BSiPhVi-1, BSiPhVi-2 and BSiPhVi-3 are compared in Fig. 3. As observed for borosiloxane oligomers synthesized from PTEOS [25] and from VTEOS [31], the ceramic residue decreases with the increase in BA concentration in the monomer feed. BSiPhVi-1, BSiPhVi-2 and BSiPhVi-3 give ceramic residue of 83, 80 and 75% respectively at 900 °C.
3.2 Borosiloxane Oligomers from PTMOS and VTEOS
Borosiloxane oligomers synthesized from BA and mixtures of PTEOS and VTEOS were insoluble in organic solvents when the byproduct, ethanol, and the solvent, diglyme, were distilled off. In an attempt to synthesize soluble vinyl-functionalized borosiloxane oligomers with improved ceramic residue, BA was reacted with PTMOS and VTEOS mixture. The reaction was carried out using BA:PTMOS:VTEOS monomer feed ratios, 1:1:1, 1.5:1:1 and 2:1:1 in diglyme at 83–87 °C for 3 h using hydrochloric acid as catalyst and the oligomers are designated as BSiPhVi-4, BSiPhVi-5 and BSiPhVi-6 respectively.
BSiPhVi-4 is soluble in THF, diglyme and dioxane and insoluble in chloroform, toluene and xylene whereas BSiPhVi-5 and BSiPhVi-6 are insoluble in these organic solvents. The GPC curve of BSiPhVi-4 is shown in Fig. 4. It shows a bimodal molecular weight distribution with \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 3650 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1860.
The IR spectra of the oligomers indicate the presence of B-O-Si at 698 cm−1, Si-vinyl at 1603, 1410, 970 and 738 cm−1, Si-OR at 1135 cm−1, Si-Ph at 1520 cm−1 and Si-OH at 3424 cm−1. Comparison of the IR spectra of the oligomers suggests that as the concentration of BA is increased in the monomer feed the peaks corresponding to Si-vinyl groups become feeble. A broad peak exists around 1028 cm−1 indicating the presence of Si-O-Si linkages.
The 1H-NMR spectrum of BSiPhVi-4 is shown in Fig. 5. The broad peak observed in the region 6.8–8 ppm is assigned to Si-Ph group and the peak observed in the region 5.5–6.3 ppm is due to Si-vinyl. The broad peak observed in the region 3.5–3.8 ppm is due to Si-OH and B-OH groups. The two peaks observed at 3.3 and 3.4 ppm are probably due to residual Si-OCH2CH3 and Si-OCH3 groups. The peak at 1.3 ppm is assigned to residual Si-OCH2CH3 present in the oligomer. Based on the intensities of phenyl and vinyl protons, the ratio of phenylsiloxy units to vinylsiloxy units is calculated to be 1:1. The 13C-NMR spectrum of BSiPhVi-4 (Fig. 6) shows peaks at 128, 130 and 134 ppm due to phenyl carbons. The signal at 136 ppm corresponds to CH2 of vinyl group. The signal due to CH of vinyl group has merged with the signal at 130 ppm due to phenyl carbon resulting in line broadening.
29Si-NMR spectrum of BSiPhVi-4 is shown in Fig. 7. Soraru et al. [24, 37] studied in detail the 29Si-NMR spectra of borosiloxane oligomers synthesized by sol–gel process of BA and organoalkoxysilanes and assigned the chemical shifts to the structural units, T1, T2 and T3, where Ti indicates the unit with ‘i’ siloxane (O-Si) bonds attached to the central silicon atom. It is reported [24, 37] that the Si-O-Si and the Si-O-B bonds do not differ in their 29Si-NMR chemical shifts. This indicates that the chemical shift values of T1, T2 and T3structures are not influenced much whether they contain Si-O-Si or Si-O-B bonds. Based on the chemical shift values reported for the sol-gels prepared from organotrialkoxysilanes and those from BA and organotrialkoxysilanes, the broad peaks in the region − 64 to − 72 and − 72 to − 90 ppm, can be assigned to T2 and T3 structures respectively [29, 30]. The splitting of T2 and T3 peaks is due to different possible structures arising due to the presence of all phenylsiloxy units, all vinylsiloxy units, and both vinylsiloxy and phenylsiloxy units.
A typical pyrogram of BSiPhVi-4 at 700 °C is shown in Fig. 8 and the pyrolysis GC data of BSiPhVi-4, BSiPhVi-5 and BSiPhVi-6 are compared in Table 2. It is noticed that the ratio of area of benzene peak to area of C2, C3 carbon peak is 0.5:0.5 for BSiPhVi-4. As the ratio of PTMOS:VTEOS in the monomer feed is same for BSiPhVi-4, BSiPhVi-5 and BSiPhVi-6, it is expected that for all the three oligomers, the ratio of area of benzene peak to area of C2, C3 carbon peak should be the same. From Table 2, it is seen that for BSiPhVi-5 the ratio is almost the same as that of BSiPhVi-4. However, for BSiPhVi-6 the ratio is 0.77:0.23 suggesting that phenylsiloxy units are incorporated more than vinylsiloxy units when BA:alkoxysilane ratio is increased from 1.5:2 to 1:1 which may be due to the preferential reaction of BA with PTMOS rather than with VTEOS when the concentration of BA is increased in the monomer feed.
The TG curves of BSiPhVi-4, BSiPhVi-5 and BSiPhVi-6 are compared in Fig. 9. It is observed that BSiPhVi-4 synthesized using BA:alkoxysilane molar ratio 1:2 gives the maximum ceramic residue (~ 86%). It is also noticed that the ceramic residue decreases with the increase in BA concentration in the reaction mixture. A weight loss of ~ 3% is observed around 170 °C for BSiPhVi-4. The initial weight loss is found to increase with increase in BA concentration in the feed. This weight loss is probably due to the loss of borate esters formed during the reaction [25].
In order to understand the effect of variation of PTMOS:VTEOS ratio on the processability, thermal stability and ceramic residue, borosiloxane oligomers BSiPhVi-7 and BSiPhVi-8 were synthesized using the monomer feed ratios (BA:PTMOS:VTEOS) 1:1.67:0.33 and 1:0.33:1.67 respectively in diglyme at 83–87 °C for 3 h.
BSiPhVi-7 is soluble in THF, diglyme and dioxane and insoluble in chloroform, toluene and xylene whereas BSiPhVi-8 is insoluble in all these solvents. The GPC curve of BSiPhVi-7 shows a bimodal molecular weight distribution with \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) of 2540 and \({\overline{\text{M}}}_{{\text{n}}}\) of 1700.
BSiPhVi-7 was also characterized by1H-, 13C- and 29Si-NMR and thermal analysis. The observations made with 1H- and 13C-NMR spectra of BSiPhVi-7 are similar to those of BSiPhVi-4. Based on the intensity of phenyl and vinyl protons, the ratio of phenylsiloxy units to vinylsiloxy units is estimated to be 0.83:0.17 as against the calculated ratio of 0.835:0.165. The 29Si-NMR spectrum of BSiPhVi-7 is shown in Fig. 10. The 29Si-NMR spectrum shows two broad peaks centered at − 68 and − 77 ppm and they are assigned to T2 and T3 structures respectively.
The oligomers, BSiPhVi-7 and BSiPhVi-8 were characterized by pyrolysis GC. The pyrolysis GC data of BSiPhVi-4, BSiPhVi-7 and BSiPhVi-8 along with the ratio of phenylsiloxy to vinylsiloxy units as obtained from 1H-NMR are summarized in Table 3. It is noticed that for BSiPhVi-7 and BSiPh-4, the ratio of PhSiO to ViSiO units as calculated from 1H-NMR, and the ratio of area of benzene to area of C2, C3 hydrocarbons are close to each other. Thus, the ratio of area of benzene to area of C2, C3 hydrocarbon could be equated to the mole ratio of PhSiO to ViSiO units present in borosiloxane oligomer. For BSiPhVi-8 which is insoluble, the ratio of area of benzene to C2, C3 hydrocarbons is 0.34:0.66. From this data, it could be inferred that more of PhSiO units are incorporated in the oligomer than expected.
The TG curves of BSiPhVi-4, BSiPhVi-7 and BSiPhVi-8 are compared in Fig. 11. It is noticed that the ceramic residue increases with the increase in VTEOS concentration in the monomer feed. BSiPhVi-8 synthesized using BA, PTMOS and VTEOS in 1:0.33:1.67 mol ratio gives the maximum ceramic residue (~ 89%).
3.3 Comparison of Solubility and Ceramic Residue of Borosiloxane Oligomers
From the results presented in the previous sections, it is evident that the solubility in organic solvents and the ceramic residue of borosiloxane oligomers depend on monomer feed ratio, i.e., BA:PTEOS/PTMOS:VTEOS. Solubility, meltability and ceramic residue are the important attributes to be considered for choosing a particular borosiloxane oligomer for application as matrix resin for CMCs and as precursors for ceramic coatings and adhesives. As the borosiloxane oligomers discussed in this investigation are not meltable, the focus is on selecting an oligomer which gives the highest ceramic residue among the oligomers which are soluble in organic solvents. Solubility and ceramic residue data of borosiloxane oligomers are summarized in Table 4. It is seen that BSiPhVi-4 and BSiPhVi-7 are the two oligomers which are soluble in organic solvents. As BSiPhVi-4 gives higher ceramic residue than that of BSiPhVi-7 the former was chosen for ceramic conversion studies.
3.4 Ceramic Conversion Studies of the Borosiloxane Oligomer from PTMOS and VTEOS Mixture
BSiPhVi-4 was converted to ceramics following the procedure explained in the experimental section. IR spectra and XRD patterns of the heat-treated samples are shown in Figs. 12 and 13 respectively.
It is seen from the IR spectrum of the oligomer heat-treated at 900 °C that four peaks are observed at 1550, 1320, 1070 and 490 cm−1. The peaks at 1070 and 490 cm−1 are due to the stretching and bending vibrations of Si-O-Si linkage respectively. The presence of peaks at 1550 and 1320 cm−1 is probably due to residual organic moieties/partially degraded organic moieties present in the pyrolyzed sample suggesting that the pyrolysis is incomplete at 900 °C. It is worth noting that the IR spectrum of the sample heat-treated at 1500 °C shows a major peak at 1070 cm−1 and the other two peaks at 1550 and 1320 cm−1 seen in IR spectrum of the pyrolyzed sample disappear, suggesting that the organic moieties have reacted with Si and B present in the oligomer resulting in the formation of a ceramic. The sample heat-treated at 1650 °C, shows a broad peak at 823 cm−1 and this is attributed to the conversion of amorphous ceramic to crystalline SiC [29,30,31]. The broadness of the peak is attributed to presence of SiOC or SiBOC glassy phase. The samples heat-treated at 900 and 1500 °C show a broad XRD pattern due to the amorphous SiBOC phase [30, 31] whereas the sample heat-treated at 1650 °C shows diffraction lines at 2θ = 36, 61 and 72.5 corresponding to 111, 220 and 311 planes of β-SiC respectively (Table 5)[37].The transformation of SiBOC to β-SiC by increasing the heat treatment temperature from 1500 to 1650 °C can be explained by adopting the crystal growth mechanism of β-SiC from SiOC glass. The pyrolyzed borosiloxanes is primarily a boron modified SiOC phase in which SiCO3, SiC2O2, SiC3O, BC2O and BCO2 units may be present and the relative concentration of each unit would depend on the monomer feed ratio. The redistribution of these structural units results in the formation of SiC4, SiO4 and BO3 units [23]. In addition to this exchange mechanism, carbothermic reduction involving nano domains of SiO2 rich and carbon rich phases can also take place [31].
4 Conclusions
Based on the present investigation the following conclusions have been drawn:
-
(i)
The borosiloxane oligomers synthesized from BA, PTEOS/PTMEOS and VTEOS are soluble in the reaction medium, diglyme irrespective of the monomer feed ratio. On removal of the solvent, diglyme and the byproduct, methanol and/or ethanol, two of the oligomers prepared from BA, PTMOS and VTEOS for the monomer feed ratios 1:1:1 and 1:1.67:0.33, were soluble in organic solvents such as diglyme, dioxane and tetrahydrofuran.
-
(ii)
In general, ceramic residue at 900 °C in argon increases with the increase in VTEOS concentration in the monomer feed ratio and this is attributed to increase in the crosslinking resulting from ViSiO units.
-
(iii)
Increase in BA in the monomer feed increases the initial weight loss up to 170 °C and this is attributed to the increase in formation of borate esters. Due to this the ceramic residue at 900 °C decreases with the increase in BA concentration in the monomer feed.
-
(iv)
Pyrolysis GC studies reveal that the oligomers produce C2, C3 hydrocarbons and benzene as the main pyrolysis products along with small amounts of other high molecular weight silicon substituted products. From the data, it could be inferred that more of PhSiO units as compared to ViSiO units are incorporated in the oligomers.
-
(v)
1H- and 13C-NMR spectral studies of soluble oligomers, BSiPhVi-4 and BSiPhVi-7 confirm the presence of Si-vinyl groups. 29Si-NMR spectra reveal the presence of T2 and T3 structures. The splitting of T2 and T3 peaks is due to different possible structures arising due to the presence of all phenylsiloxy units, all vinylsiloxy units, and both vinylsiloxy and phenylsiloxy units.
-
(vi)
GPC studies reveal that BSiPhVi-4 and BSiPhVi-7 are low molecular weight oligomers exhibiting bimodal molecular weight distribution and both \(\overline{{\text{M}}}_{{_{{\text{W}}} }}\) and \({\overline{\text{M}}}_{{\text{n}}}\) are higher for BSiPhVi-4 than for BSiPhVi-7. Ceramic residue at 900 °C is also higher for BSiPhVi-4 than that of BSiphVi-7. Thus, it is desirable to choose the monomer feed ratio (BA:PTMOS:VTEOS) of 1:1:1 for getting soluble borosiloxane oligomer capable of giving maximum ceramic residue.
-
(vii)
Ceramic conversion studies reveal that at 900 and 1500 °C, SiBOC ceramic is formed which undergoes crystallization at 1650 °C resulting in the formation of β-SiC.
Thus, the present study has resulted in arriving at a suitable monomer feed ratio for synthesizing vinyl-functionalized soluble borosiloxane oligomer (BSiPhVi-4) which gives high ceramic residue (86% at 900 °C) and this oligomer is expected to find application as precursors for SiBOC and SiC coating and as matrix resins for ceramic matrix composites.
References
J.E. Mark, H.R. Allcock, R. West, Inorganic Polymers, 2nd edn. (Oxford University Press, New York, 2005).
A.H. McKinney, Inorganic-Organic Hybrid Polymers for High-Temperature Applications, Technical Briefs, vol. 42, issue 1 (Naval Research Laboratory, Washington DC, 2018)
K.J.D. MacKenzie, Innovative Applications of Inorganic Polymers (Geopolymers), Chapter 28 in Handbook of Alkali-Activated Cements, Mortars and Concretes (Elsevier, Amsterdam, 2015), pp. 777–805
K.A. Williams, A.J. Boydston, C.W. Bielawski, Main-chain organometallic polymers: synthetic strategies, applications, and perspectives. Chem. Soc. Rev. 36, 729–744 (2007)
P. Colombo, G. Mera, R. Riedel, G.D. Soraru, Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 93, 1805–1837 (2010)
S. Packirisamy, Decaborane(14)-based polymers. Prog. Polym. Sci. 21, 707–773 (1996)
S. Packirisamy, D. Schwam, M.H. Litt, Atomic oxygen resistant coatings for low earth orbit space structures. J. Mater. Sci. 30, 308–320 (1995)
D. Devapal, S. Packirisamy, R.M. Korulla, K.N. Ninan, Atomic oxygen resistant coating from poly (tetramethyldisilylene-co-styrene). J. Appl. Polym. Sci. 94(6), 2368–2375 (2004)
D. Devapal, S. Packirisamy, C.P.R. Nair, K.N. Ninan, Phosphazene-based polymers as atomic oxygen resistant materials. J. Mater. Sci. 41, 5764–5766 (2006)
P. Innocenzi, B. Lebeau, Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 15, 3821–3831 (2005)
S. Packirisamy, K.J. Sreejith D. Devapal, B. Swaminathan, Polymer-derived ceramics and their space applications, in Handbook of Advanced Ceramics and Composites. ed. by Y. Mahajan, J. Roy (Springer Nature, Cham, 2000), pp. 975–1080
S. Fu, M. Zhu, Y. Zhu, Organosilicon polymer-derived ceramics: an overview. J. Adv. Ceram. 8, 457–478 (2019)
A. Xia, J. Yin, X. Chen, X. Liu, Z. Huang, Polymer-derived si-based ceramics: recent developments and perspectives. Crystals 10(9), 824 (2020)
A. Viard, D. Fonblanc, D. Lopez-Ferber, M. Schmidt, A. Lale, C. Durif, S. Bernard, Polymer derived Si-B-C-N ceramics: 30 years of research. Adv. Eng. Mater. 20, 1800360 (2018)
E. Ionescu, S. Bernard, R. Lucas, P. Kroll, S. Ushakov, A. Navrotsky, R. Riedel, Polymer-derived Ultra-High Temperature Ceramics (UHTCs) and related materials. Adv. Eng. Mater. 21, 1900269 (2019)
G.D. Soraru, E. Dallapiccola, G. Dandrea, Mechanical characterization of sol–gel-derived silicon oxycarbide glasses. J. Am. Ceram. Soc. 79, 2074–2080 (1996)
J. Parmentier, G.D. Soraru, F. Banonneau, Influence of the microstructure on the high temperature behavior of gel-derived SiOC glasses. J. Eur. Ceram. Soc. 21, 101–108 (2001)
G.D. Soraru, L. Pederiva, J. Latournerie, R. Raj, Pyrolysis kinetics for the conversion of a polymer into an amorphous silicon oxycarbide ceramic. J. Am. Ceram. Soc. 85, 2181–2187 (2002)
A. Saha, R. Raj, Crystallization maps for SiCO amorphous ceramics. J. Am. Ceram. Soc. 90, 578–583 (2007)
C. Stabler, E. Ionescu, M. Graczyk-Zajac, I. Gonzalo-Juan, R. Riedel, Silicon oxycarbide glasses and glass ceramics: “all-rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 101, 4817–4856 (2018)
B.V. Manoj Kumar, Y.-W. Kim, Processing of polysiloxane-derived porous ceramics: a review. Sci. Technol. Adv. Mater. 11, 044303 (2010)
K. Lu, D. Erb, Polymer derived silicon oxycarbide-based coatings. Int. Mater. Rev. 63, 139–161 (2017)
G.D. Soraru, F. Babonneau, S. Maurina, J. Vicens, Sol–gel synthesis of SiBOC glasses. J. Non-Cryst. Solids 224, 173–183 (1998)
G.D. Soraru, N. Dallabona, C. Gervais, F. Babonneau, Organically modified SiO2-B2O3 gels displaying a high content of borosiloxane (B-O-Si) bonds. Chem. Mater. 11, 910–919 (1999)
G. Ambadas, S. Packirisamy, K.N. Ninan, Synthesis, characterization and thermal properties of boron and silicon containing preceramic oligomers. J. Mater. Sci. Lett. 21, 1003–1005 (2002)
C. Gervais, F. Babonneau, N. Dallabonna, G.D. Sorarù, Sol–gel-derived silicon-boron oxycarbide glasses containing mixed silicon oxycarbide (SiCxO4−x) and boron oxycarbide (BCyO3−y) units. J. Am. Ceram. Soc. 84, 2160–2164 (2004)
M.A. Schiavon, C. Gervais, F. Babonneau, G.D. Soraru, Crystallization behavior of novel silicon boron oxycarbide glasses. J. Am. Ceram. Soc. 87, 203–208 (2004)
M.A. Schiavon, N.A. Armelin, I. Yoshida, Novel poly(borosiloxane) precursors to amorphous SiBCO ceramics. Mater. Chem. Phys. 112, 1047–1054 (2008)
D. Devapal, S. Packirisamy, K.J. Sreejith, P.V. Ravindran, B.K. George, Synthesis, characterization and ceramic conversion studies of borosiloxane oligomers from phenyltrialkoxysilanes. J. Inorg. Organomet. Polym. Mater. 20, 666–674 (2010)
K.J. Sreejith, P.V. Prabhakaran, K.P. Laly, R. Dimple, S. Packirisamy, Vinyl-functionalized poly(borosiloxane) as precursor for SiC/SiBOC nanocomposite. Ceram. Int. 42, 15285–15293 (2016)
D. Devapal, K.J. Sreejith, B. Swaminathan, S. Chinthalapalli, S. Bhuvaneswari, S. Packirisamy, Influence of heat treatment temperature on the microstructure evolution of poly(vinylborosiloxane) derived ceramics. J. Inorg. Organomet. Polym. Mater. 30, 2224–2233 (2020)
S. Rubinsztajn, New facile process for synthesis of borosiloxane resins. J. Inorg. Organomet. Polym. Mater. 24, 1092–1095 (2014)
D. Devapal, Studies on inorganic and organometallic polymers. PhD thesis, Mahatma Gandhi University, Kottayam (2007)
P.V. Prabhakaran, Studies on non-oxide ceramics derived from polymers and their applications. PhD thesis, University of Kerala, Thiruvananthapuram, 2008
K. J. Sreejith, Polymer derived ceramics and their high temperature applications. PhD thesis, University of Kerala, Thiruvananthapuram, 2010
J.A. Perry, Introduction to Analytical Gas Chromatography: History, Principles and Practice (Marcel Dekker, New York, 1981).
G.D. Soraru, F. Babonneau, C. Gervais, N. Dallabona, Hybrid RSiO1.5/B2O3 gels from modified silicon alkoxides and boric acid. J. Sol–Gel. Sci. Technol. 18, 11–19 (2000)
Acknowledgements
The authors thank the authorities of VSSC for granting permission to publish this work. Help received from the members of the Analytical and Spectroscopy Division for the thermal, chemical and spectral analysis of the samples is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest with respect to the data presented in the paper. There is also transparency of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devapal, D., Varughese, G., Radhakrishnan, T.S. et al. Studies on Borosiloxane Oligomers from Mixtures of Vinyltriethoxysilane and Phenyltrialkoxysilanes. J Inorg Organomet Polym 31, 2672–2681 (2021). https://doi.org/10.1007/s10904-021-01964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01964-9