Abstract
Medicinal plants produce several natural bioactive molecules and compounds called secondary metabolites that have very important biological properties. The objective of this work is to determine the chemical composition of essential oils (EO) obtained from the leaves of three Tunisian species of Eucalyptus (E. oleosa, E. pimpiniana, E. polyanthemos) and to evaluate their biological activities. As a result, 45 different compounds were identified: 26 from E. pimpiniana, 15 from E. polyanthemos, and 39 from E. oleosa, which represent 99.4%, 99.4% and 98.6%, respectively, of the entire essential oil constituents. The analyses showed that 1,8-cineole (35.3%) and β-eudesmol (25.5%) were the main components in E. pimpiniana essential oil, whereas 1,8-cineole (71.6%) and globulol (13.2%) characterized E. polyanthemos leaf oil. Also, 1,8-cineole (13.4%), spathulenol (11.9%), and β-eudesmol (8.5%) were found to be the main constituents of E. oleosa EO. Other compounds, such as phellandral, p-cymen-7-ol (syn. cumin alcohol), carvacrol, myrtenal, cumin aldehyde and cryptone, are specific to the EOs of E. oleosa, making it distinct from the other Eucalyptus species studied. The essential oils showed low antioxidant capacity, but significant antifungal activity against five Fusarium spp. Indeed, E. oleosa essential oil exhibited the highest level of antifungal activity. Additionally, herbicidal activity has only been proved in a preliminary in vitro test against 3 weed species (Sinapis arvensis L., and Lepidium sativum) of the same family. The greatest inhibition of seed germination was obtained with the E. oleosa essential oils even at low concentrations strongly suggesting that they could have application in agriculture, particularly as antagonists against Fusarium and other fungi and for weed control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Weeds are among the major threats to agricultural production (Mabrouk et al. 2007) because they compete with crops for natural resources, harbor pests, and reduce crop yields and quality (Brown et al. 2019; Ampong-Nyarko and De Data 1991), all of which increase costs. Chemical control remains the most common practice for decreasing weed growth, but an overdependence on chemical application has led to serious side effects, including damage to vegetation and non-target crops, persistence of chemical residues in water and soil, and serious concerns for the environment, human health, and biosafety in general (Arias-Estevez et al. 2008). In addition, the overuse of synthetic compounds can lead to the development of herbicide resistance (Heap and Le Baron 2001). To overcome these negative effects, research is actively moving toward the development of sustainable alternatives by using biological compounds that have phytotoxic potential (Mabrouk et al. 2016).
Phytopathogenic fungi cause severe crop yield losses, making them a major problem in global agriculture (Hemissi et al. 2013). To fight against these pathogens, farmers have intensified treatments that lead to numerous unwanted environmental problems, such as groundwater and soil contamination and the development of fungal resistance (Zubrod et al. 2019). For these reasons, researchers have investigated other means of disease control and plant growth promotion such as plant-derived essential oils for use as natural herbicides and fungicides to reduce the negative effects of exogenously added chemicals (Smith and Perfetti 2020; Steven et al. 1991). Essential oils are much less persistent than synthetic compounds, and they do not significantly affect the environment. Moreover, they employ very different modes of action to prevent the development of resistance in weeds or fungal species compared to synthetic compounds (Amri et al. 2023). Essential oils are formed by complex mixtures of hydrocarbons and their oxygenated derivatives. The active components are excreted or volatilized on the surface of plant organs, such as leaves, stems, flowers, fruits, and seeds, which gives them a fortuitous ecological location with respect to plants (Amri et al. 2011; Hazrati et al. 2018). Essential oils also have numerous biological effects including antiseptic, germicidal, antioxidant, phytotoxic and antifungal properties (Ootani et al. 2017; Amri et al. 2012, 2021). Many researchers have shown that essential oils are rich in compounds with allelopathic activity not only against weeds (Alipour et al. 2016), but also against crop pests (Amri et al. 2013; Hamrouni et al. 2014; Hanana et al. 2017).
The genus Eucalyptus belongs to the family Myrtaceae with about 900 species and subspecies (Tyagi and Malik 2011). It is a tree native to Australia and is characterized by evergreen and large single leaves. It was successfully introduced from Australia and planted worldwide and accounts for approximately 27% of total wood volume (Vázquez et al. 2008). In Tunisia, Eucalyptus species represent 5% of the total forest cover (Zaibet 2016). They are cultivated for two main reasons: wood production (40%) and forest protection (60%). Indeed, Eucalyptus species are important for providing combustible biomass and directly reducing carbon dioxide levels in the atmosphere (Barton 2000). In addition, their essential oils serve as pesticides (Barton 2000) and as antioxidants (Aragão et al. 2015; Loying et al. 2019), anticarcinogenic compounds (Ibáñez and Blázquez 2019), insecticides (Tolba et al. 2015), and antibacterial (Tolba et al. 2018), antiviral (Davies et al. 2019), and antifungal agents (Abril-Sánchez et al. 2019).
This study aims to elucidate the chemical composition of the essential oils obtained from the leaves of three different Eucalyptus species growing in Tunisia and their in vitro antioxidant, antifungal, and herbicidal activities. The antifungal activity of the essential oils was assessed against five plant pathogenic Fusarium fungi (F. oxysporum (Schltdl.), F. oxysporum f. matthioli (K.F. Baker), F. oxysporum MN-2, F. oxysporum 184, and F. redolens (Wollenw), and their phytotoxic effects were tested by evaluating the extent of inhibition of seed germination and seedling growth of three test plants, Sinapis arvensis L., Lepidium sativum L., and Raphanus sativus L.
Material and methods
Plant material
Plant samples of about 2 kg each were randomly collected during December 2020 from the aerial parts of individual Eucalyptus species growing in the arboretum of Hinchir EnNaâme (Siliana). The collection site is characterized by the upper semi-arid bioclimatic stage, and it is located at longitude 9° 10′ E, latitude 36° 13′ N and at an altitude of 350 m.
Essential oil extraction
The volatile oils were obtained by hydro-distillation of the leaves for 4 h in a Clevenger type apparatus. Extracted oils were dried over anhydrous sodium sulfate and stored at 4 °C until analysis. Yields based on dried weight were calculated (w/w %).
Gas chromatography and mass spectrometry analysis
The chemical composition of essential oils was studied by gas chromatography/electron ionization-mass spectrometry (GC/EI-MS). The analysis was carried out using an Agilent 7890B gas chromatograph equipped with a quadrupole mass detector (Agilent 5977B) and an Agilent HP-5MS capillary column (coating thickness 0.25 μm; 0.25 mm × 30 m).
The analytical conditions were as follows: the oven temperature varied from 60 to 240 °C at 3 °C/min; transfer-line temperature 240 °C; injector temperature 220 °C, and the carrier gas was helium with a flow of 1 ml/min.
The acquisition parameters were as follows: full scan; scan time: 1.0 s; scan range: 35–300 m/z; threshold: 1 count. The identification of the components was made by comparing their linear retention indices (LRIs) relative to the series of n-alkanes and by the comparison of their mass spectra with those of ADAMS and NIST 14 (Adams 2007; NIST 2014) commercial libraries and from an in-house mass-spectral library.
Antioxidant potential of Eucalyptus oils
Radical scavenging activity
Radical scavenging activity was studied by using a solution of 1,1- diphenyl-2-picrylhydrazyl radical (DPPH) following the method of Hanato et al. (1988). One ml of the essential oils diluted in methanol at different concentrations was added to 1 ml of a 0.2 mmol/l DPPH methanolic solution.
The mixture was shaken and incubated in the dark and at room temperature for 30 min, after which the absorbance was measured at 517 nm. The antiradical capacity was expressed as IC50 (mg/ml), the concentration required to cause a 50% DPPH inhibition. The ability of the essential oils to reduce the DPPH radical was calculated according to the following equation:
where A0: is the absorbance of the control (sample without oils) and A1: is the absorbance of the samples containing the oils.
All samples were analyzed in triplicate, and butylhydroxytoluene (BHT) was used as a positive control.
ABTS scavenging capacity
ABTS radical-scavenging capacity of the Eucalyptus volatile oils was assessed according to the method of Re et al. (1999). The ABTS radical cation was obtained by mixing 3.3 mg of 2.45 mM potassium persulfate solution and 19.2 mg of 7 mM ABTS solution, and the resulting emulsion was incubated at room temperature for 16 h in the dark. Subsequently, the ABTS solution was diluted with distilled water to an absorbance of 0.750 at 734 nm. Next, 160 µl of ABTS solution was added to 40 µl of the sample solution (essential oil in methanol) at different concentrations (6.25, 12.5, 25, 50, 100, 200 and 400 µg/ml). All the assays were made in triplicate. The decrease in absorbance was determined 10 min after an initial mixing for all samples. BHT was used as an antioxidant standard. The inhibition capacity of each concentration was calculated relative to a blank absorbance following the equation:
where AC(0) is the absorbance for the control; and AA(t) is the absorbance of the antioxidant (oils or BHT standard).
Herbicidal activity
Effects of Eucalyptus oils on seed germination and seedling growth.
Sinapis arvensis used in this work is important weed in Tunisian crops, with a wide distribution, many of which have developed herbicide resistant biotypes (Zargar et al. 2021). Lepidium sativum L., (garden cress) and Sinapis arvensis L., (field mustard) seeds were collected in a plot that was planted with field corn seed in Sidi Ismail, Beja, Tunisia, July 2020 (36° 35′ 58.6″ N 9° 06′ 24.1″ E). Seeds of Raphanus sativus L. (radish) seeds were purchased from AGRODIS company (Ben Arous 2013, Tunisia). These herbs were chosen for phytotoxicity assays because these species are widely used for herbicidal activities, and they are characterized by high and rapid germination rates (De Martino et al. 2010).
Before beginning the germination tests, the seeds were surface-sterilized for 5 min with 5% sodium hypochlorite and then, rinsed with distilled water. For each herb and oil tested, 20 seeds were placed in petri dishes (90 mm in diameter) between two layers of filter paper. Three replicates per treatment were made, and the operation was repeated three times. For each plant species and oil tested, 20 seeds were placed in petri dishes (90 mm in diameter) between two layers of filter paper, (three replicates per treatment), and the operation was repeated three times. After treatment with different concentrations (0, 1, 2, and 3 μl/ml) of volatile oil in a solution of Tween 20 (1%) or glyphosate (positive control), the Petri dishes were sealed with parafilm and incubated in a germination room (25 °C/16 h of light and 20 °C/8 h of darkness) (Tworkoski 2002). After 7 days, seed germination was assessed, and seedling shoot and root lengths were measured.
Antifungal activity
Fungal strains
Five fungal strains belonging to genus Fusarium were used for the tests. Four of the fungal strains (F. oxysporum, F. oxysporum f. matthioli, F. oxysporum MN-2 and F. oxysporum 184) were obtained from the University of California Los Angeles (UCLA), whereas F. redolens was acquired from the laboratory of plant protection of the Tunisian National Institute of Agronomic Research (INRAT). All the fungi were stored on potato dextrose agar (PDA, Sigma) at 4 °C.
In vitro antifungal activities on mycelial growth
The antifungal properties of the Eucalyptus oils were studied in Petri dishes using the contact assay method according to Cakir et al. (2004). The oils were dissolved in a Tween 20 solution (0.1% v/v) and then, added to 20 ml of PDA (Potato Dextrose Agar medium) at 50 °C to provide the required concentrations (4–10 µl/ml). A mycelial disk of 6 mm in diameter from fresh cultures of the studied fungi was placed in a separate PDA plate (90 mm diameter) and incubated at 24 °C for 7 days. PDA plates that were treated only with Tween 20 (0.1%) were used as the negative control. The experiments were conducted with three replicates per treatment. The antifungal activity was determined as the percentage of inhibition (PI) of mycelia growth compared to the control according to the following formula:
where dc and dt are the mean diameter of control growth and oil-treated fungi, respectively.
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC).
MIC is defined as the lowest dose at which there is complete inhibition of fungal growth. To establish the minimum fungicidal concentrations, the inhibited fungal disks (MICs) were inoculated into PDA Petri plates (without essential oil) and their growth was observed. After 3 days of incubation, the MFC was obtained as the lowest MIC at which no growth observed in the plates after culturing (Adjou et al. 2012).
Statistical analysis
The results were subjected to a one-way analysis of variance (ANOVA) using the SPSS 18.0 software package. Differences between means were tested through Student Newman–Keuls, and values with P ≤ 0.05 were considered significantly different. Before performing the ANOVA, we verified two essential conditions for the analysis of variance: homoscedasticity and normality of the distribution. The verification of the homoscedasticity was performed by linear regression, whereas the verification of the normality of distribution was done by the computation of Skewness or the kurtosis Z-values. In both cases, these two conditions were verified.
Results
The results of the chemical analysis of the essential oils extracted from the aerial parts of the three Tunisian Eucalyptus species are listed in Table 1. A total of 45 different compounds were identified: 26 from E. pimpiniana, 15 from E. polyanthemos, and 39 from E. oleosa, representing 99.4%, 99.4%, and 98.6% of the entire essential oil constituents, respectively. These volatiles can be classified into 5 groups (Table 1), namely monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes and non-terpene derivatives, and are listed by their LRI.
1,8-Cineole (35.3%) and β-eudesmol (25.5%) were the main components of the essential oil of E. pimpiniana, whereas 1,8-cineole (71.6%) and globulol (13.2%) characterized E. polyanthemos essential oil, 1,8-cineole (13.4%), spathulenol (11.9%) and β-eudesmol (8.5%) were the main constituents in the E. oleosa oil. Others important components of the EO of E. oleosa were phellandral, p-cymen-7-ol (syn. cumin alcohol), carvacrol, myrtenal, cumin aldehyde and cryptone, the combination of which allowed this oil to be distinguished from the EOs of the other two Eucalyptus species.
Antioxidant activity
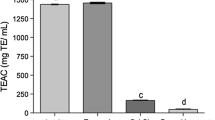
The antioxidant activities of the three essential oils were determined using two test systems, namely the ABTS and DPPH assays (Fig. 1). The results revealed moderate antioxidant activities for the three essential oils. No significant differences between the essential oils were observed in the ABTS assay. Conversely, these differences were statistically significant in the DPPH assay. In the latter case, the essential oil of E. oleosa was the most active, with an IC50 value of 92.179 ± 2.484, followed by E. pimpiniana (163.593 ± 15.122) and E. polyanthemos (359.688 ± 2.778).
Herbicidal activity
The herbicidal activities of the three essential oils are presented in Tables 2, 3, 4. A significant inhibition was observed on the germination of the seeds of the tested plants in a dose-dependent way and on the type of essential oil tested. In summary, the essential oils of two species (E. pimpiniana and E. oleosa) completely inhibited the germination of Sinapis arvensis and Raphanus sativus at 1 μl/ml. Conversely, E. polyanthemos essential oils inhibited the germination of the seeds of the same species by 75 and 55%, respectively. However, the essential oil from E. oleosa was the most active regarding seed germination resulting in complete inhibition of Sinapis arvensis at 0.5 μl/ml. For L. sativum, only E. oleosa essential oil reduced seed germination by 20–30% at 0.75 and 1 μl/ml, respectively. In contrast, the essential oils of E. pimpiniana and E. polyanthemos had no effect on seed germination on L. sativum at all the tested concentrations.
A significant reduction in the length of S. arvensis, L. sativum and R. sativus seedlings caused by treatment with the essential oils was observed at 0.5 μl/ml (Table 3). Inhibition was observed for more than 50% of the treated seedlings. For S. arvensis, no growth was observed after treatment at 1 μl/ml for each of the three oils. At all concentrations, a partial reduction in shoot growth was noted for R. sativus treated with E. polyanthemos essential oil. Seedling root lengths in the three species were significantly shortened using1µl/ml of any of the essential oils. E. pimpiniana and E. oleosa essential oils affected root lengths more than did E. polyanthemos EOs. The greatest inhibition of root length was observed for the E. oleosa EOs.
Antifungal activity
In vitro inhibition of fungal mycelium growth by essential oils of the three Eucalyptus species.
The effects of increasing concentrations of the three essential oils on mycelium growth of the different fungal strains are shown in Fig. 2 and summarized in Tables 5 and 6. All the essential oils inhibited the growth of the tested five fungal strains in a dose-dependent manner. The highest inhibitory activity was exhibited by the E. oleosa essential oil, with 100% inhibition of mycelium growth at 6 µl/ml for all fungal strains (Table 5). The fungal strains F. oxysporum MN-2 and F. oxysporum 184 were the most sensitive to the three essential oils when used at 6 µl/ml.
Minimum inhibitory concentration and minimum fungicidal concentration.
The minimum inhibitory concentration (MIC) is the lowest concentration capable of 100% inhibition of fungal mycelium growth (Table 6). The E. polyanthemos and E. pimpiniana oils MIC exhibited a range of 6–10 µl/ml for three of the five pathogens tested. To achieve a 100% inhibition of mycelial growth of F. oxysporum and F. oxysporum f. matthioli, a concentration of these oils greater than 10 µl/ml (Table 5) was required. For the E. oleosa oil, the MIC was 6 µl/ml for all the fungal strains. E. oleosa essential oil was shown to be the most lethal, with a minimum fungicidal concentration (MFC) between 6 and 8 µl/ml for the different pathogens. The second most toxic was E. pimpiniana oil, with an MFC between 6 and 10 µl/ml against the three out five fungal strains (Table 6). Eucalyptus polyanthemos oil exhibited a fungicidal activity on F. oxysporum 184 at the highest concentration (10 µl/ml). Overall, F. oxysporum and F. oxysporum f. matthioli were more tolerant of the three tested EOs.
Discussion
In this report, we describe the chemical composition of E. pimpiniana and E. polyanthemos essential oils and compare them to E. oleosa, which grows in the same region. Few studies on E. oleosa essential oil exist in the literature, and only one study has been published that is comparable to our results (Ben Marzoug et al. 2011). These authors identified 38 compounds accounting for 99.1% of the essential oil isolated from the leaves of E. oleosa. Their results further showed that the oil contained about twice the amount of sesquiterpenes compared to monoterpenes (44.3% and 28.7%, respectively). The main oxygenated sesquiterpenes were spathulenol (16.1%) and γ-eudesmol (25.0%), followed by the monoterpenes p-cymene (10.6%), 1,8-cineole (8.7%), p-cymen-8-ol (4.4%), cis-sabinol (4.2%), p-cymen-7-ol (4.0%) and verbenone (3.7%). In our study, the oil contains the same compounds, but in very different proportions. These differences depend on various factors, such as geographical origin, soil conditions, harvesting period, and the physiological state of the plant.
According to the DPPH and ABTS assay results, moderate antioxidant activities were observed for the three essential oils, with some differences depending on the species. E. oleosa EO exhibited the highest DPPH antiradical activity (IC50 = 92.179 ± 2.484 mg/ml), which is lower than the reference antioxidant, vitamin C. Its antioxidant activity has been reported to be related to the monoterpene hydrocarbon content (Marzoug et al. 2010). The essential oil of E. oleosa leaves contains the highest level of monoterpenoids (10.7%), indicating a potential for having the highest antioxidant activity.
We found that Eucalyptus oils exhibited herbicidal activity for all three plants tested. Our results are consistent with previous work showing herbicidal effects of Eucalyptus essential oils against several weeds and crop plants (Batish et al. 2006; Singh et al. 2005). Thus, the compounds contained in Eucalyptus oils are strong candidates for use as natural herbicides. Nevertheless, the phytotoxic activity of Eucalyptus oils results in damage to some crops (Zengh and Li 1997). It is therefore essential to maximize the herbicidal activity of Eucalyptus oils against weeds while minimizing the negative impacts on crop growth. All the three studied species of Eucalyptus showed contain appreciable percentages of 1,8-cineole, a monoterpene with phytotoxic properties (Singh et al. 2006; Zhang et al. 2012; Romagni et al. 2000). However, based on our data, this compound alone cannot explain the differences in the inhibition of seed germination, root, and aerial part growth observed among the three different essential oils. In fact, no correlation was observed between 1,8-cineole contents and the inhibition of these plant-associated parameters. The greatest inhibition was obtained using E. oleosa essential oil, which has the lowest level of this compound, confirming that the observed herbicidal activities do not primarily depend on 1,8-cineole and further suggests that other minor compounds may be involved. In previous reports (De Martino et al. 2010; Nishida et al. 2005), many other monoterpene hydrocarbons earlier identified in eucalypt essential oils, such as α-pinene, β-pinene, and limonene, possess herbicidal activity. Recently and for the first time, the compound trans-pinocarveol was described by Li et al. (2020) for its herbicidal effect. In our study, these compounds are more abundant or present only in the eucalypt species that have the most active essential oils. In addition, we noted remarkable levels of oxygenated sesquiterpenes such as α-, β- and γ-eudesmol in both E. oleosa and E. pimpiniana, whereas they are absent in E. polyanthemos. These results strongly suggest that these constituents may confer herbicidal activities to essential oils. Moreover, the results of this study showed that the more active essential oil (E. oleosa) contains distinctive compounds, such as isospathulenol and cryptone, which are also known for their herbicidal effects (Fouad et al. 2015). All these findings confirm a synergy between the various constituents of essential oils for the observed phytotoxic effect.
To explain the mode of action of essential oils on the inhibition of germination and as reported in the literature, different physiological and biochemical mechanisms may be explained by the fact that these monoterpenes affect physiological processes, such as cell viability, enzyme activity, and organelle reduction following membrane rupture (Mahdavikia et al. 2017; Fagodia et al. 2017). Other studies have reported that essential oils cause reduced germination and seedling growth, as well as several metabolic alterations: e.g., altering the assimilation of nitrogen into amino acids, which in turn affects glutamine metabolism and, consequently, results in an excess of toxic ammonia that accumulates in the leaves. These symptoms are associated with oxidative stress and damage and decrease the efficiency of the photosynthetic apparatus and alter the photorespiratory pathway (Araniti et al. 2018).
The essential oils of the three Eucalyptus species resulted in antifungal activity for all five of the Fusarium strains tested: F. oxysporum, F. oxysporum f. matthioli, F. oxysporum MN-2, F. oxysporum 184, and F. redolens. Mycelial growth inhibition is known to be dependent on essential oil concentrations. The results of our study are consistent with those of previous studies that evaluated the antifungal activity of against various phytopathogenic fungi of essential oils of eucalyptus species with different chemical profiles. In earlier results, Katooli et al. (2011) evaluated whether E. camaldulensis EOs had a suppressive action on the growth of the mycelia of some postharvest pathogenic fungi and soil pathogenic fungi and reported complete inhibition of mycelial growth in Pythium ultimum and Rhizoctonia solani (J.G. Kühn) by all concentrations tested (25, 50, 75 and 100%). After an additional 30 days of incubation, complete inhibition of Bipolaris sorokiniana (Shoemaker) and Colletotrichum gloeosporioides (Penz. & Sacc.) occurred, but no inhibition was recorded for Penicillium digitatum (Sacc.) and Aspergillus flavus (Link). Studies on E. camaldulensis EO antifungal activity against Fusarium graminearum (Schwabe) and Fusarium sporotrichioides (Sherb.) demonstrated a dose-dependent activity ranging between 0 and 34.1% for F. sporotrichioides and between 29.1 and 41.8% for F. graminearum (Mehani et al. 2014). In another study, the inhibitory activity of E. camaldulensis EO against several strains of species of phytopathogenic fungi showed mycelial growth inhibition up to 100% at concentration of 5 mg/ml against F. oxysporum (Schltdl.) and Thanatephorus cucumeris (A.B. Frank) and against Chaetomium globosum (Kunze) at 10 mg/ml Siramon et al. 2013). More recent work on eucalyptus species in Tunisia has shown that essential oils inhibit the growth of 8 phytopathogenic fungi and inhibition can be complete at a concentration of 4 μl/ml (Amri et al. 2023). Another study showed that E. camaldulensis EOs inhibited fungal growth with MICs ranging from 7 to 12 µl/ml depending on the fungal strain (Abo Elgat et al. 2020).
In this study, only E. oleosa showed fungicidal activity against all five Fusarium strains tested, whereas EOs obtained from E. pimpiniana were fungicidal only against two of the five fungi, F. oxysporum MN-2 and F. oxysporum 184. The EOs of E. polyanthemos and E. pimpiniana, at the four different concentrations studied, did not exhibit fungicidal activity toward F. oxysporum f. matthioli and F. redolens. Consequently, higher concentrations should be tested to determine the MFC of these oils against these two fungi. Disk bioassays revealed that the three essential oils inhibited fungal growth in a dose-dependent manner. There are many reports in the literature on the concentration-dependent antifungal activity of essential oil (Rana et al. 2011; Aguiar et al. 2014).
Based on the chemical composition of the different EOs isolated, and based on the literature, antifungal activity is ascribed to α-pinene, trans-pinocarveol, pinocarvone, aromadendrene, globulol, 1-epicubenol, eudesmols (α-, β- and γ-), and spathulenol. Many researchers have described these components as antifungal agents (Tan et al. 2016). They are mainly found in E. oleosa and E. pimpiniana which, accordingly, showed the highest antifungal activities. Compounds such as 4-terpineol, myrtenal, cumin aldehyde, phellandral, p-cymene, carvacrol, 1-epicubenol, γ-eudesmol, isospathulenol, cryptone, p-cumenol and isoamyl benzoate were found only in E. oleosa and could explain the significant antifungal effect observed for its EOs. However, synergistic effects with other compounds cannot be ignored.
Conclusion
This work reports to our knowledge for the first time the antifungal and potential herbicidal effects of essential oils obtained from the leaves of three different Eucalyptus species, E. oleosa, E. pimpiniana, and E. polyanthemos were tested against five phytopathogenic fungi and one weed and two cultivated species belonging to the same family. The essential oil of E. oleosa, in particular, was found to the most active against the tested fungal pathogens and reduced seed germination rate and inhibited seedling growth. This EO also had moderate antioxidant activity. The main constituents of the essential oil of E. oleosa are the terpenes 4-terpineol, myrtenal, cumin aldehyde, phellandral, p-cymene, carvacrol, 1-epicubenol, γ-eudesmol, isospathulenol and the non-terpene derivatives cryptone, p-cumenol and isoamyl benzoate. Hence, the possibility of using these essential oils as ingredients in the formulation of sustainable herbicides to reduce the use of potentially environmentally harmful synthetic pesticides should be pursued further. Essential oils as well as plant extracts have proven their antimicrobial powers in order to use them as a bioherbicide it would be interesting to verify their effects on the environment, cultivated crops, and the beneficial microorganisms of the soil. Employing novel biomolecules in biopesticide formulations will provide a less polluting approach and more sustainable weed and fungus management alternatives for growing food for the world’s population.
References
Abo Elgat WAA, Kordy AM, Böhm M, Černý R, Abdel-Megeed A, Salem MZM (2020) Eucalyptus camaldulensis, Citrus aurantium, and Citrus sinensis essential oils as antifungal activity against Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Fusarium culmorum. Processes 8(8):1003. https://doi.org/10.3390/pr8081003
Abril-Sánchez C, Matencio A, Navarro-Orcajada S, García-Carmona F, López-Nicolás JM (2019) Evaluation of the properties of the essential oil citronellal nano-encapsulated by cyclodextrins. Chem Phys Lipids 219:72–78. https://doi.org/10.1016/j.chemphyslip.2019.02.001
Adams RP (2007) Identification of essential oil components by gas chromatography/ mass spectrometry, 4th edn. Allured Publ, Carol Stream
Adjou ES, Kouton S, Dahouenon-Ahoussi E, Sohounhloue CK, Soumanou MM (2012) Antifungal activity of Ocimumcanum essential oil against toxinogenic fungi isolated from peanut seeds in post-harvest in Benin. Int Res J Biol Sci 1(7):20–26
Aguiar RWDS, Ootani MA, Ascencio SD, Ferreira TPS, Santos MMD, Santos GRD (2014) Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci World J. https://doi.org/10.1155/2014/492138
Ampong-Nyarko K, De Data SKA (1991) Handbook for weed control in rice international rice. Research Institute, Manila Philipines
Amri I, Hamrouni L, Hanana M, Jamoussi B (2011) Chemical composition of Juniperus oxycedrus L subspmacrocarpa essential oil and study of their herbicidal effects on germination and seedling growth of weeds. Asian J Applies Sci 8:771–779. https://doi.org/10.3923/ajaps.2011.771.779
Amri I, Hamrouni L, Hanana M, Jamoussi B (2012) Herbicidal potential of essential oils from three Mediterranean trees on different weeds. Curr Bioact Compd 8:3–12
Amri I, Gargouri S, Hamrouni L, Hanana M, Fezzani T, Jamoussi B (2013) Comparative study of two coniferous species (Pinus pinaster aiton and cupressus sempervirens L var dupreziana [A Camus] Silba) essential oils: chemical composition and biological activity. Chil J Agric Res 73:259–266. https://doi.org/10.4067/S0718-58392013000300008
Amri I, Kouki H, Mabrouk Y, Hanana M, Jamoussi B, Hamrouni L (2021) Essential oils of Tunisian Pinus radiata D Don chemical composition and study of their herbicidal activity. Vietnam J Chem 59:247–252. https://doi.org/10.1002/vjch.202000103
Amri I, Khammassi M, Ayed RB, Khedhri S, Mansour MB, Kochti O, Pieracci Y, Flamini G, Mabrouk Y, Gargouri S, Hnana M, Hamrouni L (2023) Essential oils and biological activities of Eucalyptus falcata, E. sideroxylon and E. citriodora growing in Tunisia. Plants 12:816. https://doi.org/10.3390/plants12040816
Aragão FB, Palmieri MJ, Ferreira A, Costa AV, Queiroz VT, Pinheiro PF, Andrade-Vieira LF (2015) Phytotoxic and cytotoxic effects of eucalyptus essential oil on lettuce (Lactuca sativa L). Allelopath J 35:259–272
Araniti F, Costas-Gil A, Cabeiras-Freijanes L, Lupini A, Sunseri F, Reigosa MJ, Abenavoli MR, Sánchez-Moreiras AM (2018) Rosmarinic acid induces programmed cell death in Arabidopsis seedlings through reactive oxygen species and mitochondrial dysfunction. PLoS One 13(12):e0208802. https://doi.org/10.1371/journal.pone.0208802
Arias-Estevez M, Lopez-Periago E, Martinez-Carballo E (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agr Ecosyst Environ 123:247–260. https://doi.org/10.1016/j.agee.2007.07.011
Barton A (2000) The oil mallee project: a multifaceted industrial ecology case study. J Ind Ecol 3:161–176. https://doi.org/10.1162/108819899569467
Batish DR, Singh HP, Nidhi S, Shalinder K, Kohli RK (2006) Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Z Naturforsch 61:52–56. https://doi.org/10.1515/znc-2006-1-210
Ben Marzoug HN, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, Khouja ML, Bouajila J (2011) Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16(2):1695–1709. https://doi.org/10.3390/molecules16021695
Brown B, Hoshide AK, Gallandt ER (2019) An economic comparison of weed management systems used in small-scale organic vegetable production. Org Agric 9:53–63. https://doi.org/10.1007/s13165-018-0206-1
Cakir A, Kordali S, Zengin H, Izumi S, Hirata T (2004) Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour Fragr J 19:62–68. https://doi.org/10.1002/ffj.1279
Davies JH, Moses J (2019) Insect repellent composition and method of use US Patent US2019/0037840 A1
De Martino L, Mancini E, Almeida LFR, De Feo V (2010) The antigerminative activity of twenty-seven monoterpenes. Molecules 15:6630–6637. https://doi.org/10.3390/molecules15096630
Fagodia SK, Singh HP, Batish DR, Kohli RK (2017) Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: limonene and citral. Ind Crops Prod 108:708–715. https://doi.org/10.1016/j.indcrop.2017.07.005
Fouad R, Bousta D, Lalami AEO, Chahdi FO, Amri I, Jamoussi B, Greche G (2015) Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (DC) stapf, Eucalyptus cladocalyx, Origanum vulgare L and Artemisia absinthium L cultivated in Morocco. J Essent Oil-Bear Plants 18(1):112–123. https://doi.org/10.1080/0972060X.2014.901631
Hamrouni L, Hanana M, Amri I, Romane A, Gargouri S, Bassem J (2014) Allelopathic effects of essential oils of Pinus halepensis Miller: chemical composition and study of their antifungal and herbicidal activities. Arch Phytopathol Plant Protec. https://doi.org/10.1080/03235408.2014.884667
Hanana M, Ben Mansour M, Algabr M, Amri I, Gargouri S, Romane A, Jamoussi B, Hamrouni L (2017) potential use of essential oils from four Tunisian species of 1 Lamiaceae: biological alternative for fungal and weed control. Rec Nat Prod 11:258–269
Hanato T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharm Bull 36(6):2090–2097. https://doi.org/10.1248/cpb.36.2090
Hazrati H, Saharkhiz MJ, Moein MR, Khoshghal H (2018) Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal Agric Biotechnol 13:204–212. https://doi.org/10.1016/j.bcab.2017.12.014
Heap I, Le Baron H (2001) Introduction and overview of resistance. In: Powles SB, Shaner DL (eds) Herbicide resistance in world grains. CRC Press, Boca Raton, pp 1–22
Hemissi I, Mabrouk Y, Mejri S, Saidi M, Sifi B (2013) Enhanced defence responses of chickpea plants against Rhizoctonia solani by pre-inoculation with Rhizobia. J Phytopathol 161(6):412–418. https://doi.org/10.1111/jph.12071
Ibáñez MD, Blázquez MA (2019) Tea tree and wintergreen essential oils in the management of the invasive species Cortaderiaselloana and Nicotiana glauca. J Plant Prot Res 59(2):160–169. https://doi.org/10.24425/jppr.2019.129281
Katooli N, Maghsodlo R, Razavi SE (2011) Evaluation of eucalyptus essential oil against some plant pathogenic fungi. J Plant Breed Crop Sci 3(2):41–43
Li A, Wu H, Feng Y, Deng S, Hou A, Che F, Liu Y, Geng Q, Ni H, Wei Y (2020) A strategy of rapidly screening out herbicidal chemicals from Eucalyptus essential oils. Pest Manag Sci 76:917–927. https://doi.org/10.1002/ps.5597
Loying R, Gogoi R, Sarma N, Borah A, Munda S, Pandey SK, Lal M (2019) chemical compositions, in-vitro antioxidant, anti-microbial, anti-inflammatory and cytotoxic activities of essential oil of Acorus calamus L rhizome from North-East India. J Essent Oil-Bear Plants 22(5):1299–1312. https://doi.org/10.1080/0972060X.2019.1696236
Mabrouk Y, Zourgui L, Sifi S, Belhadj O (2007) The potential of Rhizobium strains for biological control of Orobanche crenata. Biologia 62:139–143. https://doi.org/10.2478/s11756-007-0021-8
Mabrouk Y, Mejri S, Belhadj O (2016) Biochemical mechanisms of induced resistance by rhizobial lipopolysaccharide in pea against crenate broomrape. Rev Bras Bot 39(1):107–114
Mahdavikia F, Saharkhiz MJ, Karami A (2017) Defensive response of radish seedlings to the oxidative stress arising from phenolic compounds in the extract of peppermint (Mentha x piperita L). Sci Hortic 214:133–140. https://doi.org/10.1016/j.scienta.2016.11.029
Marzoug HNB, Bouajila J, Ennajar M, Lebrihi A, Romdhane M (2010) Eucalyptus (gracilis, oleosa, salubris, and salmon ophloia) essential oils: their chemical composition and antioxidant and antimicrobial activities. J Med Food 13:1005–1012. https://doi.org/10.1089/jmf.2009.0153
Mehani M, Salhi N, Valeria T, Ladjel S (2014) Antifungal effects of essential oil of Eucalyptus camaldulensis plant on Fusarium graminearum and Fusarium sporotrichioides. Int J Curr Res 6(12):10795–10797
National Institute of Standards and Technology NIST/EPA/NIH Mass Spectral Library (2014) The NIST Mass Spectrometry Data Center, Gaithersburg
Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A (2005) Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J Chem Ecol 31:1187–1203. https://doi.org/10.1007/s10886-005-4256-y
Ootani MA, Reis MR, Sander A, Cangussu R, Capone A, Fidelis RR, Oliveira W, Barros HB, Caeser A, Portella F, Aguiar RS, Santos WF (2017) Phytotoxic effects of essential oils in controlling weed species Digitaria horizontalis and Cenchrus echinatus. Biocatal Agric Biotechnol 12:59–65. https://doi.org/10.1016/j.bcab.2017.08.016
Rana IS, Rana AS, Rajak RC (2011) Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L) by extraction, purification and analysis of its main component eugenol. Braz J Microbiol 42(4):1269–1277. https://doi.org/10.1590/S1517-83822011000400004
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Romagni JG, Allen SN, Dayan FE (2000) Allelopathic effects of volatile cineoles on two weedy plant species. J Chem Ecol 26(1):303–313
Singh HP, Batish DR, Setia N, Kohli RK (2005) Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann Appl Biol 146:189–194. https://doi.org/10.1111/j.1744-7348.2005.04018.x
Singh HP, Batish RD, Kaur S, Arora K, Kohli KR (2006) α-Pinene inhibits growth and induces oxidative stress in roots. Ann Bot 98:1261–1269. https://doi.org/10.1093/aob/mcl213
Siramon P, Ohtani Y, Ichiura H (2013) Chemical composition and antifungal property of Eucalyptus camaldulensis leaf oils from Thailand. Rec Nat Prod 7(1):49–53
Smith CJ, Perfetti TA (2020) A Comparison of the persistence, toxicity, and exposure to high volume natural plant-derived and synthetic pesticides. Toxicol Res Appl 4:1–15. https://doi.org/10.1177/2397847320940561
Steven GR, Thomas JM, Weber JB (1991) Influence of soil moisture on phytotoxicity of cinmethylin to various crops. Weed Sci 39(3):402–407
Tan N, Satana D, Sen B, Tan E, Altan HB, Demirci B, Uzun M (2016) Antimycobacterial and antifungal activities of selected four salvia species. Rec Nat Prod 10:593–603
Tolba H, Moghrani H, Benelmouk A, Kellou D, Maachi R (2015) Essential oil of Algerian Eucalyptus citriodora: chemical composition, antifungal activity. J Mycol Med 25:e128–e133. https://doi.org/10.1016/j.mycmed.2015.10.009
Tolba H, Moghrani H, Aboun A, Maachi R (2018) Essential oil of Algerian Eucalyptus citriodora: chemical composition and antimicrobial activities. Nat Technol J 18:19–27
Tworkoski T (2002) Herbicide effects of essential oils. Weed Sci 50:425–431. https://doi.org/10.1614/0043-1745(2002)050[0425:HEOEO]2.0.CO;2
Tyagi AK, Malik A (2011) Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem 126(1):228–235. https://doi.org/10.1016/j.foodchem.2010.11.002
Vázquez G, Fontenla E, Santos J, Freire MS, González-Álvarez J, Antorrena G (2008) Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crops Prod 28(3):279–285. https://doi.org/10.1016/j.indcrop.2008.03.003
Zaibet L (2016) Potentials of non-wood forest products for value chain development, value addition and development of NWFP-based rural microenterprises. FAO, Tunisia
Zargar M, Kavhiza NJ, Bayat M, Pakina E (2021) Wild mustard (Sinapis arvensis) competition and control in rain-fed spring wheat (Triticum aestivum L.). Agronomy. 11:2306. https://doi.org/10.3390/agronomy11112306
Zeng RS, Li PW (1997) Allelopathic Effect of Eucalytus Exserta and E Urophylla. J South China Agric Univ 18(1):6–10
Zhang J, An M, Wu H, Liu DL, Stanton R (2012) Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silver leaf nightshade (Solanum elaeagnifolium Cav) in Australia. Plant Growth Regul 68:231–237. https://doi.org/10.1007/s10725-012-9711-5
Zubrod JP, Bundschuh M, Arts G, Brühl CA, Imfeld G, Knäbel A, Payraudeau S, Rasmussen JJ, Rohr J, Scharmüller A, Smalling K, Stehle S, Schulz R, Schäfer RB (2019) Fungicides: an overlooked pesticide class? Environ Sci Technol. https://doi.org/10.1021/acs.est.8b04392
Acknowledgements
This research was supported by Laboratory project LR16CNSTN01 funded by the Tunisian Ministry of Higher Education and Scientific Research. Additional funding from a UCLA Faculty Award to AMH also supported this research. We thank Dr Rayda Ben Ayed, Laboratory of Extremophile Plants, Centre of Biotechnology of Borj-Cédria for performing the statistical analyzes of the data.
Author information
Authors and Affiliations
Contributions
IA and YM conceived and designed the experiments. HK, MS, YP and GF performed experiments and statistical analysis. IA, MS, HK and GF contributed reagents/material tools. HK, YM, IA, YP and GF analyzed the data. HK, IA, YM, AMH, and GF contributed to manuscript preparation and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kouki, H., Amri, I., Souihi, M. et al. Chemical composition, antioxidant, herbicidal and antifungal activities of leaf essential oils from three Tunisian Eucalyptus species. J Plant Dis Prot 130, 1411–1422 (2023). https://doi.org/10.1007/s41348-023-00772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00772-2