Abstract
Phytotoxicity and chemical composition of essential oils from four selected Eucalyptus species in Australia were investigated. Essential oils had stronger inhibitory effects on germination and seedling growth of silverleaf nightshade (Solanum elaeagnifolium Cav.) when compared with a commercial eucalyptus oil and with 1,8-cineole. E. salubris oil had the highest inhibition index for silverleaf nightshade germination, root growth and shoot growth, while E. spathulata had the lowest inhibitory effect except root growth. Gas chromatography-mass spectrometry analysis revealed 56 compounds present in E. salubris oil, with 1,8-cineole (57.6 %), α-pinene (10.9 %) and p-cymene (8.3 %) predominant. E. dundasii oil contained 55 identified compounds with 1,8-cineole (65.5 %) and α-pinene (19.9 %) being the richest fractions. There were 56 compounds identified from E. brockwayii oil with α-pinene (31.1 %), isopentyl isovalerate (20.2 %) and 1,8-cineole (16.9 %) as the most abundant components. E. spathulata oil contained 60 compounds, predominantly 1,8-cineole (52.9 %) and α-pinene (31.0 %). Further study is required to determine the phytoxicity of the individual identified compounds on silverleaf nightshade and whether the observed phytotoxicity is attributable to a single compound or to the synergistic effects of several compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus belongs to the myrtle (Myrtaceae) family. While typically native to Australia, a small number of species are indigenous to neighbouring countries, such as Papua New Guinea and Indonesia (Coppen 2002). Essential oils from eucalyptus have many traditional uses and potential commercial implications (Kandasamy et al. 2000; Zhang et al. 2010). They can be used as a folk medicine and have been reported to have a range of bioactivity, including antimicrobial, antiviral, fungicidal, insecticidal, anti-inflammatory, anti-nociceptive, anti-oxidant and phytotoxic activity (Duke 1983).
The phytotoxic activity of eucalyptus essential oils suggests that they may have potential commercial value as natural herbicides (Zhang et al. 2010). Setia et al. (2007) reported that volatile essential oils from E. citriodora were phytotoxic to the germination and growth of a number of weed species, such as Bidens pilosa, Amaranthus viridis, Rumex nepalensis and Leucaena leucocephala. Similarly, Ramezani et al. (2008) reported that essential oils from E. nicholii strongly inhibited the germination of Amaranthus retroflexus, Portulaca oleracea and Acroptilon repens. The herbicidal activity of eucalyptus essential oils against Parthenium hysterophorus, Cassia occidentalis, Echinochloa crus-galli and A. viridis has also been documented (Batish et al. 2004, 2006; Singh et al. 2006).
Silverleaf nightshade (Solanum elaeagnifolium Cav.) has become a serious problem in Australia, in particular in New South Wales, Victoria and South Australia (Stanton et al. 2009). It is a deep-rooted, summer-growing perennial weed of the Solanaceae family that is a declared noxious weed in several countries, including Australia, South Africa and approximately 20 states of the USA (OEPP/EPPO 2007; USDA-NRCS 2005). The management of this weed includes cultural, mechanical, chemical and biological controls (OEPP/EPPO 2007). However, the weed is very difficult to control, possibly due to the strong regenerative ability of the root system. In the absence of reliable and effective control options, alternative control options are needed for the effective management of this weed.
Field observations have identified that there is limited vegetation within the dripline of four Eucalyptus species: E. salubris, E.dundasii, E. brockwayii and E. spathulata. The presence of these Eucalyptus species in silverleaf nightshade infested roadside areas has assisted with the management of the weed. It is suspected that essential oils from the four special Eucalyptus species may play a role in suppressing silverleaf nightshade although the suppression may also be associated with other factors such as competition for resources. Therefore, this study was conducted to firstly determine the phytotoxicity of the above four eucalyptus essential oils against silverleaf nightshade, in comparison with a commercial eucalyptus essential oil, and 1,8-cineole, one of major components in most eucalyptus essential oils. Secondly, the chemical compositions of the essential oils from the four Eucalyptus species were determined and compared.
Methods and materials
Plant materials and chemicals
Approximately 2 kg of fresh leaves of E. salubris, E.dundasi, E. brockwayii and E. spathulata were collected from the field at Ungarie (Long. 146°55′41.33″, Lat. 33°35′53.06″), New South Wales (NSW), Australia. The leaves were then stored in a cool room (10 °C) before the extraction of essential oil. Seeds of silverleaf nightshade were collected in April 2008 from a silverleaf nightshade site at Culcairn (Long. 147°10′7.75″, Lat. 35° 35′38.11″), NSW. The seeds were dried and stored in a glass jar at the room temperature prior to the seed germination bioassays in November 2009. The seeds collected had 97 % viability in a tetrazolium assay. A commercial eucalyptus oil was purchased from a local super market (Woolworths, Australia) and 1,8-cineole was purchased from Sigma-Aldrich Pty. Ltd (Castle Hill, Australia).
Extraction of essential oils
Essential oils were extracted according to Batish et al. (2006) with some modifications. Three hundred grams of fresh leaves of eucalyptus leaves were cut into 5 mm strips and subjected to steam-distillation for 2.5 h using a Pyrex oil distillation apparatus with a flat bottom flask (2 L) containing 1,200 mL distilled water to generate steam. The volatile components from the leaves were condensed through a cooling tube. A separation funnel was used to collect the distilled essential oil, which was then dried over anhydrous sodium sulfate and stored in sealed vials at 4 °C before use.
Bioassays of essential oils on weed germination and growth
A previous bioassay protocol (Batish et al. 2004) was adopted with slight modifications. Seeds of silverleaf nightshade were dipped in distilled water for 5 h prior to germination bioassays. Fifty seeds were placed in a 9-cm Petri dish lined with one layer of Whatman No.1 filter paper moistened with 5 mL of distilled water. To test the inhibitory effects of essential oils and the pure compound (1,8-cineole), an aliquot of 0, 10, 30, 90 and 270 μL essential oil were loaded using an Eppendorf micro pipette onto a piece of filter paper (2 × 2 cm) attached to the inner side of the cover of the Petri dish. The Petri dishes were then sealed with parafilm and maintained in a growth incubator with a diurnal temperature cycle of 25 °C in light and 15 °C in dark and a 12 h photoperiod. A randomized complete block design with three replicates was used. Seeds with >1 mm radical growth were considered as germinated and seedling length measured after 20 days of incubation.
Chemical analysis of essential oils
The essential oils were analysed by gas chromatography (GC)-mass spectrometry (MS) with the use of J & W DB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm) in a Varian 3800 gas chromatograph directly coupled to a Varian Saturn 2000 Ion Trap (ITD) mass spectrometer controlled by a Saturn GC/MS workstation (v5.2). Gas chromatography operating conditions followed those described by Adams (1995): 240 °C injector and transfer line temperature; 60–250 °C at 3 °C/min oven temperature, with a final hold time of 8.67 min at 250 °C (total run time 72.0 min); Helium carrier gas; 0.2 μL sample injection volume; 1:20 split ratio. Mass spectrometry acquisition parameters were: full scan with scan range 41–415 amu; 1.0 s scan time; 1 count threshold; AGC mode on; 5 microscans; 1.8 min filament delay. Column head pressure was adjusted to 13.0 psi.
Compounds were identified by comparing their Kovats indices (KI), retention times and mass spectra with Adams (1995), aided with NIST mass spectra library. Quantification of essential oil components (expressed as percentage of total peak area of chromatogram) was carried out by peak area normalisation measurements.
Statistical analysis
The dose–response data were subjected to the analysis of whole-range assessment proposed by An et al. (2005). The whole-range assessment considers overall effect/response across the whole range of application rates, instead of assessing the effect of each individual rate on test species. The program WESIA (Whole-range Evaluation of the Strength of Inhibition in Allelopathic-bioassay) developed by Liu et al. (2007) was used to calculate the inhibition index. The inhibition index is a summary of the overall biological response of an organism to a tested allelochemical or equivalent and provides a relative strength indicator of biological response. Large values indicate that the species is sensitive or that the allelochemical possesses strong allelopathic potential/biological activity, whilst small values indicate tolerance or weak potential/biological activity.
Principle Component Analysis (PCA) was used to analyze the chromatogram profiles and to provide supplementary analysis on the chemical differences between essential oils tested. The software for PCA followed Hammer et al. (2001).
Results
Bioassays of essential oils on weed germination and growth
All essential oils tested inhibited the germination of silverleaf nightshade, depending on the species (Table 1). The inhibition varied between the essential oils of different Eucalyptus species. The essential oil from E. salubris had the highest inhibitory activity on silverleaf nightshade germination, with a germination inhibition index of 73 %, whereas the commercial essential oil purchased from the market was the least, with an inhibition index of only 38 %. The essential oils of the four selected Eucalyptus species had a higher inhibition than that of the commercial essential oil or the pure 1,8-cineole. The inhibition potential was ranked in a decreasing order as E. salubris oil, E. dundasii oil, E. brockwayi oil, E. spathulata oil, 1,8-cineole and commercial oil based on the whole range assessment. These results are similar to the reported effects of other eucalyptus essential oils on weeds (Batish et al. 2004, 2006; Setia et al. 2007) although neither the phytotoxicity of eucalyptus essential oils on silverleaf nightshade nor the phytotoxic effects of essential oils from these four eucalyptus species on other weeds have been reported previously.
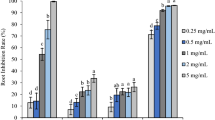
The inhibitory effect increased as the dose of the essential oil increased (Fig. 1). The germination of silverleaf nightshade was decreased by more than 50 % at a dose of 270 μL/dish for all oils.
The bioassays also showed that the seedling root growth of silverleaf nightshade was suppressed by all essential oils (Table 1, Fig. 2). Increasing essential oils dose levels resulted in higher inhibitory effects on silverleaf nightshade. E. salubris essential oil was the most inhibitory oil, reducing root growth by 84 % when applied at 10 μL/dish. The commercial essential oil showed the least inhibitory activity, causing only 41 % reduction in root length at the same dose and only 59 % reduction at 270 μL/dish. The inhibition potential was ranked in a decreasing order as E. salubris oil, E. dundasii oil, E. spathulata oil, E. brockwayii oil, 1,8-cineole and commercial essential oil (Table 1) based on the whole range assessment.
The essential oils also significantly suppressed the shoot growth of silverleaf nightshade seedling (Table 1, Fig. 3). This inhibition became more severe with increased dose used, but different degrees of inhibition were observed between the essential oils. The application of the essential oil of E. salubris at 10 μL/dish resulted in more than 80 % inhibition on the shoot growth of silverleaf nightshade, while the commercial essential oil had only 54 % reduction in the shoot growth even at the highest dose of 270 μL/dish. The inhibition potential was ranked in a decreasing order similar to the germination inhibition reported above.
Chemical analysis of essential oils by GC–MS
Each essential oil has a distinct chemical profile (Fig. 4). The composition of essential oils, the content of main compounds and ratio of each individual component varied considerably between the species (Table 2). This was further demonstrated by the comprehensive PCA analysis (Fig. 5), which showed that five essential oils were distinctly separated from each other by the PCA first principle component, accounting for 83 % of the total variance. 1,8-Cineole was the most abundant component for all essential oils except E. brockwayii oil. The selected Eucalyptus species in the decreasing order of 1,8-cineole content were E. dundasii, E. salubris, E. spathulata and E. Brockwayii (Table 2). The commercial eucalyptus oil contained a higher 1,8-cineole level than the four species tested.
A total of 55 compounds were identified in the essential oil extracted from the leaves of E. dundasii. The dominant components were 1,8-cineole (65.5 %), and α-pinene (19.9 %). This result did not quite agree with the previous work by Bignell et al. (1996a), who reported that 1,8-cineole content was 34.4 %. A number of factors could contribute to this variation such as the sub-species variant, sites and extraction method/time (Zhang et al. 2010). The essential oil of E. salubris consisted of 56 identifiable compounds, with the 1,8-cineole (57.6 %), α-pinene (10.9 %) and p-cymene (8.3 %) being the main component. The content of 1,8-cineole is a little higher than that in the previous report (Bignell et al. 1996b), in which the value is 48.8 %. There were 60 compounds identified in the essential oil of E. spathulata, with the predominant compounds being 1,8-cineole (52.9 %) and α-pinene (31.0 %). Our result was in agreement with the previous work (Fathi et al. 2009) but 37 more compounds were identified. In the essential oil of E. brockwayii, 56 compounds were identified with α-pinene (31.1 %), isopentyl isovalerate (20.2 %) and 1,8-cineole (16.9 %) as the most abundant components. Instead of 1,8-cineole, α-pinene was the dominant constituents. This composition pattern was similar to the work by Bignell et al. (1996b).
Discussion
All essential oils tested inhibited the germination and seedling growth of silverleaf nightshade, but different degrees of inhibition were observed between the essential oils. The essential oils from E. salubris oil, E. dundasii oil, E. brockwayi oil and E. spathulata had stronger phytotoxic effect on silverleaf nightshade compared with the commercial eucalyptus oil. Among these four species, E. salubris oil had the highest inhibition index for silverleaf nightshade germination, root and shoot growth. Moreover, the bioactivity of the four essential oils on other weeds such as wild radish and barley grass was also under testing and more phytotoxic effects were found. This preliminary study supports essential oils playing a crucial role in suppressing understory growth of silverleaf nightshade within the driplines of the four selected Eucalyptus species in the field. The planting of suitable Eucalyptus species could be an alternative management strategy for the effective control of this intractable weed. E. salubris may serve as a potential source for developing a natural herbicide for the control of silverleaf nightshade and other weed species.
As one of major components found in the essential oils tested, 1,8-cineole could contribute to the bioactivity of oils tested as the herbicidal activity of 1,8-cineole has been demonstrated on other weeds (Singh et al. 2002; Romagni et al. 2000). 1,8-Cineole has been successfully used as a lead compound in the development of an morphogenetically active grass herbicide for use in broadleaf crops such as soybeans [Glycine max (L.) Merr.] (Baum et al. 1998). Our study also showed that 1,8-cineole inhibited the germination and seedling growth of silverleaf nightshade (Table 1, Fig. 1, 2, 3).
However, the inhibition index of 1,8-cineole on either germination or seedling growth of silverleaf nightshade was lower than that of the extracted essential oils (Table 1), and similar to the inhibition index of commercial oil which was composed of 77 % 1,8-cineole. The essential oil of E. brockwayii had the lowest content of 1,8-cineole (16.9 %), but higher activity than the commercial oil. Similarly, the best herbicidal activity was obtained with E. salubris oil, which had only a moderate 1,8-cineole content but the highest p-cymene content (8.3 %). These results suggest that the herbicidal activity of essential oils tested against silverleaf nightshade may not be associated solely with a single major compound, but may result from the synergistic effects of several bioactive compounds. Chemical and PCA analysis showed that the content of major compounds varied among oils tested, which may be responsible for the differences in phytotoxicity between these oils. The potential elevated phytotoxicity due to the synergistic effects of mixtures could assist in the development of natural herbicides in the future and would warrant further investigation.
References
Adams RP (1995) Identification of essential oil components by gas chromatography/mass spectroscopy, 2nd edn. Allured publishing Co, Carol Stream, IL
An M, Pratley JE, Haig T, Liu DL (2005) Whole-range assessment: a simple method for analysing allelopathic dose-response data. Nonlinearity Biol Toxicol Med 3:245–260
Batish DR, Setia N, Singh HP, Kohli RK (2004) Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Prot 23:1209–1214
Batish DR, Singh HP, Nidhi S, Shalinder K, Kohli RK (2006) Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Z Naturforsch 61c:52–56
Baum SF, Karanastasis L, Rost TL (1998) Morphogenetic effect of the herbicide Cinch on Arabidopsis thaliana root development. J Plant Growth Regul 17:107–114
Bignell CM, Dunlop PJ, Brophy JJ, Jackson JF (1996a) Volatile leaf oils of some south-western and southern Australian species of the genus Eucalyptus. Part X. Flavour Frag J 11:101–106
Bignell CM, Dunlop PJ, Brophy JJ, Jackson JF (1996b) Volatile leaf oils of some south-western and southern Australian species of the genus Eucalyptus. Part XI. Flavour Frag J 11:107–112
Coppen JJW (2002) Eucalyptus: The genus Eucalyptus. Taylor and Francis, London
Duke AS (1983) Handbook of Energy Crops. http://www.hort.purdue.edu/newcrop/duke_energy/Eucalyptus.html. Accessed 20 February 2011
Fathi E, Sefidkon F, Abravesh Z (2009) The effects of drying and extraction methods on essential oil content and composition of Eucalyptus spathulata. J Essent Oil-Bearing Plants 12:189–197
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological satistics software package for education and data analysis. Palaeontol Electron 4:1–9
Kandasamy OS, Mohamed Yassin M, Babu RC (2000) Biology, ecology, silviculture and potential uses of Eucalyptus—an overview. J Med Arom Plant Sci 22:30–335
Liu DL, An M, Wu H (2007) Implementation of WESIA: whole-range evaluation of the strength of inhibition in allelopathic-bioassay. Allelopathy J 19:203–214
OEPP/EPPO (2007) Solanum elaeagnifolium. Bull OEPP/EPPO Bull 37:236–245
Ramezani S, Saharkhiz MJ, Ramezani F, Fotokian MH (2008) Use of essential oils as bioherbicides. Essent Oil-Bearing Plants 1:319–327
Romagni JG, Allen SN, Dayan FE (2000) Allelopathic effects of volatile 1,8-cineoles on two weedy plant species. J Chem Ecol 26:303–313
Setia N, Batish DR, Singh HP, Kohli RK (2007) Phytotoxicity of volatile oil from Eucalyptus citriodora against some weedy species. J Environ Biol 28:63–66
Singh HP, Batish DR, Kohli RK (2002) Allelopathic effect of two volatile monoterpenes against billy goat weed (Ageratum conyzoides L.). Crop Prot 21:347–350
Singh HP, Batish DR, Shalinder K, Kohli RK, Komal A (2006) Phytotoxicity of the volatile monoterpene citronellal against some weeds. Z Naturforsch 61c:334-340
Stanton RA, Heap JW, Carter RJ, Wu H (2009) Biology of silverleaf nightshade (Solanum elaeagnifolium), In: Panetta FD (ed) The biology of Australian weeds Panetta, vol. 3. RG and FJ Richardson, Melbourne, pp 274–293
USDA-NRCS (2005) The PLANTS Database, Version 3.5 National Plant. Data Center, Baton Rouge (US). http://plants.usda.gov. Accessed 20 February 2011
Zhang JB, An M, Wu H, Stanton R, Lemerle D (2010) Chemistry and bioactivity of Eucalyptus essential oils. Allelopathy J 25:313–330
Acknowledgments
Authors are grateful for the financial support from Meat and Livestock of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., An, M., Wu, H. et al. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul 68, 231–237 (2012). https://doi.org/10.1007/s10725-012-9711-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9711-5