Abstract
Chemical composition and antioxidant and antifungal action of the oils from leaves and wood bark of two chemotypes of Cinnamomum verum J. Presl were evaluated. Plants were sampled in the cities of São Luís and Santa Inês, state of Maranhão, Brazil. GC–MS and GC-FID, DPPH radical scavenging, and in vitro test against the phytopathogenic fungus Colletotrichum musae were used to perform these analyses. Cinnamomum verum is worldwide known as Cinnamon, highlighted for its extensive use in the cooking of diverse cultures of the world, and as a medicinal plant to treat environmental viral diseases. In the leaf oil of São Luís chemotype, eugenol (93.6%) was the main constituent, while in Santa Inês chemotype, it was benzyl benzoate (95.3%). In the bark wood oil of São Luís chemotype, (E)-cinnamaldehyde (89.3%) was the main constituent, while in Santa Inês chemotype, they were benzyl benzoate (23.3%), linalool (14.0%), (E)-caryophyllene (9.1%), caryolan-8-ol (7.2%) and borneol (4.7%). Leaf oils from both chemotypes showed strong to moderate antifungal activity, reaching 100% efficacy in eugenol-containing oils and above 70% in benzyl benzoate oils. In the antioxidant evaluation, the chemotype with a high eugenol content presented an inhibitory concentration higher than 80%, compared to Trolox. The leaf oils of the two C. verum chemotypes showed significant antifungal and antioxidant potential, considering their economic use as a functional and nutraceutical food supplement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cinnamomum belongs to Lauraceae. It is represented by trees and shrubs and comprises about 250 species which are distributed in tropical and subtropical regions of Southeast Asia, Australia, and North, Central and South America (Jayaprakasha et al. 2003; Wang et al. 2009). Cinnamomum verum J. Presl (syn. Camphora mauritiana Lukman, Cinnamomum zeylanicum Blume, Laurus cinnamomum L.) (Missouri Botanical Garden, www.tropicos.org/Name/17800682), is a native species grown mostly in Sri Lanka, India, and Seychelles and Madagascar islands. It is worldwide known as Cinnamon, has been highlighted for its extensive use in the cooking of diverse cultures of the world, as well as to possess aromatic, digestive, stimulant, antibacterial, astringent, antioxidant, and antinociceptive pharmacological properties (Wang et al. 2009; Gupta 2010). The species was introduced in Brazil during the slavery period, and it is commonly known as Canela, Canela-da-Índia, or Canela-do-Ceilão, popularly used as a stimulant, tonic, carminative and anti-spasmodic (Pio Corrêa 1984).
Concerning the C. verum oil, some reports have shown diversity in its composition, displaying some chemical types. The leaf and bark oils of specimens of C. zeylanicum growing in Sri Lanka, India, Fiji Islands and Malaysia presented eugenol and cinnamaldehyde content, respectively, as the principal constituents (Senanayake et al. 1978; Raina et al. 2001; Mallavarapu and Rao 2007; Patel et al. 2007; Jantan et al. 2008; Subki et al. 2013; Chakraborty et al. 2015). Besides, benzyl benzoate (Nath et al. 1996), linalool (Jirovetz et al. 2001), and cinnamaldehyde, cinnamyl acetate, and cinnamyl benzoate (Boniface et al. 2012) have been identified as the main compounds in the leaf and bark oils from other Cinnamon specimens occurring in India and Africa, respectively.
A number of biological properties have been attributed to C. verum leaf and bark oils, including the antimicrobial (El-Baroty et al. 2010; Boniface et al. 2012) antifungal (Simic et al. 2004; Jantan et al. 2008), mosquitocidal (Samarasekera et al. 2005), antioxidant (Simic et al. 2004; Schmidt et al. 2006), and cytotoxic activities (Unlu et al. 2010).
The substitution of synthetic pesticides for natural products less toxic to humans has stimulated their use in the control of phytopathogens. Alternatives to reduce pests and diseases in agriculture are the oils and extracts of medicinal plants, which has increased in recent years and proving to be potential fungicidal and fungistatic agents, as well as contributing to the activation of plant defense mechanisms. In Brazil, the action of essential oils on phytopathogenic fungi that attacks economically important crops is still little known, and this knowledge can contribute alternatively to the control of some diseases, as well as in the development of new products. In this regard, one example is the post-harvest sanitary control of bananas.
Banana is cultivated on a large scale in many locations of Brazil due its nutritional value and export potential. Storage of banana is made difficult by the growth of fungi, as Colletotrichum musae, which causes anthracnose disease. Banana producers are looking for new alternative post-harvest treatments that are pesticides free and acceptable to consumers.
Antioxidants from natural sources have received attention from researchers. Efforts have been made to identify compounds which can prevent oxidative body damage and be formulated as new functional foods and nutraceuticals (Hashemi et al. 2017). The leaf and bark oils of C. zeylanicum and eugenol, its main constituent, were tested in vitro models of peroxynitrite-induced nitration and lipid peroxidation, showing a significant antioxidant property in both models, higher than Trolox (Chericoni et al. 2005). The C. zeylanicum leaf oil demonstrated significant scavenger activity against the DPPH radical, at concentrations lower than the eugenol (Schmidt et al. 2006). Also, eugenol showed a most potent radical-scavenging activity in comparison to butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol, and Trolox (Gülçin 2011).
The objective of this work was to determine the variability in the composition of the essential oils of leaf and bark wood of two C. verum specimens, sampled in the municipalities of Santa Inês (SI) and São Luís (SL), Maranhão state, and to test their antifungal and antioxidant action, with a view to its economic utilization as functional food and nutraceutical.
Materials and methods
Chemicals
The reagents and solvents, dimethyl sulfoxide (DMSO), ethanol, 1,1-diphenyl-2-picrylhydrazyl (DPPH), tris hydrochloride (tris-HCl), and 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were all from Sigma-Aldrich, USA. The potato dextrose agar medium was from Millipore, USA.
Plant material and collection data
The C. verum leaves and barks wood were collected from municipalities of Santa Inês (SI) (3° 41′ 0′′ S/45° 23′ 12′′ W) and São Luís (SL) (02° 31′ 47′′ S/44° 18′ 10′′ W), State of Maranhão, both at 9 am, January 2014. Samples of the two specimens were sent to the João Murça Pires Herbarium, at the Museu Emílio Goeldi (MPEG), city of Belém (PA), and identified by comparison with an authentic sample (MG 165477) previously deposited.
Essential oil extraction
The leaves and barks (50 g, each) of C. verum were ground and submitted to hydrodistillation using a Clevenger-type apparatus (3 h), after drying at room temperature (3 days). The oils were dried over anhydrous sodium sulfate, and their yields were calculated by the plant dry weight. The moisture content of the samples was calculated using an Infrared Moisture Balance for water loss measurement. The procedure was performed in duplicate.
Oil composition analysis
Analysis of the oils were carried on a GC–MS Thermo-Electron, model Focus DSQ II, under the following conditions: DB-5 ms (30 m × 0.25 mm; 0.25 mm film thickness) fused-silica capillary column; programmed temperature, 60–240 °C (3 °C/min); injector temperature, 250 °C; carrier gas, helium, adjusted to a linear velocity of 32 cm/s (measured at 100 °C); injection type, split (1.0 μL), from 1:1000 hexane solution; split flow was adjusted to yield a 20:1 ratio; septum sweep was a constant 10 mL/min; EIMS, electron energy, 70 eV; temperature of the ion source and connection parts, 200 °C. The quantitative data regarding the volatile constituents were obtained by peak area normalization using a FOCUS GC/FID operated under similar conditions for the GC–MS, except the carrier gas (nitrogen). The retention index was calculated for all the volatiles constituents using a homologous series of n-alkanes (C8–C32, Sigma-Aldrich), according to Van den Dool and Kratz (1963). The oil components were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with those existing in the GC–MS system libraries and literature spectra (Adams 2007; NIST 2011; Modello 2011).
Antifungal assay
The leaf oils of C. verum (Cvl-SL and Cvl-SI) were tested against the fungus C. musae (Berk. & M.A. Curtis) Arx, a plant pathogen that causes severe damage in a ripe banana. The isolate of C. musae (MGSS85) was obtained from the mycology collection of Laboratório de Fitopatologia, Universidade Estadual do Maranhão, São Luís, MA. The oils were dissolved in DMSO (50% v/v) and incorporated to the PDA culture medium at concentrations 0.5, 1.0, 2.0, 3.0 and 4.0 μL/mL. The media (20 mL) containing the samples was poured into separately Petri dishes and inoculated in the center with a 6 mm diameter disk containing the fungal mycelia. The plates were incubated in a BOD incubator, under a 12 h photoperiod and 25 °C. Negative control was composed by plates containing the media and fungus mycelia, but without the oil. The effect of the oil on the mycelial growth (mm) was determined by measuring the radial growth of the fungus in intervals from the 1st to 11th day, after the inoculation. The in vitro fungitoxic activity was expressed by the inhibition percentage of mycelial growth, calculated by the equation, (I%) = [(NC − TP)/TP] × 100, where NC and TP are the mycelial growth of the negative control and the treated plate, respectively. The experimental design was completely randomized with five treatments and six replications. Three replicates were performed and the data were also evaluated by mycelial growth rate index (MGRI), expressed in mm/day and calculated by the equation, MGRI = (D − Db)/n, where D and Db are the diameters of the current day and the previous day, respectively, and n the number of days of incubation (Nascimento et al. 2013).
Antioxidant assay
A stock solution of DPPH (1,1-diphenyl-2-picrylhydrazyl, 0.5 mM) was prepared in ethanol. The solution was diluted to approximately 60 µM, measuring an initial absorbance of 0.62 ± 0.02, at 517 nm and room temperature. The radical scavenging activity was expressed as milligrams of Trolox equivalent per gram of oil (mg TE/g), and it was calculated for the leaf oils of C. verum (Cvl-SL and Cvl-SI). The reaction mixture was composed of 900 μL of tris-HCl (100 mM, pH = 7.4), 40 μL of ethanol, 50 μL of Tween 20 solution (0.5% m/v), 10 μL of Trolox in ethanol at concentrations of 0.25, 0.375, 0.50, 0.75, 1.00 and 1.25 mg/mL, followed by 1 mL of DPPH. The absorbance was measured at the start of the reaction, every 5 min during the first 20 min and, then, at 10 min intervals until the constant absorbance value. The DPPH inhibition percentage was calculated by the equation, IDPPH% = [1 − (AbsA/AbsB)] × 100, where AbsA and AbsB are the absorbance values of the sample and the control (blank) at the end of the reaction, respectively. The Trolox equivalent was obtained by replacing the Trolox solution with 10 µL of oil sample and it was calculated by the equation, TE = (A − B)/(A − C) × 25/1000 × 250.29/1000 × 1000/10 × D, where A, B and C are the blank, sample and Trolox absorbance values in the reaction end, and D is the dilution factor (da Silva et al. 2010).
Statistical analysis
All data were submitted to analysis of variance (ANOVA) and compared by Tukey’s test at 5% probability, using the Prisma 4.0 software. Results were expressed as mean ± standard deviation. All samples in each experiment were performed in triplicate.
Results and discussion
Oil yield and composition
The average values of oil yields were determined in triplicate, and they are presented in Table 1. Leaf oils yield was higher when compared to previous samples of C. verum (Wang et al. 2009; Chakraborty et al. 2015; Boniface et al. 2012). On the other hand, the bark wood oils yields obtained for the two analyzed specimens were lower in comparison with other reported works (Li et al. 2013; Unlu et al. 2010). The yield and composition of essential oils can be influenced by genetic and environmental factors, among them, the age and plants growth stage. It has been seen that younger plants show higher activity while growing, with an increase in their metabolism.
The oil compositions of leaves (Cvl-SL and Cvl-SI) and wood barks (Cvb-SL and Cvb-SI) for two analyzed specimens are shown in Table 1. In total, forty-four constituents were identified. In general, the phenylpropanoid compounds predominated in the cinnamon oils analyzed, with an average value of 94.8%, except for the wood bark of the specimen harvested in Santa Inês, whose percentage was only 27.2%, below the value observed for the oxygenated monoterpenes, which was 32.7%, due to the significant percentual of linalool, camphor and others. For the leaf oil of São Luís specimen, eugenol (93.6%) was the principal component. For the wood bark oil of São Luís specimen, (E)-cinnamaldehyde (89.3%) was the major constituent. Benzyl benzoate (95.3%) was the main component in the leaf oil of Santa Inês specimen. In the wood bark oil of Santa Inês specimen, benzyl benzoate (23.3%), linalool (14.0%), (E)-caryophyllene (9.1%), caryolan-8-ol (7.2%) and camphor (6.8%) were the main constituents.
The leaf (Cvl-SL) and wood bark (Cvb-SL) oils of the C. verum specimen collected in the city of São Luís (MA), with the predominance of eugenol and (E)-cinnamaldehyde, respectively, are comparable to those previously described for specimens sampled in Sri Lanka, India, Fiji Islands and Malaysia, which showed the same principal constituents (Senanayake et al. 1978; Raina et al. 2001; Mallavarapu and Rao 2007; Patel et al. 2007; Jantan et al. 2008; Subki et al. 2013; Chakraborty et al. 2015). Similarly, the leaf (Cvl-SI) and bark (Cvb-SI) oils of the C. verum specimen harvested in Santa Inês (MA), with significant percentages of benzyl benzoate and linalool, were very like to those previously reported for specimens existing in India and Africa, with the same main compounds (Nath et al. 1996; Jirovetz et al. 2001). Considering the results with the cinnamon oils sampled in the cities of São Luís and Santa Inês, the similarity with the composition of the oils previously reported and the significant presence of new constituents such as (E)-caryophyllene, camphor and caryolan-8-ol, it was assumed the existence of at least two C. verum chemotypes, with the occurrence in Maranhão state, Brazil.
Antifungal activity
The C. verum oil (Cvl-SL), resulting from the specimen collected in the city of São Luís (MA), and eugenol, its main constituent, displayed total inhibition of the mycelial growth of C. musae, in all oil concentrations tested. On the other hand, the C. verum oil (Cvl-SI) extracted from the Santa Inês (MA) specimen, rich on benzyl benzoate, displayed a 100% inhibition only at concentrations above 3.0 μL/mL. The benzyl benzoate standard showed a 55% maximum inhibition at 4.0 µL/mL (Table 2).
Colletotrichum species are known as the significant plant pathogens worldwide, spreading the anthracnose disease in many crop plants and leading to significant yield loss (Rabari et al. 2017). Some studies on antifungal activity of Cinnamomum oils and its main compounds have been reported as an alternative to control post-harvest diseases. Jantan et al. (2008) correlated high levels of cinnamaldehyde, eugenol, geraniol, benzyl benzoate, and methyl cinnamate, in combination with their minor volatile components, as responsible for the significant antifungal activity of cinnamon oils.
In vitro antifungal activities of Cinnamon oils, (E)-cinnamaldehyde and eugenol were evaluated during conidial germination and mycelial growth of Colletotrichum gloeosporioides, the causal agent of anthracnose in pepper fruit. Mycelial growth displayed a high inhibition when treated by the indirect vapor of the oil samples reducing the lesion diameter on the C. gloeosporioides-inoculated immature green pepper fruits at similar levels. In vitro conidial germination, it was most drastically inhibited by vapor treatments with cinnamon oil and (E)-cinnamaldehyde (Hong et al. 2015).
The antifungal property and potential mechanism of the clove oil, rich in eugenol (78.0%), were studied in vitro and in vivo against C. gloeosporioides, isolated from sweet cherry (Prunus avium L.). The values of minimal inhibitory concentration (MIC) in the air (by fumigation) and contact phases were 80 and 300 μL/L, respectively. Furthermore, the microscopy analysis of C. gloeosporioides after exposure to clove oil showed a deleterious morphological and ultrastructural alterations. These observations confirmed the disruption of the fungal cell wall and endomembrane system, resulting in an increase of permeability and causing the loss of the intracellular constituents (Wang et al. 2019).
Essential oils from leaves of Cinnamomum cassia composed by (E)-cinnamaldehyde (66.3%) and benzyl benzoate (10.2%) and oils from the bark of C. zeylanicum, rich in (E)-cinnamaldehyde (64.1%) and linalool (10.3%), were tested against C. gloeosporioides extracted from an infected mango. The fungal suspension was spread on potato dextrose agar and tested with 5 µL of these different oils. The values of percentages of inhibition were of 72.7% to C. cassia, and 65.3% to C. zeylanicum, respectively (Rabari et al. 2017).
Effects of cinnamon bark and clove bud essential oils were investigated, in vitro, on the Colletotrichum acutatum mycelial growth, in conidial germination, in appressoria formation, and, in vivo, on strawberry fruit anthracnose incidence. The oil samples inhibited mycelial growth, showed a fungistatic effect at a concentration of 667 µL/L, and wholly prevented conidial germination at the lowest concentrations of 1.53 and 76.5 µL/L of air fumigation, respectively. Also, the treatments with oils had disabled the appressoria formation at a concentration of 1.53 µL/L of air fumigation. On inoculated strawberry fruit, only cinnamon bark oil reduced the anthracnose incidence at 76.5 µL/L, by air fumigation (Duduk et al. 2015). Furthermore, leaf oils from C. zeylanicum, rich in eugenol (76.9%), and bark oils, rich in cinnamaldehyde (50.5%) and cinnamyl acetate (8.7%), were previously tested against C. musae. The values of minimum inhibitory concentration (MIC) and minimum lethal concentration (MLC) displayed ranges of 0.03 to 0.05% (v/v) and 0.04 to 0.07% (v/v), respectively (Ranasinghe et al. 2002).
Antioxidant activity
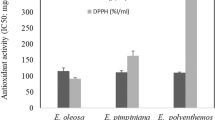
The DPPH antioxidant test was used for the sole purpose of evaluating and comparing the different C. verum chemotypes existing in the state of Maranhão, Brazil. Leaf oils tested in the DPPH assay displayed different ranges of inhibition after 30 min of reaction. Due to its reactivity, the oil extracted from the São Luís (Cvl-SL) specimen and the eugenol standard were diluted in the proportion of 1:10 (oil:ethanol). However, the leaf oil obtained from the Santa Inês (Cvl-SI) specimen and the benzyl benzoate standard were tested pure in the reactional mixture. The percentage of inhibition of DPPH radicals was used to calculate the Trolox Equivalent Antioxidant Capacity (TEAC, mg TE/mL). The highest antioxidant activity was observed to Cvl-SL oil and eugenol, its main constituent, with TEAC values of 1438.1 ± 5.6 and 1456.8 ± 8.0 mg TE/mL, respectively. On the other hand, the Cvl-SI oil displayed a low activity, at 165.2 ± 2.6 mg TE/mL, about nine times less than Cvl-SL oil. Benzyl benzoate standard, also the main constituent of Cvl-SI oil, was considered inactive, at 47.0 ± 3.3 mg TE/mL (Fig. 1).
The antioxidant capacity of oils from Cinnamomum species and its main constituents have been reported using various methods and mechanism of actions. The bark oil of C. zeylanicum rich in eugenol (46.5%) and cinnamaldehyde (32.7%), and eugenol standard, showed potent activities in two in vitro models of peroxynitrite-induced nitration and lipid peroxidation with half-maximal inhibition concentration (IC50) values lower than ascorbic acid and Trolox, two reference standards (Chericoni et al. 2005). In other study, a C. zeylanicum leaf oil, rich in eugenol (74.9%) and including β-caryophyllene (4.1%) and benzyl benzoate (3.0%), demonstrated scavenger activity against the DPPH radical at concentrations which are lower than the concentrations of the standards eugenol, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA). Also, this oil showed a significant inhibitory effect on hydroxyl radicals, acting as an iron ion chelator, inhibiting the formation of conjugated dienes and the generation of secondary products of the lipid peroxidation efficiently, at a concentration equivalent to that of the standard BHT (Schmidt et al. 2006).
Leaf oils of C. walaiwarense, unexplored wild cinnamon from India, displayed high amount of benzyl benzoate (65.0 to 89.8%). The DPPH and ABTS assays evaluated the free radical scavenging potential of the oils and the standard benzyl benzoate. Benzyl benzoate (IC50, 10.0 mg/mL) was about three times less active than the oils (IC50 ≈ 3.7 mg/mL) of C. walaiwarense (Sriramavaratharajan and Murugan 2018). Some commercial oils of clove bud, rich in eugenol (76.1%), and jasmine absolute, rich in benzyl acetate (32.3%) and benzyl benzoate (22.9%), as well as the standards of eugenol, benzyl acetate and benzyl benzoate, were submitted to antioxidant assays by different methods. Clove bud oil, jasmine absolute, and eugenol were active in the β-carotene bleaching test and as scavengers of ABTS and DPPH radicals. However, the standards benzyl acetate and benzyl benzoate were inactive in all assays (Wang et al. 2017).
Conclusion
Considering the results with the cinnamon oils sampled in the cities of São Luís and Santa Inês, the similarity with the composition of the oils previously reported and the significant presence of new constituents, such as (E)-caryophyllene, camphor and caryolan-8-ol, it was assumed the existence of at least two C. verum chemotypes, with the occurrence in Maranhão state, Brazil. C. verum leaf oil from São Luís and the standard eugenol, its main constituent, displayed total inhibition of the mycelial growth of C. musae, in all oil concentrations tested. C. verum oil from Santa Inês, rich on benzyl benzoate, displayed a 100% inhibition only at concentrations above 3.0 μL/mL. The highest antioxidant activity was observed to C. verum oil from São Luís and the eugenol standard. C. verum oil from Santa Inês displayed a low activity, about nine times less than the C. verum oil from São Luís, while benzyl benzoate standard was considered inactive.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream
Boniface Y, Philippe S, de Lima HR, Noudogbessi JP, Alitonou GA, Toukourou F, Sohounhloue D (2012) Chemical composition and antimicrobial activities of Cinnamomum zeylanicum Blume dry leaves essential oil against food-borne pathogens and adulterated microorganisms. Int Res J Biol Sci 1:18–25
Chakraborty A, Sankaran V, Ramar M, Chellappan DR (2015) Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J Chem Pharm Sci 8:476–479
Chericoni S, Prieto JM, Iacopini P, Cioni P, Morelli I (2005) In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J Agric Food Chem 53:4762–4765
da Silva JKR, Andrade EHA, Kato MJ, Carreira LMM, Guimarães EF, Maia JGS (2010) Antioxidant capacity and larvicidal and antifungal activities of essential oils and extracts from Piper krukoffii. Nat Prod Commun 6:1361–1365
Duduk N, Markovic T, Vasic M, Duduk B, Vico I, Obradovic A (2015) Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J Essent Oil Bear Plants 18:529–537
El-Baroty GS, Abd El-Baky HH, Farag RS, Saleh MA (2010) Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr J Biochem Res 4:167–174
Gülçin I (2011) Antioxidant activity of eugenol: a structure–activity relationship study. J Med Food 14:975–985
Gupta M (2010) Pharmacological properties and traditional therapeutic uses of important Indian spices: a review. Int J Food Prop 13:1092–1116
Hashemi SMB, Khorram SB, Sohrabi M (2017) Antioxidant activity of essential oils in food. In: Hashemi SMB, Khaneghah AM, Sant’Ana AS (eds) Essential oils in food processing: chemistry, safety and applications. Wiley, New York, pp 247–265
Hong JK, Yang HJ, Jung H, Yoon DJ, Sang MK, Jeun Y-C (2015) Application of volatile antifungal plant essential oils for controlling pepper fruit anthracnose by Colletotrichum gloeosporioides. Plant Pathol J 31:269–277
Jantan IB, Moharam BAK, Santhanam J, Jamal JA (2008) Correlation between chemical composition and antifungal activity of the essential oils of eight Cinnamomum species. Pharm Biol 46:406–412
Jayaprakasha GV, Rao LJM, Sakariah KK (2003) Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem 51:4344–4348
Jirovetz L, Buchbauer G, Ruzicka J (2001) Analysis of Cinnamomum zeylanicum Blume leaf oil from South India. J Essent Oil Res 13:442–443
Li Y-Q, Kong D-X, Wu H (2013) Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind Crops Prod 41:269–278
Mallavarapu GP, Rao BRR (2007) Chemical constituents and uses of Cinnamomum zeylanicum Blume. In: Jirovetz L, Dung NX, Varshney VK (eds) Aromatic plants from Asia their chemistry and application in food and therapy. Har Krishan Bhalla & Sons, Dehradun, pp 49–75
Missouri Botanical Garden. www.tropicos.org/Name/17800682. Accessed Nov 2019
Mondello L (2011) Flavors and fragrances of natural and synthetic compounds, Mass Spectral Database (FFNSC 2). Wiley, New York
Nascimento JM, Serra AP, Bachii LM, Gavassoni WL, Vieira MC (2013) Inibição do crescimento micelial de Cercospora calendulae Sacc. por extratos de plantas medicinais. Rev Bras Plant Med 15:751–756
Nath SC, Pathak MG, Akhil Baruah A (1996) Benzyl benzoate, the major component of the leaf and stem bark oil of Cinnamomum zeylanicum Blume. J Essent Oil Res 8:327–328
NIST (National Institute of Standards and Technology) (2011) Mass Spectral Library (NIST/EPA/NIH), v.2.0d. The NIST Mass Spectrometry Data Center, Gaithersburg
Patel K, Ali S, Sotheeswaran S, Dufour JP (2007) Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji Islands. J Essent Bear Plants 10:374–377
Pio Corrêa M (1984) Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Imprensa Nacional, Rio de Janeiro
Rabari VP, Chudashama KS, Thaker VS (2017) In vitro screening of 75 essential oils against Colletotrichum gloeosporioides: A causal agent of anthracnose disease of mango. Int J Fruit Sci. https://doi.org/10.1080/15538362.2017.1377666
Raina VK, Srivastava SK, Aggarwal KK, Ramesh S, Kumar S (2001) Essential oil composition of Cinnamomum zeylanicum Blume leaves from Little Andaman, India. Flav Fragr J 16:374–376
Ranasinghe L, Jayawardena B, Abeywickrama K (2002) Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana. Lett Appl Microbiol 35:208–211
Samarasekera R, Kalhari KS, Weerasinghe IS (2005) Mosquitocidal activity of leaf and bark essential oils of Ceilon Cinnamomum zeylanicum. J Essent Oil Res 17:301–303
Schmidt E, Jirovetz L, Buchbauer G, Eller GA, Stoilova I, Krastanov A (2006) Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J Essent Oil Bear Plants 9:170–182
Senanayake UM, Lee TH, Wills RBH (1978) Volatile constituents of cinnamon (Cinnamomum zeylanicum). J Agric Food Chem 26:822–824
Simic A, Sokovic MD, Ristic M, Grujic-Jovanovic S, Vukojevic J, Marin PD (2004) The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother Res 18:713–717
Sriramavaratharajan V, Murugan R (2018) Chemical profile of leaf essential oil of Cinnamomum walaiwarense and comparison of its antioxidant and hypoglycemic activities with the major constituent benzyl benzoate. Nat Prod Commun 13:779–782
Subki SYM, Jamala JA, Husaina K, Manshoora N (2013) Characterisation of leaf essential oils of three Cinnamomum species from Malaysia by gas chromatography and multivariate data analysis. Pharmacogn J 5:22–29
Unlu M, Ergene E, Unlu GV, Zeytinoglu HS, Vural N (2010) Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem Toxicol 48:3274–3280
Van Den Dool H, Kratz PDJA (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr A 11:463–471
Wang R, Wang R, Yang B (2009) Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov Food Sci Emerg Technol 10:289–292
Wang H-F, Yih K-H, Yang C-H, Huang K-F (2017) Antioxidant activity and major chemical component analyses of twenty-six commercially available essential oils. J Food Drug Anal 25:881–889
Wang D, Zhang J, Jia X, Xin L, Zhai H (2019) Antifungal effects and potential mechanism of essential oils on Collelotrichum gloeosporioides in vitro and in vivo. Molecules 24:3386
Acknowledgements
This study was supported by CAPES/Brazilian Government and FAPEMA/Maranhão Government through the concession of scholarships and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farias, A.P.P., Monteiro, O.S., da Silva, J.K.R. et al. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J Food Sci Technol 57, 3176–3183 (2020). https://doi.org/10.1007/s13197-020-04288-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04288-7