Abstract
Nowadays, there are increasing concerns about the bioavailability of neonicotinoids in the environment and possible exposure of nontarget organisms to these insecticides, their residues having been detected at different concentrations in many matrices, i.e., pollen, nectar, soil, water. Regarding the risk assessment process, there are still some information gaps about the exposure pathways and the possibility of various sublethal effects on insect pollinators. Recently, a clear rapprochement between the sublethal effects of different endpoints under laboratory conditions and field-realistic exposure level has been demonstrated. Here, we attempt to draw general portrayal about the current debate of the exposure to neonicotinoids and their impacts on pollinators. Depending on our extracted data from the published literature, we show that the lowest observed effect concentration under realistic field conditions in the most cases is higher than under laboratory conditions, which indicate that further long-term field research is required with consideration that our good understanding of the pollinators’ responses to sublethal exposure should be taken into account in the future experimental design in order to establish vigorous conclusions. We review currently available information in the published literature, presenting the reports about detected residues in relation to multiple ways of exposure and their potential consequences on insect pollinators and community dynamics. Nevertheless, we attempt to classify the sublethal effects depending on the different biological levels from genes to population. Moreover, we consider the field-realistic exposure level and critically analyze the laboratory as well as field studies to specify their physiological and behavioral effects. Additionally, synergistic effects of different factors, including exposure to neonicotinoids and their hazards on bees, will find special attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of the neonicotinoids as a new class of insecticide, there has been a huge body of literature assessing their effects on bees. This increasing interest indicates that there are many gaps in our knowledge about the potential effects of these insecticides on nontarget beneficial insects, especially pollinators. This resulting, hugely varying information comes from laboratory as well as field studies, which in turn led to some difficulties in analyzing their impacts. The concerns about pollinators’ exposure to neonicotinoids depend on their high toxicity, persistence in soil and water, and wide application. Also, their systemic properties lead in turn to their diffusion through the xylem in growing plants, thus contaminating nectar, pollen [26, 74, 110, 118], and guttation water [55, 69], which were collected by bee foragers and transported to the nest. As a result, neonicotinoids are considered as insecticides bioavailable to insect pollinators at sublethal concentrations through the potential uptake from crops and wild plants.
Recently, these concerns about negative effects of neonicotinoids on bees have led to 2-year restrictions on the use of three neonicotinoids (clothianidin, imidacloprid, and thiamethoxam) as seed treatment in bee-attractive crops in the European Union to evaluate their potential environmental impacts [46]. However, this process will also play an important role in evaluating the present and future of the pest control strategies.
Therefore, the potential exposures and effects on pollinators have been the subject of numerous studies. Nowadays, there is increasing attention being paid to sublethal effects due to their subsequent impacts on the development of the insect pollinators. Among them, Apis and non-Apis bees are considered as the most important pollinators worldwide, playing an important role in the maintenance of biodiversity and food production [73, 137].
Neonicotinoids have been classified depending on the pharmacophore into three main groups, which are N-nitroguanidines (imidacloprid, thiamethoxam, clothianidin, and dinotefuran), nitromethylenes (nitenpyram), and N-cyano-amidines (thiacloprid and acetamiprid) [44].
According to their different ways of application, including soil treatment, seed treatment, and spray, they have since become the most used class of insecticides (26 % of the insecticide market in 2010) [18] and are licensed in more than 120 countries for more than 1000 uses in treating a wide range of plants [41].
The neonicotinoids’ mode of action is known as acetylcholine mimics, and they act as agonists of nicotinic acetylcholine receptors (nAChR), which in turn activate persistently the cholinergic receptors, leading to hyper-excitation and death in the end [68]. Sublethal effects are defined as physiological and/or behavioral effects on individuals who survive after exposure to a pesticide at a dose with no apparent mortality in the experimental population.
The risk assessment of nontarget organisms, especially bees, to pesticide exposure had been developed in many countries to take into account the sublethal effects on the different levels of the organism’s development.
We focus on sublethal impacts of neonicotinoids and review currently available information in the published literature. We attempt to classify these effects depending on the different biological levels. So, in this review we present the reports about detected residues in relation to multiple ways of exposure and their potential consequences on insect pollinators and community dynamics. Moreover, we take into account the field-realistic exposure and critically analyze the laboratory as well as field studies to specify their physiological and behavioral effects. In addition, the synergization of different factors, including exposure to neonicotinoids and their hazards for bees, will be given special attention.
The exposure routes related to ways of application
The bioavailability of neonicotinoids is considered to be at a high level throughout the year depending on the respective pest control profiles in a wide range of agricultural and horticultural plants, where they exhibit long persistency in soil and a high ability to diffuse throughout the plants (e.g., the half-life of clothianidin in soil is between 148 and 6900 days [107] and imidacloprid 40–997 days). In turn, there is a potential accumulation in the soil after repeated applications and contamination of other growing plants [62]. On the other hand, Van Dijk et al. [138] reported that imidacloprid could travel far beyond the fields via surface and ground water. Therefore, the exposure of insect pollinators at very low doses to various sources of different neonicotinoids is very likely. The potential exposure pathways of insect pollinators are shown in Fig. 1. According to these pathways, we summarize the range of detected concentrations under field conditions based on the latest published studies (Table 1). Several studies were performed worldwide to determine exposure levels to neonicotinoid residues, where either large surveys in different sites [21, 113], sampling from different crops (i.e., maize and oilseed rape) over many years [101], or only from one crop in one season [26, 102] were conducted.
Under realistic conditions in the field, only a little information is known about the level of oral or contact exposure either through contaminated food (nectar, pollen, and water) or other treated surfaces. It is assumed that different exposure levels occur in the bee’s colony (honeybees or bumblebees) among different castes. On the other hand, there are information gaps about the amount consumed by wild bees.
Regarding oral exposure, as given in Table 1, the neonicotinoid residues in positive samples depended on the way of application. For imidacloprid, the highest residues 6.0–28.0 and 5.0–14.0 µg/kg were detected after soil treatment in the pollen and nectar of squash, respectively [126], whereas the lowest residues 0.6–2.0 µg/kg were found in the nectar of seed-treated oil seed rape and not detected in pollen [102]. Similarly, for thiamethoxam, the highest detected concentrations 5.0–35.0 and 5.0–20.0 µg/kg were found after soil treatment in the pollen and nectar of squash, respectively [126], whereas the lowest residues were found in seed-treated crops, i.e., 1.0–7.0 µg/kg in maize pollen and 0.7–2.4 µg/kg in oilseed rape [101]. On the other hand, relatively high concentrations of clothianidin were detected in seed-treated crops, where the residues in maize pollen ranged between 0.3 and 11.4 µg/kg [91] and 0.5–10.1 µg/kg in canola nectar [102], with an exception in the Krupke et al. [74] study, who reported detecting residues in pollen ranging between 1.1 and 88.0 µg/kg. Generally, the frequencies of positive samples in most studies were relatively low to medium in the collected samples, ranging from not detected to 60 %. However, the spray application of both low toxic neonicotinoids (i.e., thiacloprid and acetamiprid) leads to relatively higher residues in both nectar and pollen but remain at much lower than lethal concentration.
Water as another suggested potential oral exposure includes surface and guttation water. Currently, high levels of residues in puddles of water as a possible source for drinking water from seed-treated corn fields were detected [111]. They found that clothianidin and thiamethoxam residues ranged from 0.1 to 55.7 and 0.1 to 63.4 µg/L, respectively. Also, very low concentrations were found in different rivers in Australia [113] compared to very high levels of neonicotinoids in guttation water [7, 55, 69]. Reetz et al. [104] demonstrated that the residual concentrations in guttation water from seed-coated winter oilseed rape decreased throughout the plant development (up to 130 µg/L clothianidin during autumn, prewinter <30 µg/L, spring <15 µg/L). They also evaluated the water-foraging activity of honeybees on guttation fluid from seed-coated canola, where the thiamethoxam residues in honey-sac contents at concentrations ranging from 0.3 to 0.95 µg/L were detected.
However, this study confirms that bees could use guttation water as a source of water. Thus, the exposure levels in different crops should be evaluated.
For wild bees nesting in soil, direct or indirect contact exposure to contaminated soil is an additional pathway of concern. Stewart et al. [125] reported that the detected concentration in soil was between 1.0 and 29.0 µg/kg of imidacloprid and clothianidin and 1.0–39.0 µg/kg of thiamethoxam. Currently, assessments of clothianidin accumulation in soil and bee-relevant matrices showed no increase over time in fields receiving multiple applications of clothianidin. Relatively low residues in soil of 5.7–7.0 µg/kg, corn pollen 1.8 µg/kg, and canola nectar 0.6 µg/kg were detected [146].
Moreover, the dust drift has been taken into account as an exposure way, where the level of dust decreases relative to the distance. APENET project 2010 reported that the dust amount ranged between 2.0 and 16.0 µg/m2 for imidacloprid at a distance of 5–20 m.
Few studies analyze the residues in nesting materials, where some residues of neonicotinoids were detected in bee wax [90]. Pareja et al. [95] reported that high residue levels of imidacloprid were detected in the honeycombs (240.0–450.0 µg/kg) and propolis (20.0–100.0 µg/kg) of depopulated beehives located near treated sunflower crops in Uruguay. These indicate the possibility of accumulation in these materials.
However, until now, the field-relevant concentrations and/or doses are still not completely resolved due to a limited investigation of few pollinator-relevant plants. So, it is unexpected that residue concentrations are in a wide range over different spaces, and in turn it is difficult to conclude whether such residues exist rarely or commonly at the field level [137]. Nevertheless, there is a possibility of accumulative poisoning through the repetitive consumption of food containing low residues of neonicotinoids.
Comparison of evidences in published laboratory and field studies
During the last years (between 2014 and 2016), new evidences about the sublethal effects of exposure to neonicotinoids have been published, which lead to advance our knowledge and understanding about the potential exposure of different insect pollinators to these pesticides and their responses under realistic conditions. In the most studies, it has been considered that the exposure of pollinators to seed-treated crops occur at sublethal levels. Since the impacts of neonicotinoids depending on various factors, e.g., active ingredient (imidacloprid, clothianidin and thiamethoxam), bee species (honeybees, bumblebees and solitary bees), type of exposure (acute vs. chronic and/or oral vs. contact), and study type (laboratory or field), the generalization of the effects is very difficult. However, we take all of these factors into account to provide a comprehensive insight into the current state of this issue. Thus, we consider some criteria to compare the effects of pesticides ingestion at sublethal concentrations, which are active ingredients of neonicotinoids (Imi, clo, and thia), bee species (honeybees and bumblebees), and study type (laboratory or field). The available data about NOEC and LOEC form published laboratory and field studies are extracted wherever possible and transferred to concentration unit µg/kg of diet. However, we could not give any information about the sensitivity of different bee species to different active ingredients, since there are not enough studies on all three substances and the most studies used imidacloprid as a representative member of neonicotinoids.
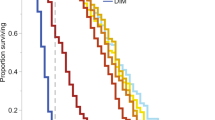
According to our previous criteria, we show that there are differences between NOEC of the active ingredient on both bee species under field conditions and laboratory conditions, where the laboratory NOEC is relatively higher than field NOEC in the most cases. An explanation for this difference is that the detected residues in the most conducted field studies to investigate the effects of exposure to neonicotinoid seed-treated crops on bees are found to be trace in pollen and/or nectar. Depending on the detected residues in pollen and nectar in the seed-treated crops, the field-realistic concentrations of these pesticides were assumed to be 1–10 µg/kg (see [24]). Nevertheless, the extracted data from the published laboratory studies indicate that there are not significant differences between NOEC and LOEC under laboratory conditions, since numerous sublethal endpoints have been developed to evaluate the exposure effects. The most of these studies are carried out at the individual level, and the effects have been reported also at the field-realistic concentrations, especially the effects related sub- and cellular functions and learning performance, etc. On the other hand, as shown in Fig. 2, the LOEC under realistic field conditions is higher than under laboratory conditions, which in turn indicate that the consequences of adverse effects in the complex context like free-flying individual and/or at colony levels related to other environmental factors are not clear. However, we cannot compare the sensitivity of solitary bees with honeybees and bumblebees, since few researches are carried out to investigate their responses to the sublethal exposure through different pathways, which mean that more studies are needed. Furthermore, the different scenarios of realistic exposure depending on the good understanding of the pollinators’ responses to sublethal exposure should be taken into account in the future experimental design in order to establish vigorous conclusions.
a–c Comparison of NOEC and LOEC of neonicotinoids for honeybees (HB) and Bumblebees (BB) under laboratory and field conditions. Different active ingredients a Imidacloprid, b clothianidin, and c thiamethoxam are compared. Extracted data are transferred to concentration unit µg/kg of diet and compared with field-realistic concentrations (FRC). Superscript ‘a’ in Tables 2, 3, and 4 indicate the corresponding references
Side effects of neonicotinoid exposure
As we interpret the different potential exposure pathways and detected concentrations under field conditions, sublethal effects might occur at low concentrations of neonicotinoids. These sublethal impacts could involve several successive modifications at different biological levels from genes to population (Fig. 3). Nowadays, at subcellular levels, there are various new approaches using new techniques, including transcriptomics, proteomics, and metabolomics. These rapid developments of new technologies are involved in ecotoxicology during the risk assessments. They play an important role in explaining the complex interactions between responses from cellular mechanisms to the whole organism and then to the population level. So, determining any alterations could be used to evaluate the impacts of very low concentrations at the individual level.
Sub- and cellular functions
Although the investigation of biochemical changes at the subcellular level might be valuable as an additional sublethal toxicity endpoint, only some studies have been carried out on the effects of neonicotinoids at the sublethal level on gene expression and enzyme activities in insect pollinators. These possible modifications in the biological processes, i.e., gene pathways, after exposure to pesticides could be associated with various impacts on the detoxification capacity, immune function, and behavioral maturation. Therefore, these studies could prove useful to evaluate the detoxification capacity and/or sensitivity of exposed bees to neonicotinoids under both field and laboratory conditions (Table 2).
Derecka et al. [35] reported several changes in the metabolic networks of honeybee larvae taken from treated colonies with imidacloprid, e.g., an overrepresentation of E-box elements in the promoter regions of genes, increased RNA levels for a cluster of genes encoding detoxifying P450 enzymes, and a reduction in the expression of the environmentally responsive Hsp90 gene, which could affect the developmental process. Furthermore, sublethal chronic exposure of honeybees to imidacloprid or Nosema ceranae imidacloprid decreases the expression of some genes relative to controls, where a significant down-regulation of immunity-related genes was observed [8]. Currently, Brandt et al. [15] reported that oral exposure to neonicotinoids for 24 h influenced the individual immunocompetence of honeybees, where a reduction in hemocyte density, the encapsulation response, and antimicrobial activity was observed. Additionally, immunohistochemical data of honeybees exposed to sublethal doses of thiamethoxam and/or to N. ceranae showed that thiamethoxam exposure only had a minor synergistic toxic effect on midgut tissue when applied as a low dose simultaneously with N. ceranae, in comparison with the effect caused by both stressors separately [59].
Mainly measurements of the enzymes’ activity after or during exposure were used to investigate any changes related to the treatment. Usually at the individual level, a large set of metabolic enzymes will be inducted into the detoxification process to protect the insect against the harmful effects of pesticides. For instance, [67] suggested that a reduced toxicity of acetamiprid and its metabolites was related to increased metabolism by cytochrome P450 monooxygenases, but no quantitative measures were taken. Alptekin et al. [6] reported a significant increase in the expression of genes encoding detoxification enzymes [P450s and carboxyl/cholinesterase (CCE)] of thiacloprid-treated bees compared with untreated bees.
On the other hand, the regulatory role of various enzymes in the honeybee workers after being exposed to sublethal doses of thiamethoxam [11] or acetamiprid and dinotefuran [10] was investigated. Different changes were observed, where the low doses induced nearly the strongest effect on some tested enzymes activities. Another study showed an increase in acetylcholinesterase AChE activity in honeybee under both field and laboratory conditions after chronic exposure to relatively low doses of neonicotinoids [13]. It is assumed that these increases in AChE activity are attributed to a typical substrate-enzyme cellular response resulting in occupying the binding site of acetylcholine and in turn an accumulation in the synapses.
Most recently, a study suggested that vitellogenin (Vg) could be used as a biomarker to determine the energy stress and sublethal effects of pesticides on honeybees, where bees exposed to imidacloprid exhibited a significant decrease in the titer of Vg which could correlate with the increased energy usage [1].
Peng and Yang [96] found that imidacloprid-treated bees during their larval stage exhibited a reduction in the density of their synaptic units in the region of the calyces, which are responsible for olfactory and visual functions. Thus, this finding confirmed that the development of the nervous system in regions responsible for both olfaction and vision is affected by exposure to imidacloprid during the larval stage.
In bumblebees (Bombus impatiens), Samson-Robert et al. [112] reported that at the beginning of the planting, AChE mRNA expression was increased in the samples collected from the neonicotinoid seed coating corn field and then decreased throughout the planting season to reach a similar level to that of bumblebees from control sites.
It should be considered that although these changes in the enzymes’ activity play an important role in the detoxification process to protect the insect against the harmful effects of pesticides, these enzymes are also very important in the metabolism of endogenous compounds such as hormones and pheromones [22]. Thus, any changes in the activity of this system might have various subsequent effects on honeybee sensitivity to pesticides, physiological homeostasis, natural behavior, and in turn weakness of individual immune systems.
Organ and system functions
The neurophysiological basis of exposure to low concentrations of neonicotinoids as cholinergic pesticides has been recently investigated using cultures of Kenyon cells (KCs) from dissociated bees’ mushroom bodies. KCs are the major neuronal component of the mushroom bodies, a higher order of a bee’s brain, and comprise over 40 % of neurons in the honeybee brain [109].
To assess age-related neuronal sensitivity to imidacloprid, cultured KCs of 1- and 13-day-old bumblebee workers (B. impatiens) were exposed to imidacloprid for 24 h. The results showed that 13-day-old nurses and foragers were more sensitive toward imidacloprid than 1-day-old workers [144].
Furthermore, whole-cell voltage-clamp and current-clamp recordings were obtained from mushroom body KCs in an acutely isolated honeybee brain to investigate the effects of different concentrations of bath-applied imidacloprid and clothianidin via an extracellular solution [94]. Both tested neonicotinoids cause a depolarization block of neuronal firing and inhibit nicotinic responses at low concentrations. The depolarization effect of clothianidin was larger than imidacloprid depending on their respective actions as full and partial nAChR agonists [16]. Recently, Moffat et al. [87] showed rapid mitochondrial depolarization of nicotinic acetylcholine receptor dependent in cultured neurons of bumblebees (Bombus terrestris audax) after acute exposure to clothianidin and after chronic exposure to imidacloprid.
However, the direct exposure of individual cells to the full dose of the pesticides makes it difficult to interpret the previously observed effects in relation to behavioral effects, because there are many metabolic and biological barriers that could modulate the achieved concentration of pesticides in the brain neurons [144]. On the other hand, these results could be useful in understanding the sensitivity of different insect pollinators when exposed to sublethal concentrations under realistic conditions.
Although neonicotinoids are considered primarily as neurotoxins, they could have impacts on secondary targets during their diffusion in the organism. Some studies investigated the cytotoxicity of these insecticides in different organs of the treated honeybees. Oliveira et al. [92] reported that newly emerged workers of Africanized honeybees orally exposed to sublethal doses of thiamethoxam exhibit morphological and histochemical alterations in the brain as well as in the midgut depending on the exposure period. They found that low doses required less time to induce morphological alterations, including the presence of condensed cells in the mushroom bodies and optical lobes compared with the higher doses. Additionally, the cellular Xylidine-Ponceau staining was intense in mushroom bodies as well as optical lobes at the beginning of the treatment and decreased over time suggesting an expression of heat shock protein to protect cells against adverse effects. In the midgut, the digestive and regenerative cells from treated bees also showed various alterations, like cytoplasm vacuolization, increased apocrine secretion, and increased cell elimination. Cytotoxic impacts were also observed in midgut and Malpighian tubules of Africanized honeybees orally exposed to sublethal doses of thiamethoxam [19]. At a relatively high applied dose of imidacloprid 8.09 ng/bee (LD50/10), various cytotoxic effects were observed in mushroom bodies, whereas in optic lobes these effects were found at lower doses indicating a higher sensitivity of optic lobes to low doses of imidacloprid [28]. In another study, numerous cytotoxic activities of imidacloprid in Malpighian tubules were observed [29]. However, none of those studies provided any information about the frequency of observed alterations in the tested bees (i.e., in all five or six tested bees per group or only in some of them).
Furthermore, other researchers have looked at the effect of imidacloprid on hypopharyngeal gland (HPG) development either in nurse bees [66] or bees of different ages [122]. Significantly smaller HPG acini were observed in treated bees compared with untreated bees. However, the authors did not determine the consumed doses of imidacloprid during the exposure period. Similar effects were found in the newly emerged caged bees chronically exposed to imidacloprid, where the HPG acini were 14.5 and 16.3 % less in 9- and 14-day-old honeybees, respectively, compared with same-aged untreated bees [64]. This modification could induce earlier field activities.
All of the previously observed changes at the cellular level due to exposing bees to sublethal doses indicate that many physiological processes could be impaired and subsequently lead to abnormal of different functions in the organism (Table 2).
Whole organism
The sensitivity of an individual after being exposed to a pesticide correlates with its ability to sequester or eliminate the metabolites from its body. Therefore, the detection of any adverse effect of pesticide exposure before populations are negatively affected plays an important role in the risk assessment process. Subsequently, various bioassays used development in vitro or in vivo to investigate the sublethal effects at the individual level. Most recently, there has been a debate about the volubility of such bioassays and attempts to standardize them. Here, we interpret the potential endpoints at the individual level that could be considered in the risk assessment (Table 3).
Cognitive performance
Cognition is very complex and covers essential functions, including the interaction processes of an individual with various environmental cues and responding to life requirements. For instance, forager bees visiting a flower show the proboscis reflex as a result of different receptors’ stimulation from the reward (nectar and/or pollen) as well as the odors and color cues. This process then induces memorization that in turn facilitates flower recognition during the next trips [86].
Regarding cognition’s involvement in various behavioral types, investigating and assessing the sublethal effects of the neurotoxins ‘neonicotinoids’ on bees are considered an attractive topic. As we describe above, the exposure to sublethal doses could cause alterations in the neural processes which in turn affect the bees’ response and behavior. To investigate the sublethal effects on bees, many in vivo and in vitro approaches were developed. We will outline the related effects of bee exposure to these pesticides.
Associative and non-associative learning and memory
To investigate the associative learning and memory of bees, there are several well-established approaches under laboratory as well as field conditions honing in on conditioning cues. The widely used method depends on the proboscis extension reflex behavior of honeybees.
An impairment in olfactory associative learning performances and memory formulation of honeybees exposed to neonicotinoid insecticides was observed [4, 31–34, 43, 63, 128, 143, 147]. These effects depended on the dose, administration way, exposure duration, and season (see Table 3). Given the different effects of different substances, it should be considered that they are not always the same for all the insecticide family. An acute oral sublethal dose of imidacloprid had no adverse effect on the learning and memory of honeybees [141]. Nevertheless, oral subchronic doses of imidacloprid impaired various aspects of olfactory learning and memory formation of honeybees [143]. Oral subchronic doses of thiamethoxam induced a slight and nonsignificant reduction in learning and memory performance, whereas topical application decreased the learning performance and eLTM of tested bees. No adverse effects of applied doses of acetamiprid on learning and memory were observed [4]. Another study failed to observe any effects on tested bees after acute exposure to thiamethoxam, while oral sublethal doses of acetamiprid impaired the long-term retention of olfactory learning in contrast to topical application that had no effects on learning and memory performance [43]. Decourtye et al. [34] demonstrated that a lower concentration of imidacloprid was required to elicit adverse behavioral effects on summer bees compared to winter bees.
Moreover, consistent results were observed under both semi-field and laboratory conditions, where a reduction in the foraging bees’ activity and at the hive entrance was associated with a decrease in olfactory performance [32]. On the other hand, Han et al. [63] supplied a new approach relying on the T-maze test to assess the sublethal effects of pesticides on the visual associative learning of honeybees. They found that oral chronic exposure to imidacloprid induced a reduction in visual learning capacities in a T-tube maze and olfactory learning performances measured with PER. Recently, Alkassab and Kirchner [5] reported that chronic oral exposure of winter honeybees to clothianidin had no effects on their learning performance, whereas specificity of early long-term memory (24 h) at 15 µg/kg was affected.
Furthermore, exposing bees during the larval stage to a sublethal dose of imidacloprid showed a decrease in their associative learning ability. These results suggested that subsequent effects are not excluded [147].
Most recently, Tan et al. [128] found that exposing Asian honeybees (Apis cerana) as larvae or as adults to actual sublethal doses of imidacloprid showed an impairment in olfactory learning. Through different exposure stages, the adults of imidacloprid-exposed larvae exhibited poorer short-term memory compared with the control, whereas the adults exposed to imidacloprid showed poorer long-term memory.
Effects on non-associative learning are not well documented. An example for such behavior is PER habituation, which is induced by stimulation of one antenna using a sucrose solution. This learning behavior indicates the bee’s ability to avoid the energy-dispersive resulting from a wrong response.
Topical acute sublethal exposure of honeybees to imidacloprid caused a reduction in the needed trials to observe habituation [75]. Another study demonstrated that topical application of imidacloprid at different ages showed contrasting effects, where in ≤7-day-old bees the number of trials for habituation increased, and in ≥8-day-old bees, the effect was a reduction in the needed trials 15–60 min post-treatment, with an increase 4 h post-treatment [61]. These contrasting effects may refer to the existence of different subtypes of nAChR with different affinities to imidacloprid (e.g., [12, 40, 132]) or its metabolites [60].
On bumblebees, Stanley et al. [124] reported memory impairment following exposure to 2.4–10 ppb thiamethoxam for 24 days. Nevertheless, they could not observe any effects on memory performance after acute exposure. Another study showed that chronic exposure to 1 ppb clothianidin had no significant effects on the associative learning and memory of bumblebees [100].
Chemical senses (olfaction and gustation)
For insect pollinators, these chemical senses play a very important role, since they are involved in various behavioral functions and tasks, e.g., the detection of food sources, recruitment of foragers (e.g., [52, 56, 82].
Applied pesticides could be attractive, repellent, or neutral for a pollinator, directly affecting their behavior before being exposed. Indirect effects could also occur after exposure as effects on the neural processes.
Neonicotinoids’ direct effects (pre-exposure) on bees have not been well investigated. Bortolotti et al. [14] reported that imidacloprid showed no repelling effect at field-relevant concentrations and had repelling effects at 500 µg/L.
Consistently, other studies demonstrated that both honeybees and bumblebees cannot distinguish between solutions uncontaminated and contaminated with imidacloprid, thiamethoxam, and clothianidin. Moreover, bees consumed more sucrose solutions when these contained imidacloprid, thiamethoxam but not clothianidin [71].
Post-exposure effects of neonicotinoids on bees’ chemical senses could trigger different alterations in their behavioral responses. Using the PER assay, bees exposed to neonicotinoids showed alterations in their gustatory threshold to sucrose [4, 42, 43, 75]. Imidacloprid led to an increase in the gustatory threshold to sucrose after oral or topical exposure [42, 75], while acetamiprid and thiamethoxam reduced the threshold after oral exposure but not by contact [4, 43].
Navigation and homing flight
Bees’ ability to search, find food and return to the nest requires integrating multiple cognitive skills, especially different forms of memory [85].
Regarding their impacts on memory formation, exposure to neonicotinoids could cause homing failure and/or longer foraging flight. These behavioral functions are essential for the bee’s life as well as nest development; therefore, there is a debate on taking this endpoint into account during the pesticides’ risk assessment. Recently, it has been attempted to standardize the methods employing a catch-and-release paradigm that proves the bee’s navigation ability after being exposed to pesticides.
Honeybees orally treated with imidacloprid-contaminated sugar solution after being trained on an artificial feeder showed a delay in their homing behavior depending on the concentration [14, 148].
Matsumoto [83] observed a reduction in successful homing flights of clothianidin-treated bees, but no effects on the homing time of the returning bees compared to control bees.
To supply more accurate details about sublethal impacts in comparison with the traditional observation of marked bees, new approaches were developed to automatically register the bees’ activity, including harmonic radar and radiofrequency identification (RFID). Henry et al. [65] reported that fewer bees returned to the colony after being treated with thiamethoxam than untreated bees. Another study demonstrated that both imidacloprid and clothianidin reduced the foraging activity and increased the foraging flights [119]. Most recently, a field study was conducted to assess the homing behavior of honeybees during their foraging on seed-treated canola using RFID. Under the experimental conditions, the authors found no effect on the flight activity or the homing ability of the exposed bees compared to control groups [134].
On the other hand, various parameters of the navigation process of honeybees could be investigated using the harmonic radar technique. Regardless of applied doses, the analysis of the navigation of bees treated with neonicotinoids (imidacloprid, clothianidin, or thiacloprid) showed modifications in the length and directional components of vector flight and homing flight. These alterations indicated that sublethal doses of the tested neonicotinoids either block the retrieval of a remote memory or alter this form of navigation memory [50].
Motor functions
Various motion activities of Apis and non-Apis bees achieved by muscular constriction are involved in different behavioral (e.g., foraging and communication) as well as physiological (e.g., digestion and respiration) aspects. The impacts of pesticides on bees’ mobility were studied by investigation of the locomotion modifications and foraging activity.
At high doses, the neonicotinoids cause numerous symptoms, which are easy to recognize by visual observation, e.g., trembling, uncoordinated movements, hyperactivity [75, 84, 127].
At low sublethal doses, alterations in the motor functions might occur to different degrees, which require efficient tests to determine and quantify them.
Grooming
Grooming behavior is an essential hygiene behavior, especially against parasites, at the individual level and in the nest. The observation of this behavior is very difficult within a teeming honeybee colony [9]. In observation hives, it is time-consuming to observe both grooming and allogrooming behaviors [97, 131]. Therefore, few in vitro studies have been conducted to point out the effects of sublethal doses on grooming behavior. Williamson et al. [142] reported that bees exposed to thiamethoxam spend more time grooming, had more bouts of grooming, and had a longer duration of grooming bouts, while imidacloprid impaired the grooming behavior in the tested bees at a higher exposure dose. An explanation for these differences may lie in the presence of different receptor subtypes in the nervous system affected by different substances.
Locomotor activity
Preliminary visual observation was performed by Lambin et al. [75], showing that contact-treating bees with imidacloprid increased the motor activity at a low applied dose (1.25 ng/bee) even after 15 min of the treatment in the tested arena, whereas an impairment of the movement was observed at higher doses.
Acute contact administration of acetamiprid at sublethal doses increased locomotor activity, whereas thiamethoxam had no effect on the treated bees [4, 43].
On the other hand, bees orally treated with sublethal doses of neonicotinoids (imidacloprid, thiamethoxam, and clothianidin) exhibited no significant changes in their motor functions, including walking, sitting, and flying. Nevertheless, exposed bees spend more time laying on their backs and had difficulties in righting themselves, due to a loss of postural control [142].
Moreover, a video-tracking experiment was used as an efficient tool to investigate the sublethal effects of pesticides on bees. Honeybees orally treated with imidacloprid showed a reduction in the distance moved [130]. Additionally, ingestion of high doses of imidacloprid during the larval stage of stingless bee (M. quadrifasciata anthidioides) affected the walking behavior, including distance walked, walking velocity, and number of stops of adults after 4 and 8 days of emergence, but not after 1 day [136].
Foraging behavior
The link between the cognitive performance and motor functions of individual bees leads to an effective foraging trips, considered as an essential behavioral function that enables optimal development of the bee populations by supplementing the necessary food.
Since the exposure to pesticides could cause several alterations in both cognitive and motor functions, investigating the foraging capacity of bees exposed to pesticides should also be carried out under semi- and field conditions to relate them to the laboratory tests.
The used protocols include the observation of the activity either on an artificial feeder or a directory on the plants, where the frequency of visits, number of active bees, intervals between visits, and the amount of food taken up are considered.
For honeybees, a tunnel experiment using small honeybee colonies (nucleus) showed that sublethal concentrations of imidacloprid reduced the proportion of active bees 4 days post-exposure [23]. Also observed was a decrease in the foraging activity in a flight cage during the exposure period with a recovery of the foraging activity after the treatment [103]. Moreover, bees orally treated with imidacloprid exhibited delays in their return visits to the feeder [119, 148].
On the other hand, various studies investigating the foraging activity on neonicotinoid seed-treated crops, e.g., maize, canola, and sunflowers, under semi- and field conditions showed no effects on the foraging activity [26, 27, 118].
On bumblebees, Gill et al. [54] found that chronic exposure to imidacloprid at field-realistic levels reduced the foraging success, particularly the pollen collecting efficiency, of worker bumblebees. Another study demonstrated no effects on the nectar foraging efficiency of bees treated with imidacloprid, whereas treated bees brought significantly less pollen back than control bees [49].
Fauser-Misslin et al. [48] observed a significant reduction in sugar water collection by neonicotinoid-treated bees in addition to a decline of pollen collection per bee over time relative to untreated bees. To the contrary, Stanley and Raine [123] observed that thiamethoxam-treated colonies collected pollen more often than controls.
Reproductive performance
Regarding long-term exposure to neonicotinoids, hazard evaluations of the side effects on the reproductive performance of different insect pollinators have received some attention recently. Such information would be very helpful to determine the long-term impacts of dietary sublethal doses. Actually, many quantification parameters related to reproductive success are more determinable for insect pollinators with annual and/or less complex life cycles like bumblebees and solitary bees compared to the complex perennial life cycle like honeybees.
Brood amount and fecundity
For honeybees, some studies investigated the reproductive performance of honeybees after being exposed to neonicotinoids, where feeding honeybee colonies with sublethal concentrations of imidacloprid in sugar syrup during the summer led to changes in the capped brood area in the treated colonies [47]. Otherwise, no negative effects of different neonicotinoids on the brood development of healthy bee colonies were found after exposure to seed-treated canola with different neonicotinoids [26, 102]. Moreover, a 4-year field program investigating the long-term effects of repeated exposure of honeybee colonies to thiamethoxam-treated maize and canola had no effects on the brood amounts [101]. Another study showed that honeybee colonies chronically exposed to thiamethoxam and clothianidin through feeding contaminated pollen over two brood cycles exhibited a decreased brood amount (−13 %), but colonies recovered in the medium term and overwintered successfully [116].
Chaimanee et al. [20] topically treated honeybee queens with sublethal doses of imidacloprid and assessed the effects on the viability of sperm stored in spermatheca. They found a significant reduction (50 %) in the sperm viability 7 days post-treatment first at 20 ppb.
For bumblebees, several reports showed adverse effects on fecundity, indicating that bumblebees could be more sensitive to neonicotinoids than honeybees. Under laboratory conditions, queenless B. terrestris micro-colonies were exposed to thiamethoxam in both pollen and honey water. Significantly fewer eggs were laid and no larvae produced at 10 μg/kg over the 28-day experimental period [45]. Another study demonstrated that exposing queenless microcolonies of bumblebee workers for 12 days to a range of imidacloprid concentrations can reduce worker fecundity by at least one-third. In contrast, ovary development was unimpaired by dietary imidacloprid except at 125 ppb. However, the workers in microcolonies exposed to 63.5 ppb imidacloprid developed their ovaries but did not lay eggs [80]. On the other hand, long-term exposure (80 days) of bumblebees (B. impatiens) to clothianidin in the pollen/sugar water mixtures showed no effects on the amount of brood or the number of workers, males, and queens at each dose [51]. Another experiment was conducted using queenright colonies of B. impatiens consisting of a queen and 30–50 workers placed in greenhouses; the results showed significantly less living brood after 11 weeks of oral exposure to imidacloprid or clothianidin depending on the dose [120]. Furthermore, Tasei et al. [129] chronically (up to 10 weeks) exposed bumblebees (B. terrestris) in micro-colonies (three workers) to imidacloprid-contaminated sugar water and pollen. Both treatments significantly affected the brood production and number of larvae ejected by workers. Queenless microcolonies of worker bumblebees exposed to thiamethoxam for 17 days showed no detectable effect on the brood production at low applied concentrations and a reduction in brood production after being exposed to high concentrations [78].
Laycock and Cresswell [79] investigated the effects of pulsed exposure (14 days ‘on dose’ followed by 14 days ‘off dose’) of bumblebees (B. terrestris) in small, standardized experimental colonies (a queen and four adult workers) to imidacloprid-contaminated pollen syrup. They estimated that 14-day exposures to dietary imidacloprid between 0.3 and 10 ppb may reduce brood production in B. terrestris colonies by between 18 and 84 %, and after 14 days without exposure, the drop in brood is ameliorated to between 2 and 19 %.
One study conducted on red mason bees (Osmia bicornis) showed that chronic and dietary exposure to thiamethoxam and clothianidin had severe detrimental effects on solitary bee reproductive output, including a reduction in total offspring production and a significantly male-biased offspring sex ratio [115].
Ontogenetic development
Special attention has recently been paid to evaluating the risk of chronic neonicotinoid exposure in the ontogenetic phases, including larval and pupal development. Some studies have been performed to investigate sublethal impacts of neonicotinoids on larval development in insect pollinators. From those, we have excluded studies using high concentrations compared with field-relevant concentrations (e.g., [57, 58], where concentrations of imidacloprid were used at 200 and 400 ppm, respectively).
For honeybees, Yang et al. [147] investigated the capped brood, pupation, and eclosion rates of the honeybee larvae after treating them directly in the hive with different dosages of imidacloprid over 4 days. No significant effects were found on the capped brood, pupation, and eclosion rates after treating larvae at low exposure doses, but at higher doses, such effects occurred. Furthermore, worker honeybee larvae reared in a brood comb containing 17 different pesticides (including residues of several neonicotinoids) expressed delayed development at day 4 and day 8 [145]. When the larvae of stingless bees (M. quadrifasciata anthidioides) were exposed to imidacloprid, a lower survival rate was found. However, no significant impacts on developmental time or on fresh body mass were observed at the white-eyed pupa stage [136].
For bumblebees, Tasei et al. [129] found no effect on the required duration for the emergence of the first male of bumblebees (B. terrestris) after being exposed chronically to imidacloprid-contaminated sugar water and pollen in micro-colonies (three workers).
For solitary bees, larvae of Osmia lignaria were exposed to imidacloprid-contaminated pollen and left either under field or laboratory conditions. Under field conditions, only medium and high treatments showed various sublethal effects including longer time needed to reach the last larval stage, complete spinning a cocoon in males only, to fully darkening of a cocoon, but no effects were found on the time until emergence and weight. No effects on the investigated parameters were observed under laboratory conditions. Moreover, exposure of alfalfa leafcutter bees (Megachile rotundata) to clothianidin had no impacts either on cocoon completion and darkening or on emergence and weight [2]. Another study conducted on red mason bees (O. bicornis) showed a lower proportion of offspring that completed larval development and/or could hatch after hibernation due to oral chronic exposure to thiamethoxam and clothianidin [115]. Generally, more quantitative and field studies at this endpoint are needed.
Adult longevity
Numerous studies have investigated the influence of prolonged exposure to neonicotinoid residues on the lifespan of the bees. These studies include laboratory, semi-field, and field experiments.
Laboratory experiments showed no significant effect on the worker honeybee longevity during 11 days after oral or contact exposure to acetamiprid and thiamethoxam [4]. Decourtye et al. [34] reported a difference between winter bees and summer bees when reacting to chronic lethal doses. Schmuck [117] did not observe an increased mortality of worker honeybees from different ages exposed to imidacloprid in contrary to Suchail et al. [127], due to various differences in experimental methodology and/or the physiological state of the tested bees. Moreover, long-term oral exposure of caged bees (over 60 days) to imidacloprid resulted in a higher mortality compared to a control after 30 days [30].
In a tunnel-feeding experiment, exposing honeybee colonies to contaminated sunflower honey with a range of imidacloprid concentrations over 39 days had no effect on the daily mortality in the tested colonies [118]. Faucon et al. [47] exposed the honeybee colonies to imidacloprid throughout 33 days and reported no increase in mortality. Moreover, under field conditions, no increased mortality was observed in the colonies placed in clothianidin-treated canola [26, 27], thiamethoxam-treated maize, and canola [101].
Exposure of bumblebees over a relatively long period (up to 10 weeks) can be performed using queenless micro-colonies (3–5 workers). Tasei et al. [129] chronically (up to 10 weeks) exposed bumblebees (B. terrestris) in micro-colonies (three workers) to imidacloprid-contaminated sugar water and pollen but did not find any effects on the longevity of the tested bees. Another experiment was conducted using colonies of B. impatiens consisting of a queen and 30–50 workers. After 11 weeks of oral exposure to a range of imidacloprid or clothianidin, the results showed a significant change of the queens’ mortality by week 6 for both imidacloprid and clothianidin at high concentrations but not at low concentrations [120]. Nevertheless, during the 28 days of the thiamethoxam exposure period, the life span of the tested bumblebees was not affected [45]. Laycock et al. [78] reported that bumblebee workers survived fewer days in queenless micro-colonies when exposed over 17 days to a high concentration (98 μg/kg) of thiamethoxam.
For solitary bees, Sandrock et al. [115] demonstrated that no effect on adult females’ longevity of the red mason bee (O. bicornis) exposed to thiamethoxam and clothianidin for 35 days was observed.
Given the results of those studies, the period of exposure played a key role in addition to different sensitivities of bee species to the tested neonicotinoids. However, various factors affect this sensitivity, including methodology and/or the physiological state of the tested bees.
Population dynamic
Bee population development is a complex process, where different strategies (i.e., increasing the brood amount, shifting the foraging activity) could succeed in maintaining the right functions of the population against external stressors. On this point, the differences between the Apis and non-Apis bees’ biology and behavior should be considered due to their different capacity to interact with the stressors. Within a population, the rapid alterations in their performance in response to stressors could enhance an adaptive process to avoid the adverse effects. But the chronic exposure to stressors could prove more problematic for population fitness. Here, we attempt to highlight the reports that investigated the related effects of neonicotinoids on the whole bee population under field conditions see (Table 4).
Intra-specific interactions within the population
Social interaction plays a critical role in social bees. Nevertheless, colony fitness depends on the communication efficiency. Therefore, any disruption in these processes could lead to a reduction in collected pollen and/or nectar; accordingly, this could affect the colony’s survival [121]. Over the period of exposure to pesticides, various social interactions, including antennation, trophallaxis, allogrooming, and nestmate recruitment by dance language, could change. To date, little is known about the effect of neonicotinoids on such interaction processes, since only two studies have investigated the effects of imidacloprid on honeybee communication. Kirchner [72] found that bees treated with imidacloprid showed trembling dancing at a concentration of 20 ppb, which in turn may decrease the recruitment of foragers and foraging activity. Another study showed a reduction in the waggle dance performance of bees treated with 0.21 ng of imidacloprid [42]. To our knowledge, no study has been carried out to investigate other social interactions, i.e., antennation, trophallaxis, and allogrooming, of bees treated with neonicotinoids compared with untreated bees. Future studies are needed to determine whether sublethal exposure to neonicotinoids affects honeybee communication.
Population development
Current debates consider whether chronic sublethal stress impairing individual bees could cause whole colonies’ failure. Further questions deal with how the cumulative effect on colony fitness could be influenced. Therefore, several studies have been conducted to investigate the performance of bee colonies related to chronic exposure to neonicotinoids. Dietary chronic exposure to pesticides could be carried out experimentally by offering contaminated food or treating plants visited by bees.
The results of field studies were sometimes conflicting, depending on the exposure period and/or the applied doses. Some long-term studies showed no observable effects on the fitness and development of the honeybee colonies after being exposed to crops treated (canola, maize, etc.) with neonicotinoids [26, 27, 101, 102]. Faucon et al. [47] did not observe adverse effects on the treated colonies with repeated sublethal doses of imidacloprid in sucrose syrup. Recently, Sandrock et al. [116] found negative short-term and long-term effects on colony performance exposed to thiamethoxam and clothianidin at sublethal field-relevant concentrations. Other studies reported adverse impacts on the treated colonies using imidacloprid-contaminated syrup at relatively high applied concentrations (up to 20 μg/kg) [38, 81].
In bumblebee colonies, individual performance is linked strongly with overall colony fitness due to their smaller size and annual colonies compared to large size and perennial honeybee colonies. Thus, bumblebees could be more sensitive to neonicotinoid exposure than honeybees [93]. Several semi-field and field experiments demonstrated that field-realistic chronic exposure to neonicotinoids (imidacloprid and clothianidin) significantly decreased colony growth rates by impairing the provisioning efficiency [49, 54, 76, 120, 140]. Moreover, the results of laboratory experiments using microcolonies were clearly comparable with other semi-field studies, where adverse effects were observed on several endpoints of colony performance [45, 80, 88]. On the other hand, some studies suggested that proper use of neonicotinoids will not influence the bumblebee colonies [25, 51, 89, 129].
However, different bee species exhibit differences in their risk profile regarding neonicotinoids. The capacity of a large colony of honeybees to buffer any reductions in foraging performance is more properly compared to a small colony of bumblebees or solitary bees, where only one female is responsible for provisioning the offspring.
Thus, effects at the population level could conversely be related to levels of sociality. Most recently, Rundlöf et al. [110] found that clothianidin-treated canola caused a reduction in the density of solitary bee (O. bicornis) and bumblebee colony growth as well as decreasing reproduction under field conditions, but no adverse effects on honeybee colonies were observed.
Generally, these findings about the side effects of sublethal neonicotinoid exposure should be taken into account to optimize the use of pesticides and avoid any possible adverse effects on the pollinator’s population.
Overwintering success
Although the overwintering phase is considered as an essential and sensitive period for successful development of perennial honeybee colonies, the long-term effects of neonicotinoids have received relatively little attention. However, some field studies indicated that chronic exposure to imidacloprid-contaminated sucrose syrup [47] or seed-treated crops with clothianidin [26, 27] and thiamethoxam [101] did not affect the overwintering success. Nonetheless, in some of these studies, relatively high losses of winter colonies (more than 30 % of tested colonies) indicated some weakness of methodological persuaders (see [27, 101]). Dively et al. [38] reported that chronic exposure to imidacloprid at the higher range of field doses (up to 20 μg/kg) could cause negative impacts on honeybee colony health and reduced overwintering success, but at field-relevant concentrations for seed-treated crops (5 μg/kg), negligible effects on colony health were observed. Recently, Rondeau et al. [108] extrapolated a possible delayed and time-cumulative toxicity of imidacloprid in some arthropods using a toxicokinetic–toxicodynamic model (TKTD). They suggested that prolonged exposure of winter bees throughout their life span (150 days) to honey contaminated with imidacloprid at 0.25 µg/kg would be lethal to a large proportion of bees nearing the end of their lives.
In conclusion, reassessment of the pesticide risk at this endpoint in relation to other possible stressors should be considered to achieve more realistic results.
No studies have been done on the overwintering success of bumblebee queens.
Synergistic effects
Synergy of xenobiotics
Frequent exposure to various xenobiotics (i.e., agrochemical and veterinary products) could occur more likely as a consequence of the foraging activities of the bees. Thus, the toxicity of specific pesticides should be tested in combination with other pesticides that exist under realistic conditions. In ecotoxicological studies, the complex interactions of pesticides could lead in some cases to enhance the toxicity of one when another is present.
Only two studies investigated the synergistic effects of neonicotinoids with other pesticides on honeybees. Iwasa et al. [67] reported synergistic effects between neonicotinoids and compounds, which inhibit the P450s involved in their metabolism. The toxicity of thiacloprid and acetamiprid was found to increase several 100-fold synergistically with triazole fungicide, but only a minimal synergistic effect between them and imidacloprid was found.
Other studies reported low levels of synergism (less than threefold maximum) between ergosterol biosynthesis inhibitor fungicides and the range of neonicotinoid insecticides (thiamethoxam, clothianidin, imidacloprid, and thiacloprid) [133].
However, understanding the synergistic mechanisms between applied xenobiotics is very important for the limitations of using some defined mixtures. Moreover, there are no systematic monitoring studies on the effects of mixture pesticide exposure on colony health and bees’ behavior; therefore, this specific issue should be given special attention.
Interaction with diseases and parasites
Generally, there are widely known diseases and parasites on bees which could negatively affect the development and health of the bees. Nowadays, increasing concerns about potential effects of pesticides on the susceptibility of bees to diseases is a vital issue [36, 114]. However, growing evidence from several recent studies demonstrated that interactive effects between various pathogens and pesticides increase the adverse impacts on the bees’ health. Most investigations were conducted between two pathogenic infections (Nosema and viral infection) on honeybees and one pathogen (Crithidia bombi) on bumblebees. Several laboratory studies showed a relationship between exposure to neonicotinoids and the Nosema load in the treated bees. Exposure to imidacloprid or thiacloprid affected the Nosema spore count and reduced honeybee worker survival [3, 39, 99, 105, 139]. However, Pettis et al. [99] reported a reduction in Nosema spore counts related to imidacloprid exposure under field conditions. Another study also suggested that neonicotinoids (acetamiprid, imidacloprid, and thiacloprid) reduce the risk of Nosema infection [98]. On the other hand, no impacts were found on the levels of Nosema infection in honeybee colonies placed close to neonicotinoid-treated canola compared to colonies at other sites [102]. More recently, a field study concluded that there are no interactions between thiacloprid and a Nosema infection [106].
However, Nosema could also be present in healthy colonies, but usually honeybees can resist it. Nevertheless, the exposure to pesticides at certain levels could affect their immune system, rendering it unable to contain the infection (see review by Sánchez-Bayo et al. [114].
For bumblebees, only one available study reported significant interaction between neonicotinoid exposure and parasite infection (C. bombi) on mother queen survival, but not in the worker bumblebees [48]. Furthermore, increased viral loads in honeybees after being exposed to neonicotinoids were observed under laboratory conditions [37, 39].
Conclusion
In the past two decades, systemic insecticides, e.g., neonicotinoids, were widely applied to provide plants with protection from root and foliar pests. On the other hand, the growing evidences from various studies has shown that sublethal effects on insect pollinators after prolonged exposure are not excluded. Therefore, this tradeoff between insecticides controlling the wide variety of agricultural pests without any threat to forager bees and/or the whole colony, which inadvertently come into contact with pesticides, is currently a vital issue in the risk assessment process.
As we reported here, sublethal impacts on bees could occur at different biological levels, where innovative and new methodologies like using molecular markers may help to address the effect mechanisms of these insecticides. Moreover, the exposure levels and detected concentrations depend on the way of application. Furthermore, various factors should be considered during the risk assessment process such as exposure duration, the season, castes, age, and developmental stage of the bees. Nevertheless, bumblebees and other bee species seem to have different exposure profiles and sensitivities compared to honeybees. Thus, the population size and its ability to regulate any behavioral changes or errors of worker performances should also be investigated in further studies under field conditions.
Finally, our comparison of evidences in published laboratory and field studies show that the lowest observed effect concentration (LOEC) under realistic field conditions in the most cases is higher than under laboratory conditions, which indicate that further long-term field research is required with consideration that our well understanding of the pollinators’ responses to sublethal exposure should be taken into account in the future experimental design in order to establish vigorous conclusions.
References
Abbo PM, Kawasaki JK, Hamilton M, Cook SC, DeGrandi-Hoffman G, Li WF, Liu J, Chen YP (2016) Effects of imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci. doi:10.1111/1744-7917.12335
Abbott VA, Nadeau JL, Higo HA, Winston ML (2008) Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J Econ Entomol 101:784–796
Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP, Le Conte Y (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12:774–782
Aliouane Y, El Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M (2009) Sub-chronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ Toxicol Chem 28:113–122
Alkassab AT, Kirchner WH (2016) Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology 25:1000–1010
Alptekin S, Bass C, Nicholls C, Paine MJI, Clark SJ, Field L, Moores GD (2016) Induced thiacloprid insensitivity in honeybees (Apis mellifera L.) is associated with up-regulation of detoxification genes. Insect Mol Biol 25:171–180
APENET (2010) Effects of coated maize seed on honey bees. Report based on results obtained from the second year of activity of the ApeNet project. CRA-API, Bologna, Italy. http://www.reterurale.it/downloads/APENET_2010_Report_EN%206_11.pdf
Aufauvre J, Misme-Aucouturier B, Vigues B, Texier C, Delbac F, Blot N (2014) Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One 9:e91686
Aumeier P (2001) Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie 32:81–90
Badawy MEI, Nasr HM, Rabea EI (2015) Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 46:177–193
Badiou-Bénéteau A, Carvalho SM, Brunet JL, Carvalho GA, Buleté A et al (2012) Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: application to the systemic insecticide thiamethoxam. Ecotoxicol Environ Saf 82:22–31
Bodereau-Dubois B, List O, Calas-List D, Marques O, Communal PY, Thany SH, Lapied B (2012) Transmembrane potential polarization, calcium influx, and receptor conformational state modulate the sensitivity of the imidacloprid-insensitive neuronal insect nicotinic acetylcholine receptor to neonicotinoid insecticides. J Pharm Exp Ther 341:326–339
Boily M, Sarrasin B, Deblois C, Aras P, Chagnon M (2013) Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: laboratory and field experiments. Environ Sci Pollut Res 8:5603–5614
Bortolotti L, Montanari R, Marcelino J, Medrzycki P, Maini S, Porrini C (2003) Effects of sub-lethal imidacloprid doses on the homing rate and foraging activity of honey bees. Bull Insectol 56:63–67
Brandt A, Gorenflo A, Siede R, Meixner M, Büchler R (2016) The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J Insect Physiol 86:40–47
Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB (2006) Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J Neurochem 99:608–615
Byrne FJ, Visscher PK, Leimkuehler B, Fischer D, Grafton-Cardwell EE et al (2014) Determination of exposure levels of honey bees foraging on flowers of mature citrus trees previously treated with imidacloprid. Pest Manag Sci 70:470–482
Casida JE, Durkin KA (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu Rev Entomol 58:99–117
Catae AF, Roat TC, De Oliveira RA, Nocelli RCF, Malaspina O (2014) Cytotoxic effects of thiamethoxam in the midgut and malpighian tubules of Africanized Apis mellifera (Hymenoptera: Apidae). Microsc Res Tech 77:274–281
Chaimanee V, Evans JD, Chen Y, Jackson C, Pettis JS (2016) Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J Insect Physiol 89:1–8
Chauzat MP, Martel AC, Cougoule N, Porta P, Lachaize J, Zeggane S, Aubert M, Carpentier P, Faucon JP (2011) An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera Apidae) to monitor pesticide presences in continental France. Environ Toxicol Chem 30:103–111
Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA et al (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15:615–636
Colin ME, Bonmatin JM, Moineau I, Gaimon C, Brun S, Vermandere JP (2004) A method to quantify and analyze the foraging activity of honey bees: relevance to the sublethal effects induced by systemic insecticides. Arch Environ Contam Toxicol 47:387–395
Cresswell JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20:149–157
Cutler GC, Scott-Dupree CD (2014) A field study examining the effects of exposure to neonicotinoid seed-treated corn on commercial bumble bee colonies. Ecotoxicology 23:1755–1763
Cutler GC, Scott-Dupree CD (2007) Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. J Econ Entomol 100:765–772
Cutler GC, Scott-Dupree CD, Sultan M, McFarlane AD, Brewer L (2014) A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2:e652
de Almeida Rossi C, Roat TC, Tavares DA, Cintra-Socolowski P, Malaspina O (2013) Effects of sublethal doses of imidacloprid in malpighian tubules of africanized Apis mellifera (Hymenoptera, Apidae). Microsc Res Tech 76:552–558
de Almeida Rossi C, Roat TC, Tavares DA, Cintra-Socolowski P, Malaspina O (2013) Brain morphophysiology of Africanized bee Apis mellifera exposed to sublethal doses of imidacloprid. Arch Environ Contam Toxicol 65:234–243
Dechaume-Moncharmont FX, Decourtye A, Hennequet-Hantier C, Pons O, Pham-Delégue MH (2003) Statistical analysis of the honeybee survival after chronic exposure to insecticides. Environ Toxicol Chem 22:3088–3094
Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S, Gauthier M, Pham-Delegue MH (2004) Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic Biochem Phys 78:83–92
Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delegue MH (2004) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol Environ Saf 57:410–419
Decourtye A, Devillers J, Genecque E, Le Menach K, Budzinski H, Cluzeau S, Pham-Delegue MH (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch Environ Contam Toxicol 48:242–250
Decourtye A, Lacassie E, Pham-Delegue MH (2003) Learning performances of honeybees (Apis mellifera L.) are differentially affected by imidacloprid according to the season. Pest Manag Sci 59:269–278
Derecka K, Blythe MJ, Malla S, Genereux DP, Guffanti A, Pavan P et al (2013) Transient exposure to low levels of insecticide affects metabolic networks of honeybee larvae. PLoS One 8:e68191
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F (2013) Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci USA 110:18466–18471
Dively GP, Embrey MS, Kamel A, Hawthorne DJ, Pettis JS (2015) Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS One 10:e0118748
Doublet V, Labarussias M, Miranda JR, Moritz RF, Paxton RJ (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol 17:969–983
Dupuis J, Louis T, Gauthier M, Raymond V (2012) Insights from honeybee (Apis mellifera) and fly (Drosophila melanogaster) nicotinic acetylcholine receptors: from genes to behavioral functions. Neurosci Biobehav Rev 36:1553–1564
EFSA (European Food Safety Authority) (2012) Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe. EFSA J 10:2752
Eiri DM, Nieh JC (2012) A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J Exp Biol 215:2022–2029
El Hassani AK, Dacher M, Gary V, Lambin M, Gauthier M, Armengaud C (2008) Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch Environ Contam Toxicol 54:653–661
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008) Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64:1099–1105
Elston C, Thompson HM, Walters KF (2013) Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 44:563–57456
European Commission (2013) European Commission, Commission Implementing Regulation (EU) No. 485/2013 of 24 May 2013 Amending Implementing Regulation (EU) No. 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off J Eur Union 139:12–26. http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32013R0485
Faucon JP, Auriéres C, Drajnudel P, Mathieu L, Ribiére M, Martel AC, Zeggane S, Chauzat MP, Aubert MFA (2005) Experimental study on the toxicity of imidacloprid given in syrup to honey bee (Apis mellifera) colonies. Pest Manag Sci 61:111–125
Fauser-Misslin A, Sadd BM, Neumann P, Sandrock C (2014) Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J Appl Ecol 51:450–459
Feltham H, Park K, Goulson D (2014) Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23:317–323
Fischer J, Müller T, Spatz AK, Greggers U, Grünewald B, Menzel R (2014) Neonicotinoids interfere with specific components of navigation in honeybees. PLoS One 9:e91364
Franklin MT, Winston ML, Morandin LA (2004) Effects of clothianidin on Bombus impatiens (Hymenoptera: Apidae) colony health and foraging ability. J Econ Entomol 97:369–373
Gawleta N, Zimmermann Y, Eltz T (2005) Repellent foraging scent recognition across bee families. Apidologie 36:325–335
Genersch E, von der Ohe W, Kaatz H, Schroeder A, Otten C, Büchler R et al (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41:332–352
Gill RJ, Ramos-Rodriguez O, Raine NE (2012) Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491:105–108
Girolami V, Mazzon L, Squartini A, Mori N, Marzaro M, Dibernardo A, Greatti M, Giorio C, Tapparos A (2009) Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. J Econ Entomol 102:1808–1815
Giurfa M (1993) The repellent scent-mark of the honeybee Apis mellifera ligustica and its role as communication cue during foraging. Insect Soc 40:59–67
Gregorc A, Ellis JD (2011) Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pest Biochem Physiol 99:200–207
Gregorc A, Evans JD, Scharf M, Ellis JD (2012) Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J Insect Physiol 58:1042–1049
Gregorc A, Silva-Zacarin EC, Carvalho SM, Kramberger D, Teixeira EW, Malaspina O (2016) Effects of Nosema ceranae and thiametoxam in Apis mellifera: a comparative study in Africanized and Carniolan honey bees. Chemosphere 147:328–336
Guez D, Belzunces LP, Maleszka R (2003) Effects of imidacloprid metabolites on habituation in honeybees suggest the existence of two subtypes of nicotinic receptors differentially expressed during adult development. Pharmacol Biochem Behav 75:217–222
Guez D, Suchail S, Gauthier M, Maleszka R, Belzunces LP (2001) Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honeybees (Apis mellifera). Neurobiol Learn Mem 76:183–191
Haith DA (2010) Ecological risk assessment of pesticide runoff from grass surfaces. Environ Sci Technol 44:6496–6502
Han P, Niu CY, Lei CL, Cui JJ, Desneux N (2010) Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 19:1612–1619
Hatjina F, Papaefthimiou C, Charistos L, Dogaroglu T, Bouga M et al (2013) Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie 44:467–480
Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Heylen K, Gobin B, Arckens L, Huybrechts R, Billen J (2011) The effects of four crop protection products on the morphology and ultrastructure of the hypopharyngeal gland of the European honeybee, Apis mellifera. Apidologie 42:103–116
Iwasa T, Motoyama N, Ambrose JT, Roe MR (2004) Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot 23:371–378
Jeschke P, Nauen R (2008) Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag Sci 64:1084–1098
Joachimsmeier I, Pistorius J, Schenke D, Kirchner WH (2012) Guttation and risk for honeybee colonies (Apis mellifera L.): use of guttation drops by honey bees after migration of colonies—a field study. Jul Kuhn. doi:10.5073/jka.2012.437.016
Jones A, Harrington P, Turnbull G (2014) Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Manag Sci 70:1780–1784
Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S et al (2015) Bees prefer foods containing neonicotinoid pesticides. Nature 521:74–76
Kirchner WH (1999) Mad-bee-disease? Sublethal effects of imidacloprid (Gaucho) on the behaviour of honeybees. Apidologie 30:421–422
Klein AM, Vaissiére BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc Biol Sci 274:303–313
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One 7:e29268
Lambin M, Armengaud C, Raymond S, Gauthier M (2001) Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch Insect Biochem Physiol 48:129–134
Larson JL, Redmond CT, Potter DA (2013) Assessing insecticide hazard to bumble bees foraging on flowering weeds in treated lawns. PLoS One 8:e66375
Lawrence TJ, Culbert EM, Felsot AS, Hebert VR, Sheppard WS (2016) Survey and risk assessment of Apis mellifera (Hymenoptera: Apidae) exposure to neonicotinoid pesticides in urban, rural, and agricultural settings. J Econ Entomol 109:520–528
Laycock I, Cotterell KC, O’Shea-Wheller TA, Cresswell JE (2014) Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol Environ Saf 100:153–158
Laycock I, Cresswell JE (2013) Repression and recuperation of brood production in Bombus terrestris bumble bees exposed to a pulse of the neonicotinoid pesticide imidacloprid. PLoS One 8:e79872
Laycock I, Lenthall KM, Barratt AT, Cresswell JE (2012) Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21:1937–1945
Lu C, Warchol KM, Callahan RA (2012) In situ replication of honey bee colony collapse disorder. Bull Insectol 65:99–106
Maisonnasse A, Lenoir JC, Beslay D, Crauser D, Le Conte Y (2010) E-β-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS One 5:e13531
Matsumoto T (2013) Reduction in homing flights in the honey bee Apis mellifera after a sublethal dose of neonicotinoid insecticides. Bull Insectol 66:1–9
Medrzycki P, Montanari R, Bortolotti L, Sabatini AG, Maini S, Porrini C (2003) Effects of imidacloprid administered in sub-lethal doses on honey bee behaviour. Laboratory tests. Bull Insectol 56:59–62
Menzel R, De Marco RJ, Greggers U (2006) Spatial memory, navigation and dance behaviour in Apis mellifera. J Comp Physiol A 192:889–903
Menzel R, Greggers U, Hammer M (1993) Functional organization of appetitive learning and memory in a generalist pollinator, the honey bee. In: Papaj DR, Lewis AC (eds) Insect learning. Chapman Hall, New York, pp 79–125
Moffat C, Pacheco JG, Sharp S, Samson AJ, Bollan KA, Huang J et al (2015) Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). FASEB J 29:2112–2119
Mommaerts V, Reynders S, Boulet J, Besard L, Sterk G, Smagghe G (2010) Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 19:207–215
Morandin LA, Winston ML (2003) Effects of novel pesticides on bumble bee (Hymenoptera: Apidae) colony health and foraging ability. Environ Entomol 32:555–563
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, Pettis JS (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honeybee health. PLoS One 5:e9754
Nikolakis A, Chapple A, Friessleben R, Neumann P, Schad T, Schmuck R et al (2009) An effective risk management approach to prevent bee damage due to the emission of abraded seed treatment particles during sowing of seeds treated with bee toxic insecticides. Jul Kühn Arch 423:132–148
Oliveira RA, Roat TC, Carvalho SM, Malaspina O (2014) Side-effects of thiamethoxam on the brain and midgut of the africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ Toxicol 29:1122–1133
Osborne JL (2012) Bumblebees and pesticides. Nature 491:43–45
Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA et al (2013) Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat Commun 4:1634
Pareja L, Colazzo M, Pérez-Parada A, Niell S, Carrasco-Letelier L, Besil N, Cesio MV et al (2011) Detection of pesticides in active and depopulated beehives in Uruguay. Int J Environ Res Public Health 8:3844–3858
Peng YC, Yang EC (2016) Sublethal dosage of imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci Rep 6:19298
Peng YS, Fang Y, Xu S, Ge L (1987) The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J Invertebr Pathol 49:54–60
Pettis JS, Lichtenberg EM, Andree M, Stitzinger J, Rose R, vanEngelsdorp D (2013) Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One 8:e70182
Pettis JS, vanEngelsdorp D, Johnson J, Dively G (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99:153–158
Piiroinen S, Botías C, Nicholls E, Goulson D (2016) No effect of low-level chronic neonicotinoid exposure on bumblebee learning and fecundity. PeerJ 4:e1808
Pilling E, Campbell P, Coulson M, Ruddle N, Tornier I (2013) A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS One 8:e77193
Pohorecka K, Skubida P, Miszczak A, Semkiw P, Sikorski P, Zagibajlo K et al (2012) Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J Apic Sci 56:115–134
Ramirez-Romero R, Chaufaux J, Pham-Delegue MH (2005) Effects of Cry1Ab protoxin, deltamethrin and imidacloprid on the foraging activity and the learning performances of the honeybee Apis mellifera, a comparative approach. Apidologie 36:601–611
Reetz JE, Schulz W, Seitz W, Spiteller M, Zühlke S, Armbruster W, Wallner K (2016) Uptake of neonicotinoid insecticides by water-foraging honey bees (Hymenoptera: Apidae) through guttation fluid of winter oilseed rape. J Econ Entomol 109:31–40
Retschnig G, Neumann P, Williams GR (2014) Thiacloprid-Nosema ceranae interactions in honey bees: host survivorship but not parasite reproduction is dependent on pesticide dose. J Invertebr Pathol 118:18–19
Retschnig G, Williams GR, Odemer R, Boltin J, Di Poto C, Mehmann MM et al (2015) Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environ Microbiol 17:4322–4331
Rexrode M, Barrett M, Ellis J, Gabe P, Vaughan A, Felkel J, Melendez J (2003) EFED risk assessment for the seed treatment of clothianidin on corn and Canola. United States Environmental Protection Agency, Washington, DC
Rondeau G, Sánchez-Bayo F, Tennekes HA, Decourtye A, Ramírez-Romero R, Desneux N (2014) Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci Rep 4:5566
Rössler W, Groh C (2012) Plasticity of synaptic microcircuits in the mushroom-body calyx of the honey bee. In: Eisenhardt D, Giurfa M, Galizia CG (eds) Honeybee neurobiology and behavior—a tribute to Randolf Menzel. Springer, Dordrecht, pp 141–153
Rundlöf M, Andersson GKS, Bommarco R, Fries I, Hederström V, Herbertsson L et al (2015) Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80
Samson-Robert O, Labrie G, Chagnon M, Fournier V (2014) Neonicotinoid-contaminated puddles of water represent a risk of intoxication for honey bees. PLoS One 9:e108443
Samson-Robert O, Labrie G, Mercier PL, Chagnon M, Derome N, Fournier V (2015) Increased acetylcholinesterase expression in bumble bees during neonicotinoid-coated corn sowing. Sci Rep 5:12636
Sánchez-Bayo F, Hyne RV (2014) Detection and analysis of neonicotinoids in river waters-development of a passive sampler for three commonly used insecticides. Chemosphere 99:143–151
Sánchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N (2016) Are bee diseases linked to pesticides?—A brief review. Environ Int 89–90:7–11
Sandrock C, Tanadini LG, Pettis J, Biesmeijer JC, Potts SG et al (2014) Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric For Entomol 16:119–128
Sandrock C, Tanadini M, Tanadini LG, Fauser-Misslin A, Potts SG, Neumann P (2014) Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS One 9:e103592
Schmuck R (2004) Effects of a chronic dietary exposure of the honeybee Apis mellifera (Hymenoptera: Apidae) to imidacloprid. Arch Environ Contam Toxicol 47:471–478
Schmuck R, Schöning R, Stork A, Schramel O (2001) Risk posed to honeybees (Apis mellifera L., Hymenoptera) by an imidacloprid seed dressing of sunflowers. Pest Manag Sci 57:225–238
Schneider CW, Tautz J, Gruenewald B, Fuchs S (2012) RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One 7:e30023
Scholer J, Krischik V (2014) Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS One 9:e91573
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press, Cambridge
Smodis Skerl MI, Gregorc A (2010) Heat shock proteins and cell death in situ localisation in hypopharyngeal glands of honeybee (Apis mellifera carnica) workers after imidacloprid or coumaphos treatment. Apidologie 41:73–86
Stanley DA, Raine NE (2016) Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct Ecol. doi:10.1111/1365-2435.12644
Stanley DA, Smith KE, Raine NE (2015) Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci Rep 5:16508
Stewart SD, Lorenz GM, Catchot AL, Gore J, Cook D, Skinner J, Mueller TC, Johnson DR, Zawislak J, Barber J (2014) Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-Southern United States. Environ Sci Technol 48:9762–9769
Stoner KA, Eitzer BD (2012) Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS One 7:e39114
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20:2482–2486
Tan K, Chen W, Dong S, Liu X, Wang Y, Nieh JC (2015) A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci Rep 5:10989
Tasei JN, Lerin J, Ripault G (2000) Sublethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manag Sci 56:784–788
Teeters BS, Johnson RM, Ellis MD, Siegfried BD (2012) Using video-tracking to assess sublethal effects of pesticides on honey bees (Apis mellifera L.). Environ Toxicol Chem 31:1349–1354
Thakur RK, Bienefeld K, Keller R (1997) Varroa defense behavior in A. mellifera carnica. Am Bee J 137:143–148
Thany SH, Gauthier M (2005) Nicotine injected into the antennal lobes induces a rapid modulation of sucrose threshold and improves short-term memory in the honeybee Apis mellifera. Brain Res 1039:216–219
Thompson HM, Fryday SL, Harkin S, Milner S (2014) Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie 45:545–553
Thompson H, Coulson M, Ruddle N, Wilkins S, Harkin S (2016) Thiamethoxam: assessing flight activity of honeybees foraging on treated oilseed rape using radio frequency identification technology. Environ Toxicol Chem 35:385–393
Thompson HM, Wilkins S, Harkin S, Milner S, Walters KF (2015) Neonicotinoids and bumblebees (Bombus terrestris): effects on nectar consumption in individual workers. Pest Manag Sci 71:946–950
Tomé HVV, Martins GF, Lima MAP, Campos LAO, Guedes RNC (2012) Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS One 7:e38406
Van der Sluijs JP, Simon-Delso S, Goulson D, Maxim L, Bonmatin JM, Belzunces LP (2013) Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr Opin Environ Sustain 5:293–305
Van Dijk TC, Van Staalduinen MA, Van der Sluijs JP (2013) Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One 8:e62374
Vidau C, Diogon M, Aufauvre J, Fontbonne R, Vigues B, Brunet JL et al (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by nosema ceranae. PLoS One 6:e21550
Whitehorn PR, O’Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352
Williamson SM, Baker DD, Wright GA (2013) Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invertebr Neurosci 13:63–70
Williamson SM, Willis SJ, Wright GA (2014) Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology 23:1409–1418
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807
Wilson DE, Velarde RA, Fahrbach SE, Mommaerts V, Smagghe G (2013) Use of primary cultures of Kenyon cells from bumblebee brains to assess pesticide side effects. Arch Insect Biochem Physiol 84:43–56
Wu JY, Anelli CM, Sheppard WS (2011) Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS One 6:e14720
Xu T, Dyer DG, McConnell LL, Bondarenko S, Allen R, Heinemann O (2016) Clothianidin in agricultural soils and uptake into corn pollen and canola nectar after multi-year seed treatment applications. Environ Toxicol Chem 35:311–321
Yang EC, Chang HC, Wu WY, Chen YW (2012) Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS One 7:e49472
Yang EC, Chuang YC, Chen YL, Chang LH (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101:1743–1748
Acknowledgments
We thank the team of behavioral biology and biology education at the Ruhr-University for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkassab, A.T., Kirchner, W.H. Sublethal exposure to neonicotinoids and related side effects on insect pollinators: honeybees, bumblebees, and solitary bees. J Plant Dis Prot 124, 1–30 (2017). https://doi.org/10.1007/s41348-016-0041-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-016-0041-0