Abstract
Mushroom bodies (MBs) are prominent neuropils in the insect brain that have been implicated in higher order processing such as sensory integration, learning and memory, and spatial orientation. Hymenoptera, like the honey bee, possess particularly large MBs with doubled MB calyces (major sensory input structures of the MBs) that are divided into compartments. In this review we focus on characteristic modular synaptic complexes (microglomeruli, MG) in the honey bee MB calyx (CA). The main components of MG comprise a presynaptic bouton from projection neurons (PNs) (e.g. olfactory, visual), numerous dendritic spines from MB intrinsic neurons (Kenyon cells, KC), and processes from recurrent GABAergic neurons. Recent work has demonstrated a remarkable structural plasticity of MG associated with postembryonic brood care, age, sensory experience, and stable long-term memory. The mechanisms and functional significance of this neuronal plasticity are discussed and related to behavioral plasticity and social organization.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Honey Bee Mushroom Bodies
The mushroom bodies (MBs) are prominent paired structures on each side of the central brain of the honey bee (Fig. 3.2.1a). Anatomically, each MB is subdivided into the cup-shaped calyces – major sensory input regions of the MBs – the pedunculus (PED), and the medial (ML) and vertical lobes (VL) – the main output regions of the MBs (nomenclature after [43], Fig. 3.2.1), (see Chap. 3.1). In the honey bee and other Hymenoptera the MBs form a lateral (LCA) and medial (MCA) calyx in each brain hemisphere. Various studies in the honey bee and in other insects (e.g. Drosophila) have assigned the MBs important roles in higher sensory integration and in the organization of complex behaviors that involve learning and the formation of associative memories (e.g. [13, 20, 28]; see also Chaps. 6.1–6.5).
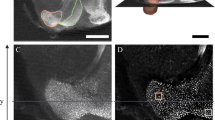
Brain of the honey bee triple-labeled with an antibody to synapsin (red), f-actin-phalloidin (green), and Hoechst nuclear marker (blue). (a) Overview of a central plane in the brain with the major neuropil regions. (b) Higher magnification of one branch of the mushroom body (MB) calyx and anatomically distinct olfactory and visual subregions. (c) and (d) Higher magnification of double-labeled microglomeruli (MG) in the lip and collar region (a physical indentation separates the lip from the collar). (e) Individual double-labeled microglomerulus with synapsin immunoreactivity in a bouton of a projection neuron (PN) in red and f-actin-phalloidin staining of Kenyon cell (KC) dendritic profiles in green. (f) and (g) Electron micrographs of a section through a MG in the lip (f) and collar (g) of a young worker bee (Large presynaptic boutons with multiple dark-labeled active zones are surrounded by numerous small profiles, most of them presumably from KC dendrites). (h) Schematic diagram of one MG (modified after [14]). For clarity, only the presynaptic bouton of a projection neuron (PN) and profiles of KC dendritic spines are shown. (i) Schematic diagram indicating putative excitatory (+) and inhibitory (−) synaptic connections between projections neurons (PN), GABAergic recurrent neurons (GABA), and Kenyon cells (KC) based on immune-electron microscopic studies by Ganeshina and Menzel [11] (scheme modified after [11]). Further abbreviations: AL antennal lobe, BR basal ring, CX central complex, DCO dense collar region, CO collar region, LA lamina, LCO loosely arranged collar region, LO lobula, LCA lateral MB calyx, LIP lip region, MCA medial MB calyx, ME medulla, and PED pedunculus. Scale bar in A = 200 μm; B = 25 μm; C, D = 10 μm; E-G = 1 μm
The total number of neurons in the brain of a worker bee was estimated with ∼850,000, and the number of Kenyon cells (KCs), with ∼184,000 on each side [30, 38, 43, 48]. This adds to ∼368,000 KCs making up more than ∼40% of the total number of neurons in the honey bee brain. For comparison, in Drosophila the total brain neuron population was estimated ∼150,000 [2] and the KC population with ∼2,000 in each brain hemisphere [3] representing less than ∼4% of the total number of brain neurons.
What is the adaptive value of very large MBs with a high number of intrinsic KC neurons as found in the honey bee? The large population of KCs and associated neuronal microcircuits is highly suggestive for an elaborated computational potential and increased neuronal plasticity and associated storage capacities [40]. It is interesting in this context that a recent developmental study suggests that the invertebrate MBs and vertebrate pallium (including the cerebral cortex) have a common origin [45].
The main focus of this chapter is to review recent work on the synaptic organization of the major input structures of the MBs, the calyces, in particular their structural and functional plasticity. Further neuroanatomical and functional aspects like neurotransmitter systems and the role of the MBs in learning and memory are summarized in other chapters of this book. Whereas in many insects the MB calyces predominantly receive olfactory input, the MB calyces in the honey bee (and in other Hymenoptera like ants) form prominent multimodal sensory input regions (Fig. 3.2.1). Dendrites from different classes of KCs receive input from projection neurons (PNs) of primary olfactory centers and from the optic lobes (OL) (e.g. [17, 30, 38, 43]), and they are innervated by PNs form the subesophageal calycal tract – mediating most likely gustatory and/or tactile input [37]. This chapter summarizes recent work on structural plasticity of synaptic complexes in the CA – the MG, in particular plasticity associated with brood care and environmental influences, sensory experience, age, and stable long-term memory. The resulting structural and functional changes in MG are assumed to contribute to long-term changes and adaptations in behavior associated with social organization.
1.1 Anatomical Subdivisions of the Mushroom-Body Calyces
In Hymenoptera, in particular in bees [14, 16, 17, 30, 43], social wasps [32], and ants (e.g. [42]), the MBs are large in relation to other brain regions. The MB calyces comprise three anatomically distinct and clearly delineated subdivisions: the lip (LIP), the collar (CO) and the basal ring (BR) receiving olfactory, visual and both sensory modalities, respectively (Fig. 3.2.1b). The lip region can further be subdivided into a cortical and central input zone innervated by olfactory PNs from two antennal lobe-protocerebral tracts (the medial and the lateral APT) ([1, 23], tract nomenclature after [10]). In the honey bee, a distinct region at the transition between the lip and collar region was shown to receive input from PNs of the subesophageal calycal tract (SCT), most likely transmitting information from gustatory and mechanosensory neurons of the proboscis or other mouthparts [37]. The basal ring (BR) is organized in distinct layers receiving input from olfactory and bilateral visual PNs [17, 23, 37]. The visual collar is further subdivided in distinct layers innervated by visual PNs that transfer chromatic, temporal, and motion sensitive input from the OLs (lobula (LO) and medulla (ME)) [34].
1.2 MG – Characteristic Synaptic Complexes in the Mushroom-Body Calyx
The different neuropil subregions of the MB calyces contain distinct synaptic complexes that were termed MG (Fig. 3.2.1e–h). Anatomical details of MG were pioneered by electron microscopic (EM) studies in ants [41]. Detailed EM studies of the synaptic organization of MG in the honey bee were performed in Randolf Menzel’s lab [11], showing synaptic microcircuits formed between olfactory PN boutons, KC dendrites and recurrent GABAergic extrinsic neurons. These investigations demonstrate the complexity of putative excitatory and inhibitory synaptic profiles in MG (Fig. 3.2.1h, i). EM studies by Yasuyama et al. [49] revealed a similar organization in the CA of Drosophila indicating that MG may represent evolutionary conserved functional units. Double staining of PN axons and KC dendritic profiles indicate that the majority of MG in the lip region comprise a central presynaptic bouton from olfactory PNs surrounded by a dense shell of numerous postsynaptic profiles (Fig. 3.2.1e, and schematic drawing in Fig. 3.2.1h), mainly formed by KC dendritic spines [9, 11, 12, 14–16, 39, 42, 49]. Double-immunolabeling with an antibody to the Drosophila synaptic-vesicle associated protein synapsin (from E. Buchner, Univ. Würzburg) in combination with f-actin-phalloidin staining and EM studies showed that KC dendritic tips are highly enriched with f-actin supporting their spine-like nature ([9, 14, 16, 42], Fig. 3.2.1e, h). In the honey bee, in addition to supply from PNs, MG microcircuits are innervated by a widely branched network of recurrent GABAergic neurons originating from the MB lobes (e.g. [18]). Furthermore, the MB calyces in the honey bee receive input from modulatory systems, in particular octopaminergic and dopaminergic neurons (e.g. [19]), which play an important role in associative learning (see also Chaps. 3.1 and 6.1–6.5). The precise synaptic connection of neuromodulatory and other extrinsic neurons into the MG microcircuits requires further investigation.
2 Structural Plasticity of MG at Different Life Stages of the Honey Bee
2.1 Influences of Postembryonic Brood Care
Cooperative brood care represents a most important feature in the organization of insect societies. Differential larval feeding and a remarkable phenotypic plasticity form the basis for the queen worker polymorphism and the determination of female castes [46]. Honey bee queens develop from fertilized eggs that are genetically not different from those that develop into workers, but they develop faster, are larger, live longer and differ markedly in their behavior. In addition to larval feeding the temperature of pupae is tightly controlled to 35 ± 0.5°C by thermoregulatory behavior of adult worker bees to ensure proper metamorphic brood development. Similarly in ants, pupae are exposed to controlled temperature ranges to regulate postembryonic development, and ant nurses respond to changes in the ambient temperature by carrying the brood to nest areas with the appropriate temperatures [47]. Recent studies in honey bees and ants have shown that pupae that developed at different temperatures within the range of naturally occurring temperatures in the brood area (33–36°C in the case of the honey bee) exhibit differences in adult behavior [4, 44, 47].
Groh et al. [14, 16] and Groh and Rössler [15] investigated effects of both larval feeding and naturally occurring variations in pupal temperature on the number of MG in freshly emerged adult bees. The results revealed that both larval feeding and pupal thermoregulation affect the adult number of MG. One day old queens, compared to workers, had significantly lower numbers of MG in both the lip and collar regions at all temperatures tested, and worker pupae raised at the lower range of naturally occurring brood temperatures (33°C compared to 34.5°C) had lower numbers of MG in the adult CA lip (but not in the collar region) compared to those raised at the natural temperature in the central brood region (34.5°C). Developmental studies showed that the temporal sequence of MG formation was substantially faster (by about 4 days) in queens compared to workers reared at the natural temperature [15]. Behavioral experiments indicate that adult bees reared at the lower range of natural occurring brood temperatures perform less well in associative memory tasks, forage later, and differ in dance-communication performance and undertaking behavior compared to bees raised at higher temperature [4, 44]. Similarly, ants that were raised at different temperatures during pupal development differed in their adult temperature thresholds that are needed to induce brood carrying behavior [47].
The results indicate that differential brood care may cause substantial changes in MG numbers in the adult CA. Whether or how these changes are causally linked to the changes demonstrated in complex behaviors including learning and memory remains to be shown. It is appealing to speculate that developmental plasticity of MG in the CA may also contribute to changes in sensory thresholds for the display of certain behaviors. This in turn may influence associative learning performance and division of labor according to a “threshold model” proposed by Pankiw and Page [33], (see Chap. 1.1). The most challenging task for future work will be to causally link differential brood-care to changes in behavior at both the individual and colony level.
2.2 Effects of Adult Behavioral Maturation, Sensory Experience and Age
Do adult sensory experience and age affect the structural plasticity of MG? A robust volume increase of the MB calyces at the onset of foraging was reported in several studies on social Hymenoptera – in bees (e.g. [5]), in ants (e.g. [42]), and in wasps, [32]. The cellular mechanism for these volume changes remained unclear. A Golgi study by [8] first indicated that in the visual subregions (collar) outgrowth of KC dendrites may contribute to volume increase in the CA during the transition to foraging. Volume studies by Ismail et al. [22] combined with pharmacological stimulation suggest that activity mediated by muscarinic cholinergic pathways trigger a volume increase comparable to the one induced by foraging experience.
What are the cellular mechanisms for these remarkable volume changes in the MBs – and how do sensory experience and age contribute to it? To address this question, effects of sensory experience and age (or an intrinsic program) need to be dissected. Due to the close contact between individuals in social-insect colonies, complete olfactory deprivation is impossible without removing the sensory organs (the antennae) or isolation. Kleineidam and Rössler [24] performed an olfactory deprivation study in Camponotus ants by unilateral removal of the antenna on the first day of adult life. This caused a substantial reduction of the volume of AL glomeruli after ∼15 days. Interestingly, no obvious changes in MG numbers were found in the olfactory subregions of the ipsilateral MB-calyx lip, which is innervated only by input from the ipsilateral antenna. Similar observations were made in the honey bee (Rössler, unpublished results). This may indicate that input from the contralateral side at the level of the MBs may compensate for unilateral loss of sensory olfactory input.
Another study focused on manipulations of the environment (natural environment versus artificially reduced environment) and/or manipulations at the colony level (experimental induction of precocious or delayed foragers) [25]. This study revealed effects on the number of MG in olfactory and visual regions of the CA, but the problem with broad manipulations of the social environment is that many variables (olfactory, visual stimuli and physical contact), are changed at the same time.
Long living honey bee queens show a remarkable age-related decrease of MG in the collar and an increase in the olfactory lip [14]. A study by Stieb et al. [42] on Cataglyphis ants was able to dissect effects of age and sensory (in this case visual) experience. Ants from dark-reared Cataglyphis fortis colonies were exposed to light precociously or delayed. The results clearly demonstrate that precocious sensory exposure (on the first day of adult life) as well as delayed light exposure (even after 6 and 12 months) triggered a decrease in MG numbers associated with CA volume change similar to that observed at the transition from indoor activities to foraging. Artificially aged ants that had lived in constant darkness over a period of 6–12 months showed a very slow increase in MG numbers, even in the collar. This is in contrast to aged honey bee queens [14], which showed a decrease of MG in the collar, indicating that caste-specific (or species-specific) differences in aging phenomena have to be considered.
Anti-tubulin staining is restricted to the dendritic shafts of KCs [42]. Combined f-actin and tubulin staining of KC dendritic processes revealed that the most drastic effect of precocious light exposure and natural maturation appears to be a massive outgrowth of KC-dendritic shafts and an associated increase in tubulin positive profiles in between MG. At the same time, the number of PN boutons per area decreased, and both processes (decease in presynaptic bouton density and KC dendrite expansion) resulted in a net expansion of the total MB-calyx volume [42]. This indicates that pruning of MG was involved, confirming an EM study in Cataglyphis ants by Seid and Wehner [39]. Future experiments need to show whether the effects on MG reorganization are causally linked to the onset of foraging behavior. To be able to further dissect mechanisms and effects of age and sensory experience on MB synaptic plasticity in the honey bee, we need to learn more about the molecular pathways involved, to combine experiments with pharmacology, RNA interference, or neuroendocrine and hormonal manipulations. Furthermore, interesting ageing models like winter bees or artificially aged dark reared (visually deprived) cohorts will offer very promising targets to dissect the mechanisms and consequences of age- and experience dependent structural synaptic plasticity in the MB calyces.
2.3 Structural Plasticity of MG Related to Stable Long-Term Memory
Transcription-dependent formation of a stable long-term memory after olfactory conditioning is associated with structural synaptic plasticity of MG in the olfactory lip of the honey bee CA [21]. In this study honey bees were trained to associate a sugar reward with odor using a five-trial conditioning paradigm and the proboscis extension response. Only the bees that had received paired stimulation of the conditioned (odor pulse) and unconditioned stimulus (sugar water) (CS and US), and that were not injected with Actinomycin D (ActD, a specific transcription inhibitor) 3 h after training, retained a stable long-term memory (LTM) after 3 days when they were tested with the CS. Most importantly, the formation of a stable LTM was associated with an increase in the density of MG in the olfactory lip. The increase in MG was modality specific as only the olfactory lip, but not the visual collar was affected. Naïve (unstimulated) bees, and bees that had received unpaired stimulation, as well as paired bees that had been injected with ActD did not show memory retrieval for the rewarded odor after 3 days (and the unpaired and naïve groups showed similar MG density values). The density of MG was sampled only in a central region of the CA, but there may be a wide distribution of structural plasticity in MG across the MB-calyx lip. This is supported by the fact that PNs have widely scattered boutons ([1, 31], see also Chap. 3.1). On the other hand, individual PNs may differ substantially regarding the contribution to MG-density changes depending on their odor specificity. Transcription-independent memories, such as early-long-term memory (eLTM) did not lead to any detectable stable changes as the unpaired and paired ActD groups displayed similar MG densities. Therefore, Hourcade et al. [21] conclude that the formation of a transcription-dependent LTM is accompanied by a stable increase in the density of MG in the MB-calyx lip. They further speculate that structural synaptic rearrangements and growth of new synapses may be a common feature involved in stable LTM in mammalian as well as in insect brains.
Previous calcium imaging experiments in Randolf Menzel’s lab had shown that five spaced CS-US presentations lead to an increase in calcium activity in the lip in response to the rewarded odor [6] indicating that calcium may be correlated with structural plasticity after multi trial learning. In fact, Perisse et al. [35] showed that calcium is essential for LTM formation, which further adds to a potential role of calcium in mediating structural plasticity associated with stable LTM. The structural synaptic changes may be part of a memory trace, but whether they are actually required for memory storage remains to be determined.
3 Outlook
The results from studies over the past years provide increasing evidence that MG in the CA undergo structural reorganization related to brood care (food and thermoregulation) [14–16], behavioral maturation [25, 42], sensory experience and age [14, 42], and are associated with the formation of stable LTM [21].
Due to the well known structural and functional properties of the olfactory pathway, the CA lip provides a particularly feasible neuronal substrate to study the mechanisms and consequences of long-term plasticity. MG in the lip region receive input from olfactory PNs via two antennal lobe-protocerebral tracts [10, 23, 31], and they receive putative modulatory reinforcement via the octopaminergic VUMmx1 neuron [11, 19, 28]. Future work is necessary to identify the physiological properties and functional role of different assemblies of MG within the CA lip, which may be diverse in their composition.
Whereas structural features of the CA input from the OLs to the collar region and from the subesophageal ganglion (SEG) to a region between lip and collar is well investigated, much less is known about the function [17, 34, 37], and almost nothing is known about the multimodal function of the BR. Furthermore, reverberant activity via GABAergic neurons and its potential influence on MG plasticity requires further investigations at both the structural and functional levels [18, 36].
The presence of f-actin rich KC dendritic spines [9, 14, 16] indicates that rearrangements of cytosceletal elements are likely to mediate structural plasticity of MG, similar to activity dependent structural plasticity of synaptic spines in hippocampal neurons (e.g. [50]). Interference with actin dynamics and associated molecular pathways will be very elusive to investigate the molecular mechanisms. Another interesting point is the potential role of epigenetic changes on neuronal networks in the MBs. Recent studies have shown a remarkable role of DNA methylation in controlling queen-worker polymorphism ([26], see also Chap. 5.4). Structural plasticity in CA MG will provide an ideal substrate to investigate how epigenetic modifications may affect synaptic microcircuits.
Structural reorganization of MG is suggestive to play a role in long-term changes of behavior. Its enormous size in Hymenoptera brings the CA MG in a position to function as a neuronal substrate for “life-time memory” (or “life-history memory”). Whether structural rearrangements of MG are causal for the storage of long-term memory, however, remains to be shown.
The olfactory pathway in the honey bee is characterized by connections of a dual olfactory pathway to the MBs and lateral horn (LH) [10]. Long-term changes in the MB neuronal networks may contribute plastic components onto more rigid or “hard wired” parallel pathways to the LH, although it is not clear at this point (and should be investigated in the future) whether the LH neuronal circuits are more hardwired than those in the MBs. To induce changes in behavior, changes in the CA would have to be mediated to MB extrinsic (or output) neurons, like the PE1 neuron investigated by Mauelshagen [27] and Menzel and Manz [29]. In fact, these studies have demonstrated plasticity in the activity of MB output neurons associated with learning. An important future challenge will be to determine how the activity of such MB output neurons in turn will be affected by long-term structural plasticity at the MB input, in the CA MG, and whether plasticity in recurrent GABAergic neurons [18] mediates changes from the MB lobes back to the CA. These questions also represent interesting aspects for future neuroinformatics modeling approaches.
Structural plasticity in the CA MG may be related to stable long-term changes in behavior as indicated for olfactory LTM [21], whereas changes in KC output synapses in the MB lobes are thought to mediate short-term and intermediate-term memory phases (e.g. [20, 28]). Long-term plasticity does particularly make sense in insects with a long life span (months or years) and which express a high degree of behavioral plasticity (e.g. castes, age and task related polyethism, long-term memory) – both is the case in the honey bee and many other social Hymenoptera. Behavioral plasticity represents a most important aspect of social life. Whether the large size of the CA is related to such a function in long-term information storage needs to be further tested. Furthermore, central-place foragers like the honey bee (bees always return to the same place – the hive – after returning from foraging trips (see Chap. 2.5)) and other social insect colonies have to memorize profitable (or bad) food sources over extended periods of time, ideally over the entire life span. A similar argument was made for generalist beetles. Interestingly, these beetles were shown to possess large and doubled MB calyces compared to food-specialist beetles which posses only a single calyx [7]. Whether CA duplication is always correlated with a high number of KCs and associated MG needs to be shown in more comparative studies across insects.
A very interesting question is whether both genetically determined and environmentally induced differences in neuronal networks in the MBs contribute to division of labor, potentially by mediating different sensory thresholds [33] or differences in learning and memory abilities. Although the CA is likely to be not the exclusive place for mediating long-term neuronal-network changes, the substantial structural plasticity in MG and the enormous number of KC neuronal circuits are suggestive for being a suitable neuronal resource with large enough storage capacities for long-term adaptive adjustments. Future comparative studies are needed to find out where high numbers of KCs and associated MG together with a doubled CA have emerged during hymenopteran evolution.
Using concerted efforts and new tools like high resolution life- and molecular-imaging, 3D ultrastructural analyses, multi- and single unit electrophysiology, genetic and molecular interference in combination with well designed behavioral assays are promising approaches to address some of the above questions. The CA in the honey bee brain offers unique opportunities for the future to investigate neuronal plasticity and how it translates into changes in behavior. Compared to work on the mammalian hippocampus, for example, physiological imaging studies in the honey bee can be performed in live animals with intact brains (the MB calyces are close to the surface of the brain) plus using controlled sensory stimulation. New electrophysiological tools like simultaneous long-term multi-unit recordings from different brain sites will be extremely helpful to investigate temporal aspects of coding and how they may affect synaptic plasticity. The diversity of species within the Hymenoptera (social and solitary species) opens up unique opportunities to correlate CA attributes with sensory and cognitive capabilities and the evolution of sociality in comparative approaches. Finally, the already well investigated neuronal circuitry, the rich diversity in behavior, the availability of a sequenced genome, and findings on epigenetic mechanisms open up completely novel approaches for targeted interventions at the genetic, epigenetic, molecular, physiological and behavior levels. The large range of phenotypic plasticity in association with social organization make the honey bee a most promising model for future studies on the neuronal mechanisms underlying behavioral plasticity in a social context.
Abbreviations
- ActD:
-
Actinomycin D
- CS:
-
Conditioned stimulus
- LTM:
-
Long term memory
- US:
-
Unconditioned stimulus
References
Abel R, Rybak J, Menzel R (2001) Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol 437(3):363–383
Armstrong JD, Kaiser K, Müller A, Fischbach KF, Merchant N et al (1995) Flybrain, an on-line atlas and database of the Drosophila nervous system. Neuron 15(1):17–20
Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I et al (2009) The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet 23(1–2):156–172
Becher MA, Scharpenberg H, Moritz RF (2009) Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.). J Comp Physiol A 195(7):673–679
Durst C, Eichmüller S, Menzel R (1994) Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav Neural Biol 62(3):259–263
Faber T, Menzel R (2001) Visualizing mushroom body response to a conditioned odor in honeybees. Naturwissenschaften 88(11):472–476
Farris SM, Roberts NS (2005) Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. P Natl Acad Sci USA 102(48):17394–17399
Farris SM, Robinson GE, Fahrbach SE (2001) Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J Neurosci 21(16):6395–6404
Frambach I, Rössler W, Winkler M, Schürmann FW (2004) F-actin at identified synapses in the mushroom body neuropil of the insect brain. J Comp Neurol 475(3):303–314
Galizia CG, Rössler W (2010) Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol 55:399–420
Ganeshina O, Menzel R (2001) GABA-immunoreactive neurons in the mushroom bodies of the honeybee: an electron microscopic study. J Comp Neurol 437(3):335–349
Ganeshina O, Vorobyev M, Menzel R (2006) Synaptogenesis in the mushroom body calyx during metamorphosis in the honeybee Apis mellifera: an electron microscopic study. J Comp Neurol 497(6):876–897
Giurfa M (2007) Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A 193(8):801–824
Groh C, Ahrens D, Rössler W (2006) Environment- and age-dependent plasticity of synaptic complexes in the mushroom bodies of honeybee queens. Brain Behav Evol 68(1):1–14
Groh C, Rössler W (2008) Caste-specific postembryonic development of primary and secondary olfactory centers in the female honeybee brain. Arthropod Struct Dev 37(6):459–468
Groh C, Tautz J, Rössler W (2004) Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc Natl Acad Sci USA 101(12):4268–4273
Gronenberg W (2001) Subdivisions of hymenopteran mushroom body calyces by their afferent supply. J Comp Neurol 435(4):474–489
Grünewald B (1999) Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee, Apis mellifera. J Comp Physiol 185:565–576
Hammer M (1993) An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366(6450):59–63
Heisenberg M (2003) Mushroom body memoir: from maps to models. Nat Rev Neurosci 4(4):266–275
Hourcade B, Muenz TS, Sandoz JC, Rössler W, Devaud JM (2010) Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? J Neurosci 30(18):6461–6465
Ismail N, Robinson GE, Fahrbach SE (2006) Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc Natl Acad Sci USA 103(1):207–211
Kirschner S, Kleineidam CJ, Zube C, Rybak J, Grünewald B, Rössler W (2006) Dual olfactory pathway in the honeybee, Apis mellifera. J Comp Neurol 499(6):933–952
Kleineidam CJ, Rössler W (2009) Adaptations in the olfactory system of social hymenoptera. In: Gadau J, Fewell J (eds) Organization of insect societies. From genome to sociocomplexity. Harvard University Press, Cambridge/London, pp 195–219
Krofczik S, Khojasteh U, de Ibarra NH, Menzel R (2008) Adaptation of microglomerular complexes in the honeybee mushroom body lip to manipulations of behavioral maturation and sensory experience. Dev Neurobiol 68(8):1007–1017
Kucharski R, Maleszka J, Foret S, Maleszka R (2008) Nutritional control of reproductive status in honeybees via DNA methylation. Science 319(5871):1827–1830
Mauelshagen J (1993) Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J Neurophysiol 69(2):609–625
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5(2):62–71
Menzel R, Manz G (2005) Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. J Exp Biol 208(Pt 22):4317–4332
Mobbs PG (1982) The brain of the honeybee Apis mellifera. 1. The connections and spatial-organization of the mushroom bodies. Philos Trans R Soc B 298(1091):309–354
Müller D, Abel R, Brandt R, Zockler M, Menzel R (2002) Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A 188(5):359–370
O’Donnell S, Donlan NA, Jones TA (2004) Mushroom body structural change is associated with division of labor in eusocial wasp workers (Polybia aequatorialis, Hymenoptera: Vespidae). Neurosci Lett 356(3):159–162
Pankiw T, Page RE (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 185(2):207–213
Paulk AC, Phillips-Portillo J, Dacks AM, Fellous JM, Gronenberg W (2008) The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J Neurosci 28(25):6319–6332
Perisse E, Raymond-Delpech V, Neant I, Matsumoto Y, Leclerc C et al (2009) Early calcium increase triggers the formation of olfactory long-term memory in honeybees. BMC Biol 7:30
Rybak J, Menzel R (1993) Anatomy of the mushroom bodies in the honey-bee brain – the neuronal connections of the alpha-lobe. J Comp Neurol 334(3):444–465
Schröter U, Menzel R (2003) A new ascending sensory tract to the calyces of the honeybee mushroom, body, the subesophageal-calycal tract. J Comp Neurol 465(2):168–178
Schürman FW (1974) Functional anatomy of corpora pedunculata in insects. Exp Brain Res 19(4):406–432
Seid MA, Wehner R (2009) Delayed axonal pruning in the ant brain: a study of developmental trajectories. Dev Neurobiol 69(6):350–364
Smith D, Wessnitzer J, Webb B (2008) A model of associative learning in the mushroom body. Biol Cybern 99(2):89–103
Steiger U (1967) Über den Feinbau des Neuropils im Corpus Pedunculatum der Waldameise – elektronenoptische Untersuchungen. Z Zellforsch Mik Anal 81(4):511–536
Stieb SM, Muenz TS, Wehner R, Rössler W (2010) Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev Neurobiol 70(6):408–423
Strausfeld NJ (2002) Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J Comp Neurol 450(1):4–33
Tautz J, Maier S, Groh C, Rössler W, Brockmann A (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci USA 100(12):7343–7347
Tomer R, Denes AS, Tessmar-Raible K, Arendt D (2010) Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142(5):800–809
Weaver N (1957) Effects of larval age on dimorphic differentiation of the female honeybee. An Entomol Soc Am 50:283–294
Weidenmüller A, Mayr C, Kleineidam CJ, Roces F (2009) Preimaginal and adult experience modulates the thermal response behavior of ants. Curr Biol 19(22):1897–1902
Witthöft W (1967) Absolute Anzahl und Verteilung der Zellen im Gehirn der Honigbiene. Zeitschrift für Morphologie der Tiere 61:160–184
Yasuyama K, Meinertzhagen IA, Schürmann FW (2002) Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol 445(3):211–226
Yuste R, Bonhoeffer T (2004) Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci 5(1):24–34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Rössler, W., Groh, C. (2012). Plasticity of Synaptic Microcircuits in the Mushroom-Body Calyx of the Honey Bee. In: Galizia, C., Eisenhardt, D., Giurfa, M. (eds) Honeybee Neurobiology and Behavior. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2099-2_12
Download citation
DOI: https://doi.org/10.1007/978-94-007-2099-2_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2098-5
Online ISBN: 978-94-007-2099-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)