Abstract
Wastewater treatment in the textile factory produces sludge classified as toxic and hazardous waste, which is harmful if left untreated. This study assessed the potential of utilizing sludge from a textile factory in Bandung Regency, Indonesia, as a co-firing fuel in coal boiler furnaces employed in the factory. The study aimed to improve the performance of sludge to meet the required standards for fuel substitution. The analysis involved proximate, ultimate, and ash element tests with correlation of the results with calorific values. The sludge was mixed with coal bottom ash produced by the textile factory and biomass (local refuse-derived fuel) at different ratios. An environmental impact analysis was also carried out with the toxicity characteristic leaching procedure (TCLP) and air emission testing. The results showed that the sludge did not meet the fuel substitution requirements if it was used as a single material. However, the sludge could be used as a substitute for coal by mixing it with bottom ash and biomass; the optimum composition was a ratio of 20% sludge, 40% bottom ash, and 40% biomass by weight. TCLP and air emission test results showed that this mixture was safe for human health and the environment and met the fuel substitution requirements. This study provides a practical solution to the problem of reducing toxic and hazardous waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Textile manufacturing is one of the main global industries responsible for producing wastewater that pollutes the environment. The textile production process uses large amounts of water that is eventually discharged as wastewater, which requires treatment to remove pollutants before being released into the environment. Textile wastewater harms the environment and human health because it contains complex pollutants (Haryono et al. 2018). The pollutant material of textile wastewater generally comes from synthetic dyes using. Synthetic dyes often used are rhodamine B and azo that are not easy to degrade. Rhodamine B contains heavy metals (Mahatmanti et al. 2019), and dyes containing azo groups are also suspected to be carcinogenic (Eskak and Salma 2020), which can produce chloro-aniline (Suhendra and Kardena 2013). Because it is difficult to degrade, textile wastewater requires treatment that combines physical–chemical methods with biological methods to get a better result (Valerie et al. 2018).
However, the various methods applied to treat wastewater still produce sludge that requires rigorous handling. So the utilization of sludge is increasingly receiving attention. Previous studies have utilized sludge from various types of wastewaters. The high organic content in the sludge resulting from biological treatment has been used as an alternative to adding nutrients on ultisol soils (Pandapotan et al. 2017) and as an alternative energy source (Kurniawan et al. 2018) or solid biomass fuel (Awere et al. 2020). In particular, sludge of textile wastewater has also been used as constituent building materials (Anwar et al. 2018) or a mixture of bricks (Arbunowo et al. 2019). The combustion process of constituent building materials or bricks can inhibit the movement of heavy metals, oxidize organic materials completely, and eliminate pathogens of sludge (Arbunowo et al. 2019).
According to Indonesian regulation, textile industry sludge is classified as toxic and hazardous waste, which makes it an obligation for every producer to manage and utilize the waste. The sludge disposal techniques commonly used are incineration and landfilling (Anwar et al. 2018). These methods need optimization according to resource availability to reduce costs in the context of global competition. The Indonesian government has encouraged the management and utilization of industrial sludge without specifying any methods to do so. One technology that is rapidly developing is the use of toxic (Chuah et al. 2016a) and hazardous waste (Skaggs et al. 2018) as alternative fuels. Based on the government's recommendations, utilization of sludge as a direct fuel source in boilers to generate thermal energy is an appropriate application. Many studies have utilized biomass as an energy alternative, such as sawdust and oak wood mixed plastic waste (Zannikos et al. 2013); agriculture waste (Ribeiro and Dalmolin 2020); firewood and charcoal (Oladeji 2015); solid waste (Igoni and Harry 2017; Nayono 2009); bamboo species (Marafon et al. 2019), but investigations using wastewater sludge as an alternative fuel are still scarce (Anwar et al. 2018; Arbunowo et al. 2019; Putri and Sukandar 2013). Research on the utilizing of various biomass wastes as an energy alternative has been still developing.
The wastewater treatment plant (WWTP) from a textile factory in the Bandung Regency produces of sludge approximately 3 t.day−1. This factory also operates three boilers of different types to supply energy for the textile production process, which produce bottom ash approximately 650 kg.day−1. According to Indonesian regulation, bottom ash is also classified as toxic and hazardous waste. Currently, 10–30% by weight of the global production of fly ash and bottom ash (FABA) is recycled or reprocessed for raw material. So the integrated utilization of these wastes has significant potential for both saving energy and reducing greenhouse gas emissions (Damayanti 2018). The recycling of bottom ash can also reduce the factory's cost burden in toxic and hazardous waste management. However, the utilization of toxic and hazardous waste, such as sludge and bottoms ash, requires adherence to strict regulatory standards.
In addition to the problem of toxic and hazardous waste, governments and researchers are also concerned about municipal solid waste (MSW), particularly in developing countries (Santos et al. 2019). The MSW generation has caused air, water, and soil pollution without treatment (Das et al. 2019). Nearly 33% by weight of MSW from cities worldwide goes to dump sites without proper treatment (Haraguchi et al. 2019). In Indonesia, the rate of MSW generation is not proportional to the speed in its management (Mahyudin 2017). Related to contributions to global warming from solid waste management (Maalouf and El-Fadel 2019), many countries face this MSW challenge by developing technology (Xu et al. 2016).
The management of MSW has become an increasingly complex problem with population growth and changes in people's lifestyles. Consequently, waste processing technology has not kept pace with MSW generation, and the final destination of much of the solid waste is landfill. At the same time, finding suitable sites for landfill construction is challenging (Utomo et al. 2017) because they must have specific characteristics (Rugatiri 2021). To overcome these problems, many countries have adopted the 3R (reduce, reuse, and recycle) concept as the first principles of solid waste management (Peprah et al. 2015; Rugatiri 2021; Somneuk 2020). The 3R principles have progressed to 4R (reduce, reuse, recycle, and recover). The term “recover” refers to the extraction of valuable materials from solid waste or to the transformation of waste to a material of value (Hannon and Zaman 2018; Popli et al. 2017). The concept of solid waste management has further evolved to 5R (4R + refuse). The term “refuse” means reducing plastic waste and is a guiding standard for consumers, industry, and authorities to control the impact of plastic waste on health and the environment (Hussein et al. 2021; Kumar et al. 2021). The government of Indonesia also encourages the business sector to support solid waste management under the 5R principles of 3R + replace and replant, especially for food, beverages, or other industry-generated organic waste. In this case, the term “replace” is concerned with replacing hazardous material as well as plastics with eco-friendly material, and “replant” refers to the restoration of degraded land using organic waste or using organic waste as growing media (Prabawanti 2021; Wirahadi 2016).
The 3R principles are fundamental to MSW management because the cost of collecting the waste can reach 40–60% of the total landfill management costs. At the same time, in situ solid waste treatment can save money and reduce CO2 gas emissions from transport vehicles (Jouhara et al. 2017). The population of the Bandung metropolitan area is 8,679,973 (BPS West Java Province 2020). The estimate of urban solid waste per capita in Indonesia is 0.65 kg.d−1 or 2.75 L.d−1 (Yusbindar et al. 2020). So the solid waste generated in the Bandung Metropolitan is estimated at 5.64 × 106 kg.d−1 or 23.87 × 106 L.d−1. The utilization of MSW makes a significant contribution if it is supported by comprehensive policies or regulations in all regions to reduce its generation and solve land use problems. One solution is to treat MSW in situ at the temporary place storage (TPS) to generate refuse-derived fuel (RDF), which can then be used as a substitute fuel for co-firing with coal.
Many initiatives have been carried out to create renewable energy alternative. They were different on material using and its direct application. Previous study created a briquette as an alternative fuel. The briquette of mixture 60 wt% of bottoms ash and 40 wt.% of biomass (RDF) was applied as co-firing. Its trial burning test showed increase in the efficiency combustion in steam boiler (Marganingrum et al. 2000), and the air emission was below the required standard (Nurhayati et al. 2021). From the observations at the same study site, it was found that the availability of wastewater sludge is much greater than bottom ash. This study aims to increase the potential value of the textile factory’s waste sludge as a fuel by combining it with coal bottom ash and RDF. This sludge may be categorized as biomass so that it or RDF has the potential to replace other biomass resources such as agricultural residual or wood as a future fuel alternative. Obviously, the sludge addition to the first briquettes composition needs to be re-evaluated. This study used an existing formula to evaluate the characteristics and gross calorific value (GCV) of the materials within the constraints of the regulatory limits to their use as fuel.
2 Materials and methods
2.1 Sources of fuel material
This study used sludge, bottom ash, and biomass. The sludge samples were obtained from the WWTP of a textile factory in Bandung Regency. Bottom ash samples were obtained from the coal-burning boiler of the same factory. Biomass used in this study was biomass produced from urban MSW processed in the Bandung area. The biomass was produced by dry fermentation of MSW using an aerobic bio-drying method called peuyeumisasi in the local language (Marganingrum et al. 2020). Figure 1 shows the material used in this study.
2.2 Parameters and methods of analysis
This study analyzed the characteristics of sludge, bottom ash, and biomass before and after mixing them following the applicable regulations for the optimal composition of the mixture as a fuel. The analyses were conducted according to the American Society for Testing and Materials (ASTM) International test standards. The characteristics of the materials as fuel were tested using proximate, ultimate, and calorific value tests (Chuah et al. 2016b). Proximate analysis determined the percentages of moisture, ash, volatile matter (VM), and fixed carbon (FC) on a dry weight basis. The standard methods used for the analysis were ASTM D3173 for moisture, ASTM D3174 for ash, ASTM D3175 for VM, and ASTM D3172 for FC. Ultimate analysis determined the composition of the material in terms of the percentage of carbon (C), hydrogen (H), nitrogen (N), oxygen (O), and sulfur (S) by weight. The standard methods used for the ultimate analysis were ASTM D5373 for C, H, N, and O and ASTM D4239 for S. Proximate and ultimate analyses used the LECO 701 apparatus. To obtain the calorific value, a bomb calorimeter was used with the standard method of ASTM D5865. This study also analyzed the chlorine content of the material using Eschka’s mixture according to ISO 587. The proximate, ultimate, and calorific values and the chlorine content of the material were obtained at the Tekmira Laboratory of the Ministry of Energy and Mineral Resources of Indonesia.
To obtain the sulfur content in sludge, this study measured the wastewater sulfate content by tracing its source to control the total sulfur content of the sludge—water samples were taken at each stage of the wastewater treatment process. The sulfate concentration was determined by the turbidimetric method at a wavelength of 420 nm using an ultraviolet–visible spectrophotometer (UV-PharmaSpec 1700) at the LIPI Laboratory of the Research Center for Geotechnology.
This study also analyzed the heavy metal content of the material and applied the toxicity characteristic leaching procedure (TCLP) to determine whether the material complied with applicable regulations. It also ensured that the materials used as fuel substitutes in this study were safe for human health and the environment. TCLP determines the level of toxicity of waste materials and classifies them as hazardous or not (Agustini et al. 2017). TCLP testing conducted by refer to US. EPA, SW.846 Test Method 1311 only for heavy metal content. The closed acid digestion method, also known as microwave digestion, was used to determine the metal content of the material. The products of the digestion were measured using an atomic absorption spectrophotometer (AA-7000). Heavy metal analysis and TCLP were conducted only for sludge and bottom ash at the Tekmira Laboratory of the Ministry of Energy and Natural Resources, Indonesia. These tests were not performed for biomass from MSW as it is not categorized as toxic and hazardous waste.
2.3 The effects of proximate compounds and ultimate elements on calorific value
The GCV, sometimes termed higher heating value (HHV), and the composition of solid fuels are essential properties that define their energy content and determine their clean and efficient use. The ultimate analysis of fuels provides a variety of correlations for predicting HHV. A few HHV correlations from proximate analysis have appeared in the solid fuel literature in the past, but they were focused on one fuel or were dependent on the country of origin. The formula based on ultimate analysis is generally more accurate than that based on proximate analysis. The quality of the correlations based on chemical analysis was found to be very poor because of the variation in the properties of components and the chemical composition of biomass. As a preliminary study, this work introduces a general correlation based on the proximate analysis of solid fuels using direct laboratory tests and only a few data points and further experimental data points. The entire spectrum of solid carbonaceous materials like sludge, bottom ash, and biomass was considered based on proximate analysis and calculated with the coal tests of ASTM D5865, although they were not all coal-based materials. To determine the accuracy of the test, this study used a proximate correlation formula (Özyuğuran et al. 2018; Parikh et al. 2005):
where fixed carbon (FC) was 1.0–91.5%, volatile matter (VM) was 0.92–90.6%, and ash was 0.12–77.7% as the analyzed content in wt.% on a dry basis.
The average absolute error of this correlation was 3.74% and the bias error was 0.12% for the measured value of HHV, which was much less than that of previous correlations of a similar kind. The advantage of this correlation is the ability to compute HHV of any fuel simply from its proximate analysis to provide a valuable tool for modeling combustion, gasification, and pyrolysis processes (Olatunji et al. 2019; Parikh et al. 2005). By definition, sludge and biomass were categorized as biomass residue resources. The formula for biomass materials was generated from ultimate analyses, with high S content, and the ultimate formula was simplified (Toscano and Foppa Pedretti 2009; Yaman 2004) as follows:
where carbon (C), hydrogen (H), nitrogen (N), oxygen (O), and sulfur (S) were analyzed as wt.% on a dry basis and 2379.9 was a correction factor.
3 Results and discussion
The sludge from the textile industry's WWTP is categorized as a general specific source of the toxic and hazardous waste category 2 with the waste code B322-3. As a by-product of the textile industry’s wastewater treatment process, sludge poses problems for handling and storage. The Indonesian government regulates the use of toxic and hazardous waste, in either solid or liquid phase, as a substitute for energy sources under the following conditions: (1) it can be categorized as waste B3, which, when burned, produces heat and energy; (2) it is able to reduce primary fuel consumption, and (3) it meets environmental standards according to the provisions of the legislation. The other specifications that are regulated are shown in Table 1. Generally, sludge has a moisture content of more than 80% by weight. Reducing the moisture content is essential during its conversion into usable energy (Ali and Akilli 2019). An appropriate test is needed to reduce the water content in sludge to apply cost-effective drying technology, especially in developing countries like Indonesia. Sun drying is an inexpensive option (Awere et al. 2020), although it is a time-consuming process. In this study, the textile factory used a belt press to reduce the sludge water content until under 20% by weight before keeping it in temporary place storage (TPS). The maximum period for storing sludge in TPS is 90 days (government regulation number 101 of 2014 article 28). This study aims to reduce sludge waste to avoid its overaccumulation in storage.
3.1 Characterization of sludge, bottom ash, and biomass as a single material
Table 2 shows the proximate, ultimate, and calorific values of sludge, bottom ash, and biomass used in this study. Based on these analyses, none of the materials met the calorific requirement. Sludge’s sulfur content did not conform to the upper limit requirement. The analyzed sludge sample had an average total sulfur content of 1.91% by weight or almost double the mandatory requirement (≤ 1 wt%). Several attempts must be made to reduce the sulfur content in sludge before it can be used as a fuel substitute. The sulfur content of sludge is influenced by the WWTP processing chemicals as well as the characteristics of the treated liquid waste. Low efficiency of chemical use can result in a significant pollution hazard (Anwar et al. 2018).
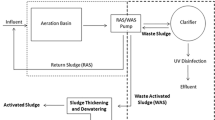
Figure 2 provides a flowchart of the waste treatment process until sludge formation and the points at which wastewater was sampled. And Fig. 3 shows the results of the test for the sulfate content in the wastewater. The textile factory in this study used aluminum sulfate [Al2(SO4)3] as a coagulant. Coagulation plays an essential role in the water treatment process by significantly reducing turbidity and colloidal particles (1–200 mµ in size) in suspension (Naje et al. 2017). The direct use of aluminum sulfate increases the sulfur content in the sludge. Based on Figs. 2 and 3, the highest contribution of sulfur came from the wet scrubber wastewater. The sulfate concentrations of the Omnical wet scrubber water were 865 mg.L−1, and from of the Thompson boiler wet scrubber water was 1115 mg.L−1.
The sulfate concentration at each sampling location in the water treatment process (see Fig. 2 for an explanation of the locations)
The wet scrubber's sulfur content is influenced by the type of coal used and the boiler's combustion performance. Wastewater from the two wet scrubbers is discharged into the equalization unit before going into the biological and chemical processing units, which produce a large quantity of sludge (Arbunowo et al. 2019). The sulfur content from both boilers will increase the sulfur concentration in the resulting sludge. Therefore, despite this wastewater treatment system recycling the wastewater extracted by the belt press into the equalization tank, if the wastewater in the equalization tank contains high levels of sulfur, the total amount of sulfur in the sludge will remain high.
The sulfur content in a fuel should not be more than 1% by weight because high levels of sulfur harm the combustion process (Chuah et al. 2015). Combustion of fuel with a high sulfur content will increase SOx gas (Sarbassov et al. 2018), a toxic aerosol emission (Zhao et al. 2016). In addition, SOx reacts readily with water to form sulfuric acid, which causes corrosion at low temperatures (Cullis and Mulcahy 1972; Srivastava et al. 2004). Aluminum sulfate undergoes a decomposition reaction at a temperature of 923–1223 K or 650–950 °C. Metal sulfate reactions are usually endothermic or absorb energy and are always reversible (Sarbassov et al. 2018). The decomposition reaction of aluminum sulfate is as follows (Ghasri-Khouzani et al. 2009; Tompkin 1976; Tagawa 1984):
During the combustion process, most (about 83–93% by weight) of the resulting sulfur trioxide is oxidized to sulfur dioxide, while a small portion remains as sulfur trioxide. The reactions that occur are as follows:
Based on these equations, the sulfur content of sludge used as a fuel should be lowered to reduce its sulfur emission value.
The GCV of all three materials used in this study was below the required standard (see Table 2). The GCV resulting from the combustion processes involved a balance between all the proximate and ultimate parameters. As bottom ash was a solid waste generated from a coal combustion process, it was difficult to burn again. The GCV of bottom ash was low because it had a low VM content. In contrast, the GCV of sludge and biomass did not meet the standard because they had a low FC content.
In general, the properties of combustible material can be assessed using a Tanner diagram. The Tanner model states that if the moisture, ash, and combustible compound (FC and VM) content of a material is within the combustible zone (the gray area in Fig. 4), so the material has the potential to burn without using additional fuel (Anggoro et al. 2017; Lombardie et al. 2015; Pasek et al. 2013; Siddiqi et al. 2019). In this study, sludge had an ash content of less than 60% by weight, moisture content of less than 50% by weight, and combustible materials above 25% by weight, and the composition of these three parameters was in the combustible zone (Fig. 4). The percentages of moisture, ash, and organic matter content of bottom ash were outside the combustible zone (Fig. 4). It means that each material cannot be burned alone without other materials. Although it was not combustible, the FC content of bottom ash was still relatively high at almost 27% by weight. The high FC content indicates that fuel processing was less efficient (James et al. 2012). The remaining carbon in bottom ash should be considered for reuse as an energy source (Marganingrum and Estiaty 2020). It would be harmful to the environment and ecologically not sustainable to discharge bottom ash without further processing.
Although the sulfur content of biomass met the requirements for a substitute fuel, its GCV was below 2,500 kcal.kg−1. This low GCV was due to biomass’s low FC content. So biomass needs other materials to meet the fuel substitution requirements. However, the Tanner diagram shows that biomass can be burned without the need for additional material. It was shown that the percentages of moisture, ash, and combustible matter content in biomass were within the gray zone (Fig. 4).
3.2 Characteristics of sludge mixed with other materials
This study sought an effective solution to change the composition of sludge to increase GCV and reduce sulfur levels to meet the requirements for fuel substitution. One of the efforts was to mix sludge with other materials to overcome this shortcoming. The high VM in sludge and the residual carbon content in bottom ash can increase GCV in mixtures of these two waste materials. Table 3 shows the results of proximate and ultimate analysis of sludge and bottom ash mixtures of various compositions. Table 4 shows the results of proximate and ultimate analysis of sludge and biomass mixtures. The reason for including biomass in the mixture was to replace bottom ash and reduce the sulfur content of sludge. It is challenging to reduce the sulfur content below 1% by weight (Fig. 4), and the sludge and bottom ash mixtures did not meet this requirement. The composition and GCV of sludge and biomass were approximately similar. Table 4 shows the results for the 40% sludge and 60% biomass mix by weight; clearly, the addition of biomass reduced sulfur levels, but it did not increase GCV. Biomass and sludge have some similar characteristics because of high VM but relatively low carbon content. So, the other material, such as bottom ash, is still needed to provide additional carbon in the mixture. This study considered various mixes of sludge, bottom ash, and biomass, and the results of their proximate, ultimate, and GCV analysis are shown in Table 5.
The results of GCV tests of the materials were compared to Eqs. 1 and 2 (Fig. 5). All samples tested by ASTM D5865 were still correlated to these formulas. According to Fig. 5, the samples with an SL:BA ratio of 30:70, 20:80, and 10:90 wt% were not suitable for use as a fuel as the ash content was at least 60% by weight (see Fig. 6); these mixes will not be further discussed.
The data in Tables 3, 4, and 5 were used to create Tanner diagrams to assess the tested sample materials to identify those that were within the areas for calorific values below and above 2500 kcal.kg−1 (Fig. 7). The number of samples was not enough to create more detailed figures. All tested samples had high ash content (at least 40% by weight), and the calorific value of biomass was usually above 2800 kcal.kg−1. This study shows that mixes with less than 35% by weight of sludge or up to 60% by weight of bottom ash were feasible as fuels.
Tanner diagrams of a mixes of sludge (SL), bottom ash (BA), and biomass (BM) to determine those with a calorific value above 2500 kcal.kg−1, and b fixed carbon (FC), volatile matter (VM), and ash to determine the mixes with a calorific value above 2500 kcal.kg−1. The gray area in b represents the combustible zone
3.3 Environmental analysis
The Indonesian regulation number 101 of 2014 (Attachment III) classifies waste as toxic and hazardous if it is explosive, flammable, reactive, infectious, corrosive, and/or toxic. Both textile factory sludge and bottom ash have the same toxicity properties because they contain heavy metals (Damayanti 2018; Kurniawan et al. 2018). Tables 6 and 7 show the heavy metal content of sludge and bottom ash used in this study. Table 6 is heavy metal content in ash and Table 7 is heavy metal content in raw material by TCLP testing.
The heavy metal content of sludge was dominated by zinc and manganese, while bottom ash was dominated by zinc and copper. This heavy metal content will increase the hazard level of the waste released into the environment (Damayanti 2018). Therefore, Indonesian regulation states that toxic and hazardous waste should not leave its source and obliges the waste producers to manage it themselves or to transfer the management to another party that has been certified to handle such waste. Toxic and hazardous waste is classified as category 1 if the heavy metal content discharged during the leaching process is more than the TCLP-A level. The hazardous waste category 1 has acute effects on exposed humans. Category 2 toxic and hazardous waste is classified as such if the heavy metal content released during the leaching process is more than the TCLP-B level but is less than the TCLP-A level. Category 2 hazardous waste has a chronic effect on exposed humans. The levels of heavy metal leaching from the material used in this study are safe for humans because they were lower than the TCLP-B levels.
In addition to TCLP, this study measured chlorine content related to dioxins (polychlorinated dibenzo-p-dioxins/PCDDs) or furans (polychlorinated dibenzofurans/PCDFs) issue. These two compounds are known as the most harmful persistent organic pollutants, which are chlorine-based compounds (Kim et al. 2005). Dioxins or furan issue related to incineration technology is due to the lack of landfill sites (Ni et al. 2009). It is an attractive alternative to treat MSW because it does not depend on biological processes that take a long time and can reduce the volume of MSW quickly up to 85–90% by volume (Chang et al. 2009; McKay 2002). Therefore the formation and control technology in gas emission that related to MSW burning has been the focus of many kinds of research (Kim et al. 2005). Dioxins or furans are produced in the combustion process by synthetic precursors. The formation rate can be affected by incomplete combustion processes due to improper air supply resulting in a lack of oxygen (Ni et al. 2009; Rathna et al. 2018). Figure 8 shows chlorine content of raw material used in this study. Based on the analysis, the chlorine content in both sludge, bottom ash, and biomass is less than 1% by weight. It means that the materials met the requirement of regulations.
To ensure that the mixture of sludge, bottom ash, and biomass (at a ratio of 20:40:40% by weight) was appropriate for co-firing and a safe fuel for the environment, a trial burning test (TBT) was conducted in the boiler of the factory concerned. The three materials were mixed, and briquettes were made using 2% by weight starch as an adhesive. This briquette fuel was substituted of 10–15% by weight for co-firing in the boiler. The emissions from the co-firing are shown in Table 8; all tested parameters had values below the relevant quality standard limits, including that for chlorine gas.
4 Conclusions
Sludge and biomass had similar characteristics, as demonstrated by their VM and FC content, but the mixing of sludge and biomass did not increase GCV because of the low carbon content of both materials. On the other hand, bottom ash had a higher carbon content, and the mixing of bottom ash with sludge or bottom ash with sludge and biomass increased GCV significantly to a value higher than that of each material on its own. The high FC and high VM in the mixtures were favorable for raising their GCV. Based on this study, the best material composition that proper of regulation was 20 wt% of sludge, 40 wt% of bottom ash, and 40 wt% of biomass. This study provides an avenue for reducing toxic and hazardous waste, a burdensome waste management challenge as well, especially for developing countries such as Indonesia. Finding of this composition needs to be reapplied on a broad and massive scale by government regulation supporting through the Ministry of Environmental and Forest of the Republic of Indonesia. These findings contribute a practical solution for governments in their efforts to encourage factories that produce wastewater sludge and use coal as an energy source to take measures to tackle the highly critical problem of industrial and urban waste. The following study was needed to decrease the moisture and sulfur content of sludge to increase the performance of sludge as an alternative fuel to ensuring the sustainability and development of clean technologies.
References
Ali AA, Akilli H (2019) Fuel characterization study and simulation of dewatered domestic wastewater sludge gasification using ASPEN plus. Acad Perspect Procedia 2(3):954–963. https://doi.org/10.33793/acperpro.02.03.107
Anggoro B, Aprilian A, Halimi B (2017) Potency of waste to energy-Bandung city case study. In: 2017 International conference on high voltage engineering and power systems (ICHVEPS), pp 135–139. https://doi.org/10.1109/ICHVEPS.2017.8225929
Anwar TB, Behrose B, Ahmed S (2018) Utilization of textile sludge and public health risk assessment in Bangladesh. Sustain Environ Res 28(5):228–233. https://doi.org/10.1016/j.serj.2018.04.003
Arbunowo AA, Purwanto PP, Budihardjo MA (2019) Waste to product: Bisolum-bricks, incorporation of WWTP sludge of textile industry into bricks for wall pairs. Jurnal Riset Teknologi Pencegahan Pencemaran Industri 10(2):29–35. https://doi.org/10.21771/jrtppi.2019.v10.no2.p29-35
Awere E, Bonoli A, Obeng PA (2020) Solids-liquid separation and solar drying of palm oil mill wastewater sludge: potential for sludge reuse. Case Stud Chem Environ Eng 2(100057):1–6. https://doi.org/10.1016/j.cscee.2020.100057
Chang YM, Hung CY, Chen JH, Chang CT, Chen CH (2009) Minimum feeding rate of activated carbon to control dioxin emissions from a large-scale municipal solid waste incinerator. J Hazard Mater 161(2–3):1436–1443. https://doi.org/10.1016/j.jhazmat.2008.04.128
Chuah LF, Aziz ARA, Yusup S, Bokhari A, Klemeš JJ, Abdullah MZ (2015) Performance and emission of diesel engine fuelled by waste cooking oil methyl ester derived from palm olein using hydrodynamic cavitation. Clean Technol Environ Policy 17(8):2229–2241. https://doi.org/10.1007/s10098-015-0957-2
Chuah LF, Yusup S, Aziz ARA, Klemeš JJ, Bokhari A, Abdullah MZ (2016a) Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Technol Environ Policy 18(2):473–482. https://doi.org/10.1007/s10098-015-1022-x
Chuah LF, Yusup S, Aziz ARA, Bokhari A, Abdullah MZ (2016b) Cleaner production of methyl ester using waste cooking oil derived from palm olein using a hydrodynamic cavitation reactor. J Clean Prod 112(5):4505–4514. https://doi.org/10.1016/j.jclepro.2015.06.112
Cullis CF, Mulcahy MFR (1972) The kinetics of combustion of gaseous sulphur compounds. Combust Flame 18(2):225–292. https://doi.org/10.1016/S0010-2180(72)80139-1
Damayanti R (2018) Abu batubara dan pemanfaatannya: Tinjauan teknis karakteristik secara kimia dan toksikologinya. Jurnal Teknologi Mineral Dan Batubara 14(3):213–231. https://doi.org/10.30556/jtmb.Vol14.No3.2018.966
Das S, Lee SH, Kumar P, Kim KH, Lee SS, Bhattacharya SS (2019) Solid waste management: scope and the challenge of sustainability. J Clean Prod 228:658–678. https://doi.org/10.1016/j.jclepro.2019.04.323
Eskak E, Salma IR (2020) Kajian pemanfaatan limbah perkebunan untuk substitusi bahan pewarna batik. Jurnal Industri Hasil Perkebunan 15(2):27–37
Ghasri-Khouzani M, Meratian M, Panjepour M (2009) Effect of mechanical activation on structure and thermal decomposition of aluminum sulfate. J Alloy Compd 472(1–2):535–539. https://doi.org/10.1016/j.jallcom.2008.05.012
Hannon J, Zaman A (2018) Exploring the phenomenon of zero waste and future cities. Urban Sci 2(90):1–26. https://doi.org/10.3390/urbansci2030090
Haraguchi M, Siddiqi A, Narayanamurti V (2019) Stochastic cost-benefit analysis of urban waste-to-energy systems. J Clean Prod 224:751–765. https://doi.org/10.1016/j.jclepro.2019.03.099
Haryono H, Faizal DM, Liamita NC, Rostika A (2018) Pengolahan limbah zat warna tekstil terdispersi dengan metode elektroflotasi. EduChemia (jurnal Kimia Dan Pendidikan) 3(1):94–105. https://doi.org/10.30870/educhemia.v3i1.2625
Hussein BA, Tsegaye AA, Abdulahi A (2021) Assessment of the environmental and health impacts of disposal plastics in Gode town, Somali regional state, Eastern Ethiopia. J Mater Environ Sci 12(3):455–471
Igoni AH, Harry SK (2017) Design models for anaerobic batch digesters producing biogas from municipal solid waste. Energy Environ Eng 5(2):37–53. https://doi.org/10.13189/eee.2017.050202
James AK, Thring RW, Helle S, Ghuman HS (2012) Ash management review-applications of biomass bottom ash. Energies 5(10):3856–3873. https://doi.org/10.3390/en5103856
Jouhara H, Czajczyńska D, Ghazal H, Krzyżyńska R, Anguilano L, Reynolds AJ, Spencer N (2017) Municipal waste management systems for domestic use. Energy 139:485–506. https://doi.org/10.1016/j.energy.2017.07.162
Kim KH, Seo YC, Nam H, Joung HT, You JC, Kim DJ, Seo YC (2005) Characteristics of major dioxin/furan congeners in melted slag of ash from municipal solid waste incinerators. Microchem J 80(2):171–181. https://doi.org/10.1016/j.microc.2004.07.022
Kumar M, Kumar S, Singh S (2021) Waste management by waste to energy initiatives in India. Int J Sustain Energy Environ Res 10(2):58–68. https://doi.org/10.18488/journal.13.2021.102.58.68 ((in progress))
Kurniawan T, Hakiki R, Sidjabat FM (2018) Wastewater sludge as an alternative energy resource: a review. J Environ Eng Waste Manag 3(1):1–12. https://doi.org/10.33021/jenv.v3i1.396
Lombardi L, Carnevale E, Corti A (2015) A review of technologies and performances of thermal treatment systems for energy recovery from waste. Waste Manag 37:26–44. https://doi.org/10.1016/j.wasman.2014.11.010
Maalouf A, El-Fadel M (2019) Towards improving emissions accounting methods in waste management: a proposed framework. J Clean Prod 206:197–210. https://doi.org/10.1016/j.jclepro.2018.09.014
Mahatmanti W, Kusumastuti E, Rengga W (2019) Membran padat kitosan-silika-PEG sebagai membran pemisah ion logam bivalen dan rhodamin B pada limbah cair industri tekstil. JC-T (journal Cis-Trans) Jurnal Kimia Dan Terapannya 3(2):12–17. https://doi.org/10.17977/um0260v3i22019p012
Mahyudin RP (2017) Kajian permasalahan pengelolaan sampah dan dampak lingkungan di TPA (Tempat Pemrosesan Akhir). Jukung Jurnal Teknik Lingkungan 3(1):66–74. https://doi.org/10.20527/jukung.v3i1.3201
Marafon AC, Amaral AFC, Lemos EEP (2019) Characterization of bamboo species and other biomasses with potential for thermal energy generation. Pesquisa Agropecuária Tropical 49:1–5. https://doi.org/10.1590/1983-40632019v4955282
Marganingrum D, Estiaty LM (2020) Value increasing of reject coal with biomass adding as bio-coal briquette. Indones J Urban Environ Technol 3(2):123–135. https://doi.org/10.25105/urbanenvirotech.v3i2.5110
Marganingrum D, Estiaty LM, Irawan C, Hidawati (2020) The biomass coal fermented (BCF) briquette as an alternative fuel. In: MSCEIS 2019 conference proceeding 1:811–819. https://doi.org/10.4108/eai.12-10-2019.2296375
McKay G (2002) Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chem Eng J 86(3):343–368. https://doi.org/10.1016/S1385-8947(01)00228-5
Naje AS, Chelliapan S, Zakaria Z, Ajeel MA, Alaba PA (2017) A review of electrocoagulation technology for the treatment of textile wastewater. Rev Chem Eng 33(3):263–292. https://doi.org/10.1515/revce-2016-0019
Nayono SE (2009) Anaerobic digestion of organic solid waste for energy production. Thesis, Karlsruhe University
Ni Y, Zhang H, Fan S, Zhang X, Zhang Q, Chen J (2009) Emissions of PCDD/Fs from municipal solid waste incinerators in China. Chemosphere 75(9):1153–1158. https://doi.org/10.1016/j.chemosphere.2009.02.051
Oladeji JT (2015) Theoretical aspects of biomass briquetting: a review study. J Energy Technol Policy 5(3):72–82
Olatunji OO, Akinlabi S, Madushele N, Adedeji PA (2019) Estimation of the elemental composition of biomass using hybrid adaptive neuro-fuzzy inference system. Bioenergy Res 12(3):642–652. https://doi.org/10.1007/s12155-019-10009-6
Özyuğuran A, Yaman S, Küçükbayrak S (2018) Prediction of calorific value of biomass based on elemental analysis. Int Adv Res Eng J 02(03):254–260
Pandapotan CD, Mukhlis M, Marbun P (2017) Pemanfaatan limbah lumpur padat (sludge) pabrik pengolahan kelapa sawit sebagai alternatif penyediaan unsur hara di tanah ultisol. Jurnal Agroekoteknologi Universitas Sumatera Utara 5(2):271–276
Parikh J, Channiwala SA, Ghosal GK (2005) A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 84(5):487–494. https://doi.org/10.1016/j.fuel.2004.10.010
Pasek AD, Gultom KW, Suwono A (2013) Feasibility of recovering energy from municipal solid waste to generate electricity. J Eng Technol Sci 45(3):241–256. https://doi.org/10.5614/j.eng.technol.sci.2013.45.3.3
Peprah K, Amoah ST, Thomas G, Achana W (2015) Assessing ‘3Rs’ model in relation to municipal solid waste management in Wa, Ghana. World Environ 5(3):112–120. http://article.sapub.org/10.5923.j.env.20150503.03.html
Popli K, Sudibya GL, Kim S (2017) A review of solid waste management using system dynamics modeling. J Environ Sci Int 26(10):1185–1200. https://doi.org/10.5322/jesi.2017.26.10.1185
Prabawanti BE (2021) Application of green marketing in the use of coffee waste as a business creative industry based on social enterprise. Jurnal Manajemen Dan Organisasi 11(3):136–142. https://doi.org/10.29244/jmo.v11i3.32886
Putri AP, Sukandar S (2013) Studi pemanfaatan limbah B3 sludge produced water sebagai bahan baku refuse derived fuel (Rdf). Jurnal Tehnik Lingkungan 19(1):1–10. https://doi.org/10.5614/jtl.2013.19.1.1
Rathna R, Varjani S, Nakkeeran E (2018) Recent developments and prospects of dioxins and furans remediation. J Environ Manag 223:797–806. https://doi.org/10.1016/j.jenvman.2018.06.095
Ribeiro AP, Dalmolin S (2020) Biomass energy as a possibility for innovative agriculture initiatives. Energy Ecol Environ. https://doi.org/10.1007/s40974-020-00201-2
Rugatiri J (2021) Assessing solid waste management strategy in Higher Education Institutions of Indonesia: a case study of IPB. Thesis, IPB University
Santos RE, Santos IFS, Barros RM et al (2019) Generating electrical energy through urban solid waste in Brazil: an economic and energy comparative analysis. J Environ Manag 231:198–206. https://doi.org/10.1016/j.jenvman.2018.10.015
Sarbassov Y, Duan L, Manovic V, Anthony EJ (2018) Sulfur trioxide formation/emissions in coal-fired air- and oxy-fuel combustion processes: a review. Greenhouse Gases Sci Technol 8(3):402–428. https://doi.org/10.1002/ghg.1767
Siddiqi MM, Naseer MN, Wahab YA et al (2019) Potential in urban Pakistan. Process 7(848):1–13. https://doi.org/10.3390/pr7110848
Skaggs RL, Coleman AM, Seiple TE, Milbrandt AR (2018) Waste-to-Energy biofuel production potential for selected feedstocks in the conterminous United States. Renew Sustain Energy Rev 82:2640–2651. https://doi.org/10.1016/j.rser.2017.09.107
Somneuk P (2020) Enabling circular economy in local solid waste management the case of Muang Kalasin Municipality, Thailand. Thesis, Uppsala University, Villavägen 16, SE- 752 36 Uppsala, Sweden.
Srivastava RK, Miller CA, Erickson C, Jambhekar R (2004) Emissions of sulfur trioxide from coal-fired power plants. J Air Waste Manag Assoc 54(6):750–762. https://doi.org/10.1080/10473289.2004.10470943
Suhendra E, Kardena E (2013) Potensi keberadaan polutan kloroanilin di Sungai Citarum akibat biotranformasi pewarna azo dari air limbah tekstil. Prosiding Seminar Nasional Pengelolaan Sumberdaya Alam dan Lingkungan:475–481.
Tagawa H (1984) Thermal decomposition temperatures of metal sulfates. Thermochim Acta 80(1):23–33. https://doi.org/10.1016/0040-6031(84)87181-6
Tompkins FC (1976) Decomposition reactions. In: Treatise on solid state chemistry. Springer, US, pp 193–231. https://doi.org/10.1007/978-1-4684-8082-5_4
Toscano G, Pedretti EF (2009) Calori value determination of solid biomass fuel by simplified method. J Agric Eng 40(3):1–6. https://doi.org/10.4081/jae.2009.3.1
Utomo HD, Yu LS, Yi DCZ, Jun OJ (2017) Recycling solid waste and bioenergy generation in MFC dual-chamber model. Energy Procedia 143:424–429. https://doi.org/10.1016/j.egypro.2017.12.706
Valerie, Wijaya JC, Pinontoan R (2018) Pemanfaatan mikroba yang berpotensi sebagai agen bioremediasi limbah pewarna tekstil. FaST-Jurnal Sains Dan Teknologi 2(1):32–47
Wirahadi M (2016) Elemen interior berbahan baku pengolahan sampah styrofoam dan sampah kulit jeruk. Jurnal INTRA 5(2):144–153
Xu S, He H, Luo L (2016) Status and prospects of municipal solid waste to energy technologies in China. In: Environmental footprints and eco-design of products and processes, pp. 31–54. https://doi.org/10.1007/978-981-10-0150-5_2
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 45(5):651–671. https://doi.org/10.1016/S0196-8904(03)00177-8
Yusbindar, Fatimah E, Suhendrayatna (2020) Aspek teknis operasional yang mempengaruhi timbulan sampah di kecamatan. Jurnal Arsip Rekayasa Sipil Dan Perencanaan 3(2):118–127. https://doi.org/10.24815/jarsp.v3i2.16562
Zannikos F, Kalligeros S, Anastopoulos G, Lois E (2013) Converting biomass and waste plastic to solid fuel briquettes. J Renew Energy 2013:1–9. https://doi.org/10.1155/2013/360368
Zhao Y, Ma Q, Liu Y, He H (2016) Influence of sulfur in fuel on the properties of diffusion flame soot. Atmos Environ 142:383–392. https://doi.org/10.1016/j.atmosenv.2016.08.001
Acknowledgements
The authors would like to thank the Ministry of Finance of the Republic of Indonesia for providing financial assistance to conduct this research through the LPDP RISPRO program in 2019–2020. The authors also thank the management and staff members of textile factory in the Bandung Regency, where this study was conducted. The authors are grateful to the Laboratory of the Research Center for Geotechnology (LIPI), the Laboratory of the Ministry of Energy and Mineral Resources, and the Laboratory for Environmental Quality Control, PDAM Tirtawening, Bandung, for their support in testing the samples.
Author information
Authors and Affiliations
Contributions
DM contributed to conceptualization, methodology, analysis on data of proximate, ultimate, calorific value, wastewater quality, TCLP, heavy metal, chlorine, air emission, and writing, review and editing; H contributed to analysis of sulfur content, and writing; SJSD contributed to conceptualization, analysis on data of proximate, ultimate, calorific value, sulfur content, and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication of this article. The authors also confirm that the data are original and the paper is free of plagiarism.
Rights and permissions
About this article
Cite this article
Marganingrum, D., Hidawati & Djaja, S.D.S. A preliminary study of fuel mixtures of industrial sludge, bottom ash, and municipal solid waste for co-firing in coal boilers. Energ. Ecol. Environ. 7, 186–198 (2022). https://doi.org/10.1007/s40974-021-00229-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-021-00229-y