Abstract
Records of Golovinomyces species new to Thailand are described on the hosts Ageratum conyzoides, Bidens pilosa, Dahlia pinnata, D. × hortensis, Helianthus annuus, Lactuca indica, Laggera crispata, Sonchus oleraceus (Asteraceae), Lygisma inflexum (Asclepiadaceae), Myosotis scopioides (Boraginaceae), Coccinia indica, Coccinia grandis (Cucurbitaceae), Vigna umbellata (Fabaceae), Torenia fournieri (Linderniaceae), Plantago major (Plantaginaceae) and Verbena × hybrida (Verbenaceae). The identifications of the particular Golovinomyces species have been performed by means of morphological examinations supplemented by molecular sequence analyses. On the basis of molecular analyses, the powdery mildew on Ocimum tenuiflorum (Lamiaceae) proved to represent a species of its own, which is referred to as Golovinomyces ocimi comb. nov. The application of Oidium ocimi, the basionym of this combination, is determined by lecto- and epitypification. Lygisma inflexum, Laggera crispata and Vigna umbellata are new host records for Golovinomyces worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Golovinomyces (U. Braun) Heluta [type species: Erysiphe cichoracearum DC. (≡ Golovinomyces cichoracearum (DC.) Heluta)] is a genus of powdery mildew having polyascal ascoma (chasmothecia) with mycelioid appendages and catenescent conidia without fibrosin bodies (Braun and Takamatsu 2000; Matsuda and Takamatsu 2003). This genus is divided into two sections, sect. Golovinomyces and sect. Depressi (U. Braun) U. Braun. Golovinomyces is a large genus comprising 45 species (Braun and Cook 2012) with an almost worldwide distribution. The host range of this genus is mostly restricted to herbaceous plants, including up to 2283 species from 58 families such as the Asteraceae, Boraginaceae, Scrophulariaceae, and Cucurbitaceae (Amano 1986). The taxonomic history of the genus was described in Braun and Cook (2012) in detail. Investigations of powdery mildews in Southeast Asia in the past 14 years, in particular in Thailand, have contributed to the discovery of new taxa and new records mainly belonging to the tribe Erysipheae (for example, Meeboon et al. 2016; Meeboon and Takamatsu 2016, 2017a, b, c, d). In this paper, three hosts of powdery mildews are reported that are new worldwide, one new combination is introduced, and 11 host-fungus combinations of the powdery mildew species (Golovinomyces ambrosiae, G. cynoglossi, G. orontii, G. sonchicola, and G. sordidus) new for Thailand are listed. The identifications of the species concerned were verified by phylogenetic methods, i.e. molecular sequence analyses.
Materials and methods
Morphological examination
Morphological examinations were carried out as outlined in Meeboon and Takamatsu (2015a). All the specimens were examined using a light microscope with phase contrast 10×, 20× and 40× objectives. Herbarium samples were rehydrated before examination by boiling a small piece of infected leaf with the fungal mycelium downwards in a drop of lactic acid on a slide (Shin and La 1993). After boiling, the rehydrated mycelium was scraped off and studied in lactic acid using a light microscope. Thirty conidia and conidiophores were measured for each specimen. Herbarium specimens were deposited at Mie University Mycological Herbarium (TSU-MUMH), Japan.
The nucleotide sequences of the rDNA internal transcribed spacer (ITS) region including 5.8S rDNA were determined in accordance with Meeboon and Takamatsu (2015b). Representative new sequences determined in this study were deposited in DNA Data Base of Japan (DDBJ) under the accession numbers LC306656–LC306669. These sequences were aligned with closely related sequences of the Erysiphaceae using MUSCLE (Edgar 2004) implemented in MEGA version 6 (Tamura et al. 2013). Alignment was further manually refined using MEGA, and deposited in TreeBASE (http://www.treebase.org/) under the accession number S21253. Phylogenetic trees were obtained from the datasets by using the maximum parsimony (MP) method implemented in PAUP* 4.0b10 (Swofford 2002) according to the procedures of Meeboon and Takamatsu (2016).
Molecular phylogeny
Whole cell DNA was extracted from mycelia using the chelex method (Walsh et al. 1991) as described in Hirata and Takamatsu (1996). The respective primer pairs of PM5/ITS4 and ITS5/PM6 (Takamatsu and Kano 2001) were used to amplify ITS fragment 1 and fragment 2, respectively. PCR reaction was conducted using KOD FX NeoDNA polymerase (Toyobo, Japan) according to the manufacturer's protocol. The PCR product was sent to SolGent Co. Ltd. (Daejeon, South Korea) for sequencing using primer pair of ITS1 and ITS4 (White et al. 1990).
New representative sequences determined in this study were deposited in DNA Data Base of Japan (DDBJ) under the accession numbers LC163909, LC163911, LC163913, LC163917 and LC163922. Sequences generated in this study were aligned with other sequences of Golovinomyces retrieved from DNA databases (DDBJ, EMBL, NCBI) using MUSCLE (Multiple Sequence Comparison by Log Expectation) (Edgar 2004) implemented in MEGA 6 (Tamura et al. 2013). The alignments were deposited in TreeBASE (http://www.treebase.org/) under the accession number S20344.
Phylogenetic trees were obtained from the data set using the maximum parsimony (MP) method performed in PAUP* 4.0b10 (Swofford 2002) with the heuristic search option using the tree bisection reconstruction (TBR) algorithm. This search was repeated 100 times with different random starting points, using the stepwise addition option to increase the likelihood of finding the most parsimonious tree. All sites were treated as unordered and unweighted, with gaps treated as missing data. Tree scores, including tree length, consistency index (CI), retention index (RI) and rescaled consistency index (RC), were calculated. The strength of the internal branches of the resulting trees was tested with bootstrap (BS) analysis (Felsenstein 1985) using 1000 replications with the stepwise addition option set to simple and a maximum tree number of 100.
Results
Phylogenetic analyses
Fourteen ITS sequences of Golovinomyces species determined in this study were aligned with other Golovinomyces sequences retrieved from DNA databases. Arthrocladiella mougeotii (Lév.) Vassilkov (AB329690) was used as outgroup taxon. Of the 510 total characters, 376 were constant, 46 were variable but parsimony-uninformative, and 88 were parsimony-informative. The MP analysis produced about 115K equally parsimonious trees with 284 steps. Topologies were almost consistent among the trees except for branching orders of the terminal branches and branch length. A typical tree is shown in Fig. 1.
Taxonomy
Golovinomyces ocimi (S. Naray. & K. Ramakr.) Meeboon & S. Takam., comb. nov. Fig. 2.
MycoBank no.: MB821878
Basionym: Oidium ocimi S. Naray. & K. Ramakr., Madras Univ. J. 37–38: 87, 1967.
Lectotype (designated here, MycoBank, (MBT377568): in Narayanaswami & Ramakrishnan (Narayanaswami and Ramakrishnan 1967: 87). Epitype (designated here, MycoBank, MBT377567): on Ocimum tenuiflorum L. (Lamiaceae), THAILAND, Chiang Rai, Wiangpapao, 15 December 2015, J. Meeboon (TNS-F-80794), TSU-MUMH6621 (isoepitype).
Gene sequence (ex epitype): LC306657 (ITS).
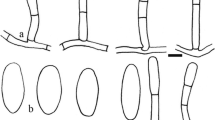
Mycelium amphigenous, effuse or in irregular patches, almost persistent or evanescent. Hyphae hyaline, walls thin, smooth or almost so, 4–9 μm wide. Hyphal appressoria nipple-shaped, sometimes poorly developed. Conidiophores terminal on the surface of mother cells, 70–173 μm long, often increasing from base to top, erect, straight or curved at the base. Foot cells 27–42 × 7–11 μm, basal septum of the foot cell mostly raised, 5–15 μm above the junction with the mother cell, foot cell followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform, 32–40 × 15–20 μm, germ tubes subapically inserted, non to one-septate, mostly short to moderately long, terminating simply or in a club-shaped appressorium.
Further collections examined – on Ocimum tenuiflorum L. (Lamiaceae), THAILAND, Chiang Mai, Su Thep, 29 December 2007, J. Meeboon, MUMH1803; Chiang Rai, Wiangpapao, 25 December 2016, J. Meeboon, MUMH6892; Chiang Rai, Mae Suai, 29 December 2016, J. Meeboon & S. Takamatsu, MUMH6903, MUMH6942.
Notes – Ocimum tenuiflorum, commonly known as holy basil, is an aromatic plant which is native to the Indian subcontinent and widespread as a cultivated plant throughout the Southeast Asian tropics. It is used in Thai cuisine as Thai holy basil (kaphrao). Golovinomyces biocellatus (Ehrenb.) Heluta was reported to occur on many host species of Lamiaceae, including Ocimum spp., in Europe, Asia, North and South America (Braun and Cook 2012). Narayanaswami and Ramakrishnan (1967) described Oidium ocimi on O. tenuiflorum (= O. sanctum) from India. The original description is rather brief and too insufficient to determine the taxonomic position of this asexual powdery mildew. Therefore, Braun (1987) listed O. ocimi under “Anamorphs of uncertain position”. Bappammal et al. (1995) collected additional Indian specimens of O. ocimi and provided a more detailed description and illustration, suggesting a close affinity of this species to Golovinomyces biocellatus. This illustration was again published in Hosagoudar and Agarwal (2009). Furthermore, Amano (1986) listed O. tenuiflorum (= O. sanctum) as host species of Erysiphe biocellata from India. Therefore, Braun and Cook (2012) reduced O. ocimi to synonymy with G. biocellatus. Identification and application of the name O. ocimi require a clarification of this species via lecto- and epitypification. Type material of this species could not be traced and is probably not preserved. Bappammal et al. (1995) also failed to locate and examine type material. Therefore, the original illustration published in Narayanaswami and Ramakrishnan (1967), which is part of the original material in the sense of the ICN, has to be taken into consideration for lectotypification (see Art. 9.2, 9.3). An epitype (new material with corresponding sequence data) is proposed to allow a phylogenetic analysis of O. ocimi which is essential for a taxonomic reassessment of this species. The G. biocellatus complex has recently been divided into four species (Scholler et al. 2016), viz, G. biocellatus, G. monardae, G. neosalviae, and G. salviae. The present collections from Thailand on Ocimum tenuiflorum are morphologically similar to these Golovinomyces species but differ in having relatively short conidiophores foot cells, 27–42 μm long, vs. 40–100(−140) μm long in G. monardae, 55–100(−130) μm long in G. biocellatus, 30–100(−120) μm long in G. salviae, 45–75(−115) μm long in G. neosalviae. Furthermore, two sequences retrieved from powdery mildew on O. tenuiflorum formed a distinct monophyletic clade. This clade grouped with the clades of G. biocellatus, G. monardae, G. neosalviae, and G. salviae with 100% BS value. The number of nucleotide differences of Golovinomyces on O. tenuiflorum was 3/486 characters (99.3% similarity, not including gaps) from G. biocellatus on Glechoma hederacea (LC076805), 3/485 characters (99.3 % similarity) from G. monardae on Monarda citriodora (LC076809), 4/485 characters (99.1 % similarity) from G. neosalviae on Salvia lavandulifolia (LC076838), and 4/486 characters (99.1 % similarity) from G. salviae on Salvia nemorosa (LC100001). These results suggest that the powdery mildew on O. tenuiflorum represents a species of its own morphologically and phylogenetically different from the allied Golovinomyces species on Glechoma, Mentha, Origanum, Rosmarinus, Salvia and Thymus, supported by the phylogenetic-taxonomic affinity and position of the host plant within the Lamiaceae [subfam. Nepethoideae tribe Ocimeae] (Walker et al. 2004). ICN (International Code of Nomenclature for algae, fungi, and plants), Art. 59.1, is applicable to this fungus. Thus, although its sexual morph is still unknown, it is assignable to Golovinomyces, which has priority over the anamorph-typified genus Euoidium that is now a heterotypic synonym of Golovinomyces (Rossman et al. 2016), and the name Oidium ocimi is used for this species (see above).
The Golovinomyces ambrosiae clade (III sensu Takamatsu et al. 2013)
This genetic assemblage is heterogeneous and unresolved. It comprises at least two morphologically distinguishable taxa that have been described as Golovinomyces ambrosiae, confined to Ambrosia, Helianthus, Iva, and Rudbeckia spp. as well as Zinnia angustifolia, all belonging to the composite tribe Heliantheae (Braun and Cook 2012). This species is characterized by having broadly ellipsoid-ovoid, doliiform to limoniform conidia, 25–45 × 15–27 μm when fresh, with a length/width ratio < 2, 1.3–1.9, mosty 1.4–1.6, and dimorphic germ tubes with a high percentage of longitubus pattern within the Euoidium type. The other taxon involved in this clade has been referred to as Golovinomyces spadiceus, which differs from G. ambrosiae in having narrower conidia, 25–40 × 14–20 μm, with a length/width ratio of 1.5–2 and a Euoidium type of the conidial germination, lacking longitubus pattern germ tubes. G. spadiceus occurs on many hosts belonging to tribe Heliantheae, including species of the genera Coreopsis, Dahlia, Xanthium, and Zinnia, but is undoubtedly plurivorous with various hosts belonging to genera of other composite tribes and possibly even to some non-composite hosts. The situation is further complicated by the fact that some of the hosts involved in this complex are colonized by several Golovinomyces species, e.g., Dahlia spp. and Helianthus spp. are also hosts of G. orontii and Zinnia spp. are known to be hosts of G. ambrosiae as well as G. spadiceus (Braun and Cook 2012). It cannot be excluded that even Helianthus spp. might be hosts of G. spadiceus. Lineage III in Takamatsu et al. (2013) is probably a complex of several species. ITS sequences are not sufficient for a reliable resolution and differentiation on species level. Furthermore, the application of the species names involved can only be considered to be tentative since Erysiphe ambrosiae and E. spadicea were described from North America on Ambrosia sp. and Xanthium sp., respectively, but reference sequences and epitypes based on North American samples are not yet available. The application of the name G. circumfusus (Schltdl.) U. Braun to a sequence retrieved from a Japanese sample on Eupatorium chinense in lineage III in Takamatsu et al. (2013) is also unclear and unproven since reference sequences obtained on the basis of European collections on Eupatorium cannabinum (type of Erysiphe circumfusa) are also still lacking. Up until a comprehensive genetic re-examination of the whole G. ambrosiae complex based on additional markers and epitypifications of the taxa involved, collections with sequences pertaining to lineage III can currently only tentatively be assigned to G. ambrosiae and G. spadiceus just based on morphology, as for instance done by Dugan (2013) in the case of Golovinomyces on Coreopsis. In this sense, Golovinomyces specimens recently collected in Thailand on Dahlia pinnata, D. × hortensis, and Laggera crispata can be assigned to G. spadiceus, and collections on Helianthus annuus to G. ambrosiae. Golovinomyces on Verbena × hybrida can only tentatively be assigned to G. spadiceus (conidiophores and conidia of the sample from Thailand are barely distinguishable from G. verbenae, but comparative sequence data retrieved from North American collection on Verbena are not yet available, i.e. the phylogenetic position of the latter species is still unknown). The identity of Golovionomyces on Ageratum conyzoides and Bidens pilosa in Thailand is unclear, since they are clearly different from G. ambrosiae and probably also distinct from G. spadiceus, and can currently only be classified as Golovinomyces sp. Further research is necessary. For the fungus on Ageratum conyzoides (Eupatorieae), the name Euoidium agerati (J.M. Yen) U. Braun & R.T.A. Cook, described on this host from Taiwan, is available (Braun and Cook 2012).
Golovinomyces ambrosiae (Schwein.) U. Braun & R.T.A. Cook, in Cook & Braun, Mycol. Res. 113(5): 628, 2009
Mycelium amphigenous, mainly epiphyllous, effuse or forming patches, thin, white, persistent or almost so; hyphae hyaline, walls thin, smooth, 4–8 μm wide. Hyphal appressoria nipple-shaped, solitary or in opposite pairs. Conidiophores arising centrally or towards one end of hyphal mother cells and from their upper surface, erect, straight, 177–298 μm long. Foot cells cylindrical, straight in the base, 71–129 × 11–18 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia broadly ellipsoid-ovoid, doliiform to somewhat limoniform, 39–60 × 23–27 μm, germ tubes terminal to subterminal, tips not swollen or only slightly swollen (Fig. 3).
Material examined – On Helianthus annuus L. (Asteraceae), Chiang Rai, Mae Suai, 30 December 2016, J. Meeboon & S. Takamatsu, MUMH6871; Chiang Mai, Mae Rim, Mon Jam, 19 January 2016, J. Meeboon, MUMH6683.
Notes – Golovinomyces ambrosiae and G. orontii have been recorded on Helianthus spp. (Braun and Cook 2012). These two species differ from each other by the shape of foot cells; G. ambrosiae has straight in the base of foot cells, G. orontii has curved foot cells. The morphological characteristics of the current specimen are more similar to G. ambrosiae than to G. orontii due to having straight foot cells and broad conidia, 25–40 × (10–)15–23(–25) μm in G. orontii vs. 25–45 × 15–27 μm in G. ambrosiae (Braun and Cook 2012). This is the first report of G. ambrosiae on Helianthus annuus from Thailand.
Golovinomyces cynoglossi (Wallr.) Heluta, Ukrayins’k. Bot. Zhurn. 45(5): 62, 1988
Mycelium amphigenous, dense, effuse or in patches, evanescent to almost persistent. Hyphae hyaline, thin-walled, smooth, 3.5–7 μm wide. Hyphal appressoria nipple-shaped. Conidiophores erect, arising from upper surface of hyphal mother cells, 90–160(–200) μm long. Foot cells straight, cylindrical, 30–110 × 10–13 μm, followed by 1–2(–3) shorter cells, forming catenescent conidia; conidia ellipsoid-ovoid, 33–45 × 18–25 μm, germ tubes arising from an end, moderately long, apex often with somewhat swollen appressorium as in Euoidium type (Fig. 4).
Material examined – on Myosotis scopioides L. (Boraginaceae), THAILAND, Chiang Mai, Su Thep, 5 January 2005, J. Meeboon, MUMH1825.
Notes – Braun and Cook (2012) described G. cynoglossi on many host species of various host genera of Boraginaceae including Myosotis throughout Europe, Asia, North and South Africa and North America. The asexual morph found on Myosotis scopioides agrees completely with G. cynoglossi and is the first record of powdery mildew on M. scopioides in Thailand.
Golovinomyces orontii (Castagne) Heluta, Ukrayins’k. Bot. Zhurn. 45(5): 63, 1988
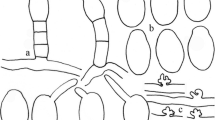
Mycelium amphigenous, mainly epiphyllous, effuse or in patches, evanescent or persistent, white; hyphae slightly flexuous, branched at right angles. Hyphal appressoria nipple-shaped, often poorly developed. Conidiophores erect, arising laterally or from the upper surface of hyphal mother cells and almost centrally or towards one end of the cell. Foot cells straight or curved in the basal half, followed by 2–3 shorter cells, forming catenescent conidia. Conidia ellipsoid, doliiform, subcylindrical, germ tubes arising from an end, occasionally from a side, straight, bent, rarely forked, apically often with a somewhat swollen appressorium, Euoidium type.
Golovinomyces orontii ex Coccinia grandis
Conidiophores erect, arising laterally or the upper surface of hyphal mother cells, 130–200 μm long. Foot cells curved, rarely straight, 60–87 × 13–16 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia subcylindrical, 36–44 × 18–20 μm (Fig. 5).
Golovinomyces orontii ex Lactuca indica
Conidiophores erect, usually arising laterally from the hyphal mother cell but occasionally from its upper surface, 100–210 μm long. Foot cells straight to curved at the base, 36–61 × 5–10 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform to subcylindrical, 28–32 × 12–14 μm (Fig. 6).
Golovinomyces orontii ex Torenia fournieri
Conidiophores erect, arising from the upper surface of hyphal mother cells, 157–290 μm long. Foot cells straight or curved, 26–124 × 9.5–13 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia ellipsoid, doliiform to subcylindrical, 30–39 × 15–20 μm (Fig. 7).
Golovinomyces orontii ex Vigna umbellata
Conidiophores erect, arising from the upper surface of hyphal mother cells, 115–235 μm long. Foot cells straight or curved, 32–57 × 13–15 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform to subcylindrical, 38–50 × 20–23 μm (Fig. 8).
Materials examined – on Coccinia grandis (L.) Voigt (Cucurbitaceae), THAILAND, Chiang Rai, 5 January 2016, J. Meeboon, MUMH6613; Lactuca indica L. (Asteraceae), THAILAND, Chiang Rai, Mae Suai, 29 December 2016, J. Meeboon & S. Takamatsu, MUMH6936; Torenia fournieri Linden ex Fourn. (Linderniaceae), Chiang Rai, Wiangpapao, 5 January 2016, J. Meeboon, MUMH6881, Vigna umbellata (Thunb.) Ohwi & H.Ohashi (Fabaceae), Chiang Rai, Mae Suai, 29 December 2016, J. Meeboon, MUMH6893.
Notes – Golovinomyces cucurbitacearum (R.Y. Zheng & G.Q. Chen) Vakal. & Kliron. is listed as powdery mildew on Coccinia spp. (Braun and Cook 2012). The asexual morph of the present sample is in good agreement with G. orontii, and the identity of this collection is confirmed by means of molecular sequence analyses (see Fig. 1). The fungus on Coccinia differs from G. cucurbitacearum in having longer foot cells and broader conidia. Golovinomyces cichoracearum, G. orontii, Leveillula lactucarum, L. lactucae-serriolae, and Podosphaera xanthii have been recorded on Lactuca spp. worldwide (Matsuda and Takamatsu 2003; Braun and Cook 2012; Takamatsu et al. 2013; Park et al. 2015; Cho et al. 2016). The asexual morph of the powdery mildew found in Thailand on L. indica having conidiophores arising laterally and from the upper surface of hyphae. However, some previous reports mentioned that the conidiophores of G. orontii obtained from Lactuca spp. are only arising laterally, thus producing curved foot cells (Braun and Cook 2012; Park et al. 2015; Cho et al. 2016). In addition, the identification as G. orontii has been confirmed by means of molecular sequence analyses (see Fig. 1). Vági et al. (2007) reported Golovinomyces sp. on Torenia fournieri from Hungary. The morphology of the specimen on Torenia fournieri from Thailand is similar to the fungus collected in Hungary, and a sequence retrieved from this collection clusters within G. orontii group 3 according to Takamatsu et al. (2013). Vigna umbellata was previously unknown as host of Golovinomyces spp. The sequences of the powdery mildews from C. grandis and V. umbellata belong to the big G. orontii complex and form a clade of its own with 94% BS support (Fig. 1). These hosts are new to Thailand.
Golovinomyces spadiceus (Berk. & M.A. Curtis) U. Braun ex Dahlia pinnata and Dahlia × hortensis
Mycelium amphigenous, forming white patches, confluent, sometimes covering entire leaves, persistent or almost so. Hyphae 4–8 μm wide, thin-walled, smooth, hyaline. Hyphal appressoria solitary, nipple-shaped, 3–8 μm diam. Conidiophores erect, arising from upper surface of hyphal mother cell and usually towards one end of it, 125–188 μm long. Foot cells cylindrical, 52–98 × 9–13 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia ellipsoid-ovoid, 27–33 × 15–25 μm. Conidial germination of the Euoidium type (Fig. 9).
Materials examined – On Dahlia pinnata Cav. (Asteraceae), Chiang Mai, Su Thep, 21 January 2005, J. Meeboon, MUMH3708; Dahlia × hortensis Guillaumin, Chiang Rai, Mae Suai, 27 December 2016, J. Meeboon, MUMH6874.
Notes – Braun and Cook (2012) described the morphological characteristics of G. spadiceus, which overlapped with our own specimens. The molecular phylogenetic analysis based on the ITS rDNA sequence of this specimen showed that the present fungus nested in the G. ambrosiae clade (III sensu Takamatsu et al. 2013) with 98% BS (Fig. 1). This is the first report of G. spadiceus on Dahlia pinnata and Dahlia × hortensis from Thailand.
Golovinomyces spadiceus (Berk. & M.A. Curtis) U. Braun ex Laggera crispata
Mycelium amphigenous, effuse or in thin, irregular patches, white, persistent. Hyphae sparingly branched, straight to moderately sinuous, 4–8 μm wide, hyaline, thin-walled, smooth. Hyphal appressoria almost indistinct to nipple-shaped, usually solitary, 2–7 μm diam. Conidiophores 156–187 μm long, arising laterally or from the upper surface of hyphal mother cells, and positioned almost centrally or towards one end of the cells, slightly curved at the base. Foot cells subcylindrical, 61–112 × 10–13.5 μm, followed by 0–3 shorter cells, forming catenescent conidia. Conidia ellipsoid-obovoid, often constricted at the ends, 35–41 × 16–21 μm. Conidial germination of the Euoidium type (Fig. 10).
Material examined – On Laggera crispata (Vahl) Hepper & J.R.I. Wood (= L. pterodonta (DC.) Sch.Bip. ex Oliv.) (Asteraceae, Inuleae), Chiang Mai, Mae Rim, 18 January 2005, J. Meeboon, MUMH1748.
Notes – This is the first report of powdery mildew on L. crispata. The morphological characteristics were typical of the asexual morph of the genus Golovinomyces, Foot cells of current material are longer than previous data, 30–80 × 9–15 μm (Braun and Cook 2012), and conidia are longer, 25–40 × 14–20 μm (Braun and Cook 2012). Based on these morphological characteristics, we identify the powdery mildew on L. crispata as G. spadiceus.
Golovinomyces spadiceus (Berk. & M.A. Curtis) U. Braun ex Verbena × hybrida
Mycelium amphigenous, effuse or in thin, irregular patches, white, persistent. Hyphae sparingly branched, straight to moderately sinuous, 4–8 μm wide, hyaline, thin-walled, smooth. Hyphal appressoria almost indistinct to nipple-shaped, usually solitary, 2–7 μm diam. Conidiophores 81–182 μm long, arising from the upper surface of hyphal mother cells, and positioned almost centrally or towards one end of the cells, straight to slightly curved at the base. Foot cells subcylindrical, 32–66 × 10–15 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform-limoniform, 38–46 × 20–26 μm. Conidial germination of the Euoidium type (Fig. 11).
Material examined – On Verbena × hybrida Groenland & Rümpler (Verbenaceae), Chiang Mai, Mae Rim, 19 January 2016, J. Meeboon, MUMH6684.
Notes – Golovinomyces verbenae (Schwein.) Heluta and G. orontii have been recorded on Verbena spp. (Amano 1986; Braun and Cook 2012). The current specimen was confirmed to be G. spadiceus by the size of foot cells and conidial shape. This is the first report of G. spadiceus on V. × hybrida from Thailand.
Golovinomyces sonchicola U. Braun & R.T.A. Cook, in Cook & Braun, Mycol. Res. 113(5): 629, 2009
Mycelium amphigenous, mainly epiphyllous, effuse or forming white patches, thin. Hyphae straight to sinuous, hyaline, thin-walled, smooth or almost so, 4–7 μm wide. Hyphal appressoria nipple-shaped. Conidiophores arising from the hyphal mother cell and towards one end of the cell, often close to a septum, rarely in the middle, 112–160 μm long. Foot cells curved, 38–70 × 12–16 μm, slightly constricted at the basal septum, followed by 1–3 shorter cells, forming catenescent conidia. Conidia ellipsoid-obovoid, 34–48 × 18–22 μm, germ tubes terminal or almost so, short to moderately long, often with a slightly swollen appressorium at the tip, Euoidium type (Fig. 12).
Material examined – on Sonchus oleraceus L. (Asteraceae), THAILAND, Chiang Mai, Su Thep, 5 January 2005, J. Meeboon, MUMH1772.
Notes – Shin (2000) described the asexual morphs of powdery mildews on Sonchus asper, S. brachyotus, and S. oleraceus collected in Korea. The ITS sequence of the powdery mildew on S. oleraceus was compared with the nucleotide sequences obtained from DNA databases. This fungus has the highest sequence similarity with G. sonchicola on S. oleaceus collected in Japan (99.8%). The present fungus formed a distinct clade with G. sonchicola on S. oleraceus (AB077623) collected in Japan with strong bootstrap support (98%) (Fig. 1). Based on the morphological and molecular characteristics, the powdery mildew on S. oleaceus is identified as G. sonchicola. This is the first report of G. sonchicola on S. oleraceus from Thailand.
Golovinomyces sordidus (L. Junell) Heluta, Ukrayins’k. Bot. Zhurn. 45(5): 63, 1988
Mycelium amphigenous, in irregular patches, almost persistent or evanescent. Hyphae hyaline, walls thin, smooth or almost so, 5–8 μm wide. Hyphal appressoria nipple-shaped or occasionally slightly lobed, sometimes poorly developed. Conidiophores arising more or less laterally from the hyphal mother cell and towards one end of the cell, often close to a septum, 98–240 μm long. Foot cells almost cylindrical, 40–100 × 11–14 μm, followed by 3–4 shorter cells, forming catenescent conidia. Conidia ellipsoid-ovoid to subcylindrical, 30–40 × 20–24 μm, germ tubes terminal or almost so, short to moderately long, ending in an unlobed, somewhat swollen appressorium, Euoidium type (Fig. 13).
Material examined – on Plantago major L. (Plantaginaceae), THAILAND, Chiang Mai, Inthanon National Park, 11 December 2014, MUMH6633.
Notes – The asexual morph of G. sordidus is the only Euoidium species occurring on Plantago spp. (Braun and Cook 2012). Although there are only minor differences in the sizes of conidiophores and foot cells, this fungus was identified as G. sordidus. This is the first report of G. sordidus on P. major from Thailand.
Golovinomyces sp. ex Ageratum conyzoides
Mycelium amphigenous, mainly epiphyllous, effuse or forming white patches, thin. Hyphae straight to sinuous, hyaline, thin-walled, smooth or almost so, 3–7 μm wide. Hyphal appressoria nipple-shaped. Conidiophores 110–237 μm long, erect, arising from upper surface of hyphal mother cell and usually towards one end of it. Foot cells cylindrical, 42–95 × 12–16 μm, followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform to subcylindrical 38–53 × 17–20 μm. Conidial germination of the Euoidium type (Fig. 14).
Material examined – On Ageratum conyzoides L. (Asteraceae, Eupatorieae), THAILAND, Lampang, Wang Nue, 13 January 2016, J. Meeboon, MUMH6739.
Notes – Ageratum conyzoides is known as host of G. cichoracearum s. lat. (Amano 1986). The foot cells and conidia of this specimen are longer than in G. cichoracearum s. str. (30–)40–80 μm long vs. 42–95 μm long and 25–42 × 14–23 μm vs. 38–53 × 17–20 μm, respectively (Braun and Cook 2012). The sequence of the powdery mildew on A. conyzoides clustered with those on Bidens pilosa in the clade of G. ambrosiae (III sensu Takamatsu et al. 2013) with 92% BS and the morphological characteristics are clearly different from G. ambrosiae, foot cells are shorter, 35–80 × 9–15 μm and conidia are broader, 25–45 × 15–27 μm (Braun and Cook 2012). Identification of Golovinomyces on A. conyzoides using solely based on the ITS rDNA sequences is insufficient. This is the first report of Golovinomyces sp. on A. conyzoides from Thailand.
Golovinomyces sp. ex Bidens pilosa
Mycelium amphigenous, mainly epiphyllous, effuse or forming white patches, thin. Hyphae straight to sinuous, hyaline, thin-walled, smooth or almost so, 4–6 μm wide. Hyphal appressoria nipple-shaped. Conidiophores arising from upper surface of hyphal mother cell and usually towards one end of it, 95–190 μm long. Foot cells 41–68 × 10–16 μm, slightly constricted at the basal septum, followed by 1–3 shorter cells, forming catenescent conidia. Conidia doliiform 34–50 × 20–25 μm. Conidial germination of the Euoidium type (Fig. 15).
Material examined – On Bidens pilosa L. Chiang Rai, Wiangpapao, 3 January 2016, J. Meeboon, MUMH6685
Notes – Bidens pilosa is known as host of G. cichoracearum s. lat. (Amano 1986). This is the first report of Golovinomyces sp. on B. pilosa from Thailand.
Golovinomyces sp. ex Lygisma inflexum
Mycelium amphigenous, forming patches or effuse, often confluent, persistent, particularly on the upper leaf surface, often evanescent on the lower surface, white or dingy greyish white. Hyphae straight to sinuous-geniculate, walls thin, smooth or almost so, hyaline, 3–8 μm wide. Hyphal appressoria almost indistinct to nipple-shape. Conidiophores arising from upper surface and usually towards one end of hyphal mother cells, 186–262 μm long. Foot cells straight to curved, cylindrical, 76–82 × 12–16 μm, followed by 1–3 shorter cells, forming catenescent conidia, often in long chains. Conidia ellipsoid-cylindrical, 35–43.5 × 20–21 μm, germ tubes short to moderately long, Euoidium type (Fig. 16).
Material examined – on Lygisma inflexum (Costantin) Kerr (Asclepiadaceae), THAILAND, Chiang Mai, Su Thep, 5 January 2005, J. Meeboon, MUMH1784.
Notes – Golovinomyces cichoracearum s. lat. has been recorded on the asclepidaceous hosts Asclepias syriaca L. and A. tuberosa L. and G. orontii on A. incarnata L. and Hoya carnosa (L.) R.Br. (Farr and Rossman 2017), but Lygisma inflexum has not yet been listed as host of any Golovinomyces species (Braun and Cook 2012). This is the first record of powdery mildews on L. inflexum. Although DNA isolation of this specimen failed, based on conidial chains with sinuous edge lines and conidia without fibrosin bodies, this powdery mildew is assigned to the genus Golovinomyces.
References
Amano HK (1986) Host range and geographical distribution of the powdery mildew fungi. Japan Scientific Societies Press, Tokyo
Bappammal M, Hosagoudar VB, Udaiyan K (1995) Powdery mildews of Tamil Nadu, India. New Botanist 22:81–175
Braun U (1987) A monograph of the Erysiphales (powdery mildews). Beihefte zur Nova Hedwigia 89:1–700
Braun U, Cook RTA (2012) Taxonomic manual of the Erysiphales (powdery mildews). CBS biodiversity. Ser. No. 11. CBS–KNAW fungal biodivers. Centre, Utrecht
Braun U, Takamatsu S (2000) Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences – some taxonomic consequences. Schlechtendalia 4:1–33
Cho SE, Choi YJ, Han KS, Park MJ, Shin HD (2016) First report of powdery mildew caused by Golovinomyces orontii on Lactuca sativa in Korea. Plant Disease 100:1015
Dugan F (2013) Golovinomyces spadiceus causes powdery mildew on Coreopsis hybrida ‘full moon’ (Heliantheae, Asteraceae) in Washington State. North American Fungi 8:1–3
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32:1792–1797
Farr DF, Rossman AY (2017) Fungal Databases, Syst. Mycol. and Microbiol. Lab., ARS, USDA. Available at: http://nt.ars-grin.gov/fungaldatabases/. Accessed 8 June 2017
Felsenstein J (1985) Confidence limits on phylogenetics: an approach using the bootstrap. Evolution 39:783–791
Hirata T, Takamatsu S (1996) Nucleotide sequence diversity of rDNA internal transcribed spacers extracted from conidia and cleistothecia of several powdery mildew fungi. Mycoscience 37:283-288
Hosagoudar VB, Agarwal DK (2009) Powdery mildews of India–check list. Associated Publishing Company, New Delhi
Matsuda S, Takamatsu S (2003) Evolution of host-parasite relationships of Golovinomyces (Ascomycetes: Erysiphaceae) inferred from nuclear rDNA sequences. Molecular Phylogenetics and Evolution 27:314–327
Meeboon J, Takamatsu S (2015a) Erysiphe takamatsui, a powdery mildew of lotus: rediscovery of teleomorph after 40 years, morphology and phylogeny. Mycoscience 56:159–167
Meeboon J, Takamatsu S (2015b) Erysiphe viburni-plicati and Podosphaera photiniae, two new species of Erysiphales (Ascomycota) from Japan. Mycoscience 56:14–23
Meeboon J, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand II. Erysiphe species on Anacardiaceae, Apocynaceae, Araliaceae, Aristolochiaceae, Bixaceae, Brassicaceae, Caprifoliaceae, Cleomaceae, Convolvulaceae, Cucurbitaceae and Euphorbiacea. Tropical Plant Pathology 41:357–369
Meeboon J, Takamatsu S (2017a) Notes on powdery mildews (Erysiphales) in Thailand III. Erysiphe species on Fabaceae, Fagaceae, Hydrangeaceae and Lamiaceae. Tropical Plant Pathology 42:239–249
Meeboon J, Takamatsu S (2017b) Notes on powdery mildews (Erysiphales) in Thailand IV. Erysiphe species on Malvaceae, Menispermaceae, Moraceae, Nyctaginaceae, Polygonaceae, Solanaceae and Urticaceae. Tropical Plant Pathology. https://doi.org/10.1007/s40858-017-0156-2
Meeboon J, Takamatsu S (2017c) New records of Erysiphe sect. Uncinula spp. (Erysiphales) from Thailand and E. liquidambaris var. acalycinae var. nov. Mycoscience 58:236–241
Meeboon J, Takamatsu S (2017d) First found of Erysiphe elevata on Eucalyptus camaldulensis and Phyllactinia lagerstroemiae sp. nov. on Lagerstroemia from Thailand. Mycoscience 58:253–260
Meeboon J, Hidayat I, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand I. Podosphaera sect. Sphaerotheca. Tropical Plant Pathology 6:142–174
Narayanaswami P, Ramakrishnan K (1967–1968) 1969 powdery mildews of Coimbatore, madras state. The Madras University Journal 37–38:84–99
Park MJ, Hong SH, Cho SE, Park JH, Shin HD (2015) First report of powdery mildew caused by Golovinomyces orontii on invasive weed Lactuca serriola in Korea. Plant Disease 99:889
Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, Romberg M, Samson RA, Seifert KA, Stone JK, Udayanga D, White JF (2016) Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 7:289–308
Scholler M, Schmidt A, Siahaan SAS, Takamatsu S, Braun U (2016) A taxonomic and phylogenetic study of the Golovinomyces biocellatus complex (Erysiphales, Ascomycota) using asexual state morphology and rDNA sequence data. Mycological Progress 15:56
Shin HD (2000) Erysiphaceae of Korea. National Inst. of Agric. Sci. and Technol, Suwon, Korea
Shin HD, La YJ (1993) Morphology of edge lines of chained immature conidia on conidiophores in powdery mildew fungi and their taxonomic significance. Mycotaxon 66:445–451
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer, Sunderland
Takamatsu S, Kano Y (2001) PCR primers useful for nucleotide sequencing of rDNA of the powdery mildew fungi. Mycoscience 42:135–139
Takamatsu S, Matsuda S, Grigaliunaite B (2013) Comprehensive phylogenetic analysis of the genus Golovinomyces (Ascomycota: Erysiphales) reveals close evolutionary relationships with its host plants. Mycologia 105:1135–1152
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Vági P, Kovács GM, Kiss L (2007) Host range expansion in a powdery mildew fungus Golovinomyces sp. infecting Arabidopsis thaliana: Torenia fournieri as a new host. European Journal of Plant Pathology 117:89–93
Walker JB, Sytsma KJ, Treutlein J, Wink M (2004) Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. American Journal of Botany 91:1115–1125
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a mediumfor simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506–513
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Acknowledgements
This work was financially supported in part by a Grant-in-Aid for Scientific Research (No. 16K07613 and 16F16097) from the Japan Society for the Promotion of Science to ST; and The JSPS postdoctoral fellowship to JM (P16097).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Meike Piepenbring
Rights and permissions
About this article
Cite this article
Meeboon, J., Kokaew, J. & Takamatsu, S. Notes on powdery mildews (Erysiphales) in Thailand V. Golovinomyces . Trop. plant pathol. 43, 202–217 (2018). https://doi.org/10.1007/s40858-017-0201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-017-0201-1