Abstract
This report provides morphological descriptions of powdery mildew specimens found on Fabaceae, Fagaceae, Hydrangeaceae and Lamiaceae and molecular phylogenetic analyses based on ITS rDNA sequences. These include 4 new host records for Erysiphe species in the world, viz., Desmodium triflorum (Fabaceae), Microtoena insuavis (Lamiaceae) and Mucuna bracteata (Fabaceae). Aeschynomene americana var. americana (Fabaceae), Sesbania grandiflora (Fabaceae) and Tamarindus indica (Fabaceae) are new host for Erysiphe trifoliorum s. lat. and Phanera purpurea (Fabaceae) is new host for Erysiphe lespedezae. In addition, 11 records of Erysiphe species new to Thailand were described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, new checklists of powdery mildews belonging to the genera Erysiphe and Podosphaera sect. Podosphaera were reported from Asia (Meeboon et al. 2016; Meeboon and Takamatsu 2016). These reports as well as those from Siahaan et al. (2016a, b) suggest that there are much more undescribed species of powdery mildews in Southeast Asia region than previously thought. This is supported by recent publications from this region, in particular from Indonesia and Thailand (To–anun et al. 2003, 2005; Divarangkoon et al. 2011; Monkhung et al. 2011, 2013; Meeboon et al. 2012a, b, c, d, 2013; Hidayat et al. 2014; Siahaan et al. 2015). In this paper, we report an additional list about Erysiphe species found on Fabaceae, Fagaceae, Hydrangeaceae and Lamiaceae in Thailand.
Materials and methods
Morphological examination
Morphological examinations were conducted according to the procedure described by Meeboon and Takamatsu (2015). Rehydration of mycelium from herbarium specimen was conducted according to the method described by Shin and La (1993). Thirty conidia and conidiophores were measured for each specimen. Specimens were deposited at Mie University Mycological Herbarium (TSU-MUMH), Japan.
Molecular phylogeny
Whole cell DNA was extracted from mycelia using the Chelex method (Walsh et al. 1991) as described in Hirata and Takamatsu (1996). The respective primer pairs of PM5/ITS4 and ITS5/PM6 (Takamatsu and Kano 2001) were used to amplify ITS fragment 1 and fragment 2, respectively. PCR reaction was conducted using KOD FX Neo DNA polymerase (Toyobo, Japan) according to the manufacturer’s protocol. The PCR product was sent to SolGent Co. Ltd. for sequencing using primer pair of ITS1 and ITS4 (White et al. 1990).
New representative sequences determined in this study were deposited in DNA Data Base of Japan (DDBJ) under the accession numbers of AB237804, AB237805, LC163907, LC163908, LC163912, LC163914, LC163915 and LC163916. Sequences generated in this study were aligned with other sequences of Erysiphe retrieved from DNA databases (DDBJ, EMBL, NCBI) using MUSCLE (Multiple Sequence Comparison by Log Expectation) (Edgar 2004) implemented in MEGA 6 (Tamura et al. 2013). The alignments were deposited in TreeBASE (http://www.treebase.org/) under the accession number of S20133. Phylogenetic trees were obtained from the data set using the maximum parsimony (MP) method in PAUP* 4.0b10 (Swofford 2002) as described in Meeboon and Takamatsu (2016).
Results
Taxonomy
Fabaceae
1. Erysiphe sp. ex Pueraria mirifica
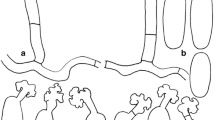
Mycelium epiphyllous, white, persistent, thin, effuse, sometimes covering entire leaves; hyphae 6–8 μm wide, branched, septate, hyaline, thin-walled, smooth; hyphal appressoria slightly to distinctly lobed, usually solitary; conidiophores up to about 160 μm long, erect, arising from the upper surface of mother cells; foot cells 40–70 × 6–9 μm, straight, occasionally slightly flexuous-sinuous, cylindrical, followed by 0–2 shorter cells, forming conidia singly; conidia 30–40 × 15–20 μm, ellipsoid to cylindrical; germ tubes subterminal, short, with lobed appressoria (Pseudoidium type) (Fig. 1).
Material: On Pueraria mirifica Airy Shaw & Suvatab. (Fabaceae), THAILAND, Chiang Mai province, Suthep, 15 January 2015, J. Meeboon, MUMH3759.
Note: Erysiphe puerariae R.Y. Zheng & G.Q. Chen is the only Erysiphe species found on Pueraria (Braun and Cook 2012). This species is endemic to China. However, there is no information on asexual morph of this species. Unfortunately, we failed to obtained DNA sequence of this fungus. This is the first report of Erysiphe sp. on Pueraria mirifica from Thailand.
2. Erysiphe lespedezae R.Y. Zheng & U. Braun ex Phanera purpurea
Mycelium amphigenous, mainly epiphyllous, white to greyish white, persistent, effuse to dense; hyphae 3.5–8 μm wide, ± straight, occasionally geniculate-sinuous; hyphal appressoria lobed to multilobed, solitary or in opposite pairs; conidiophores 48–94 μm long, erect, arising from the upper part of mother cells, position central or non-central; foot cells 35–65 × 5–9 μm, basal septum 5–20 μm distant from the branching point with the supporting hypha, slightly curved, forming conidia singly, followed by 1–3 shorter cells, with a basal septum at the branching point of the mycelium; conidia 19–41 × 10–17 μm, cylindric-ovoid; germ tubes subterminal, with lobed appressoria (Pseudoidium type) (Fig. 2).
Material: On Phanera purpurea L. (Fabaceae), THAILAND, Chiang Rai province, 28 December 2015, J. Meeboon, MUMH1816, accession number: LC163912; MUMH5742; MUMH6622, accession number: LC163921.
Note: Two powdery mildew species, i.e., Ps. bauhiniae (G.J. M. Gorter & Eicker) U. Braun & C.T.A. Cook and Ps. caesalpiniacearum (Hosag. & U. Braun) U. Braun & R.T.A. Cook have been recorded on Phanera spp. (Braun and Cook 2012). Pseudoidium bauhiniae differs from Ps. caesalpiniacearum by having multilobed appressoria. The morphological characteristics of this specimen are similar to Ps. bauhiniae (Braun and Cook 2012). Siahaan et al. (2016b) reported P. purpurea as a host of E. quercicola. Erysiphe lespedezae differs from E. quercicola by having conidia cylindric-ovoid while ellipsoid-doliiform in E. quercicola, longer conidiophores [48–94 vs. 32–72 μm in E. quercicola] and foot cells [35–65 × 5–9 μm vs. 8–30 × 5–8 μm in E. quercicola]. This report clearly indicates that two Pseudoidium species infect P. purpurea. The phylogenetic analysis based on ITS sequence showed that this fungus is identical to E. lespedezae R.Y. Zheng & U. Braun on Lespedeza thunbergii (AB015923) (Fig. 19). Based on the morphological and molecular characteristics, this fungus was identified as E. lespedezae. This is the first report of E. lespedezae on P. purpurea in the world.
3. Erysiphe trifoliorum s. lat. ex Sesbania grandiflora
Mycelium epiphyllous, white, forming dense patches; hyphae 4–8 μm wide; hyphal appressoria moderately lobed, solitary or in opposite pairs; conidiophores 75–120 μm long, erect, arising from the upper surface of mother cells; foot cells 28–56 × 8–11 μm, slightly straight to somewhat curved-sinuous, followed by 1–2 shorter cells, forming conidia singly; conidia 35–45 × 15–20 μm, ellipsoid-ovoid to cylindrical-doliiform; germ tubes almost terminal, short to long, with lobed appressoria (Pseudoidium type) (Fig. 3).
Material: On Sesbania grandiflora (L.) Poir (Fabaceae), THAILAND, Chiang Mai province, Suthep, 14 March 2014, J. Meeboon, MUMH1779, accession number: LC163908.
Note: Erysiphe sesbaniae Wolcan & U. Braun and Ps. fabacearum (Hosag.) U. Braun & R.T.A. Cook are recorded on Sesbania species worldwide (Braun and Cook 2012). Powdery mildew on S. grandiflora was previously reported as Oidium sp. in Thailand. The phylogenetic tree constructed by the parsimomy method showed that sequence of this fungus nested in the E. trifoliorum s. lat. clade (Takamatsu et al. 2015) from various hosts. Therefore, the current specimen is determined as E. trifoliorum s. lat.
4. Erysiphe trifoliorum s. lat. ex Tamarindus indica
Mycelium amphigenous, mainly epiphyllous, white, persistent, effuse or in dense, confluent patches, sometimes covering entire leaves; hyphae 3–7 μm wide, branched, septate; hyphal appressoria lobed, solitary or in opposite pairs; conidiophores 50–130 μm long, erect, arising from the upper surface of mother cells, basal septum at the junction with the mother cell or slightly elevated; foot cells 25–70 × 5–8.5 μm, straight, cylindrical, occasionally slightly curved, followed by 0–2 shorter cells, with a basal septum at the branching point of the mycelium, forming conidia singly; conidia 28–45 × 15–19.5 μm, ellipsoid-ovoid to cylindrical; germ tubes ± terminal, with lobed appressoria (Pseudoidium type) (Fig. 4).
Material: On Tamarindus indica L. (Fabaceae), THAILAND, Chiang Mai province, Suthep, 18 January 2006, J. Meeboon, MUMH1750, accession number: LC163908.
Note: Yen (1966) reported the powdery mildew on Tamarindus indica as a new variety, Oidium erysiphoides f. tamarindi J.M. Yen. Braun (1982) revised the name as O. tamarindi (J.M. Yen) U. Braun. Braun and Cook (2012) revised this name to Ps. tamarindi (J.M. Yen) U. Braun & R.T.A. Cook. This species distributes in Asia (India, Java, Singapore, Sri Lanka, Taiwan), Africa (Ghana, South Africa) and North America (Maxico). Recently, E. quercicola was found on T. indica (unpublished data by the authors). Erysiphe trifoliorum s. lat. differs from E. quercicola by having conidia ellipsoid-ovoid to cylindrical while ellipsoid-doliiform in E. quercicola. This report clearly indicates that two Pseudoidium species infect T. indica. This is the first report of E. trifoliorum s. lat. on T. indica in the world.
5. Erysiphe pisi DC. ex Pisum sativum
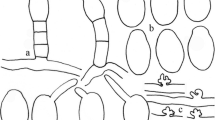
Mycelium amphigenous, persistent or evanescent, effuse or in thin, irregular patches; hyphal cells 4–7 μm; hyphal appressoria lobed, solitary, occasionally in opposite pairs; conidiophores 76–125 μm long, erect, arising mostly centrally or somewhat laterally from top of mother cell; foot cells 35–70 × 8–12.5 μm, cylindrical, straight, curved or somewhat flexuous-sinuous, followed by 0–2 cells, usually shorter, sometimes about as long as the foot cell, forming conidia singly; conidia 30–44 × 18–20 μm, ellipsoid-ovoid to doliiform (Fig. 5).
Material: On Pisum sativum L. (Fabaceae), THAILAND, Chiang Mai province, Suthep, 25 December 2013, J. Meeboon, MUMH1850, accession number: LC163915.
Note: Erysiphe glycines F.L. Tai and E. pisi DC. are recorded on P. sativum worldwide (Braun and Cook 2012). There is a record of E. pisi on P. sativum in Thailand. Falloon et al. (1989) studied asexual morph of E. pisi on P. sativum by scanning electron microscopy. The asexual morph of the present specimen are in good agreement with E. pisi. The ITS sequence showed that this fungus is identical to E. pisi on P. sativum and Lathyrus latifolius (Fig. 19). Based on the morphological characteristics and molecular analysis, this fungus is identified as E. pisi.
6. Erysiphe quercicola S. Takam. &. U. Braun ex Acacia auriculaformis
Mycelium epiphyllous, persistent or evanescent, effuse or in patches, often covering the entire surface of the leaves; hyphal cells 5–8 μm; hyphal appressoria slightly lobed to multilobed, solitary or in opposite pairs; conidiophores 80–130 μm long, erect, arising centrally or laterally from the upper surface of the mother cell; foot cells 25–45 × 10–12 μm, cylindrical, straight to sometimes curved, followed by 1–3 shorter cells or cells of about the same length, forming conidia singly; conidia 27.5–42 × 16–17.5 μm, ellipsoid-ovoid-cylindrical or doliiform; germ tubes ± terminal, with lobed appressoria (Pseudoidium type) (Fig. 6).
Material: On Acacia auriculaformis A. Cunn. (Fabaceae), THAILAND, Bangkok province, Chatuchack, 17 February 2014, J. Meeboon, MUMH1805, accession number: AB237804; MUMH5752, accession number: AB237805.
Note: Erysiphe acaciae S. Blumer, E. pisi var. pisi DC., E. polygoni DC. and E. trifoliorum (Wallr.) U. Braun have been recorded as Erysiphe s. lat. on Acacia spp. worldwide (Braun and Cook 2012; Farr and Rossman 2016). Because sexual morph was not found in the present fungus, only asexual morph was described in detail. The phylogenetic tree showed that this fungus nested in the large clade of E. quercicola from various hosts with 100% BS (Fig. 19). Based on this analysis, the current specimen is assigned as E. quercicola.
7. Erysiphe trifoliorum s. lat. ex Aeschynomene americana var. americana
Mycelium amphigenous, effuse or in patches, evanescent to persistent; hyphal cells 5–7.5 μm wide; hyphal appressoria lobed, solitary, occasionally in opposite pairs; conidiophores 100–135 μm long, erect, arising mostly centrally or somewhat laterally from top of mother cell; foot cells 30–65 × 8–11 μm, cylindrical, straight, curved or somewhat flexuous-sinuous, followed by 1–2 cells, usually shorter, sometimes about as long as the foot cell, forming conidia singly; conidia 30–45 × 12–22 μm, ellipsoid-ovoid to cylindric; germ tubes ± terminal, short, with lobed appressorium (Pseudoidium type) (Fig. 7).
Material: On Aeschynomene americana var. americana L. (Fabaceae), THAILAND, Chiang Mai province, Mae Jo, 22 December 2014, J. Meeboon, MUMH1841, accession number: LC163914.
Note: Three species of Aeschynomene, viz., A.americana, A. indica, and A. virginica, are infected with powdery mildews throughout the world (Amano 1986; Braun and Cook 2012). Shin (1988) also reported E. pisi on A. indica in Korea. Asexual morph of E. pisi were somewhat different from this fungus in its larger conidia (mostly 32–48 × 16–20) and longer conidiophores (mostly 70–120 × 7–8(−10)). The sequence from this specimen nested in the E. trifoliorum s. lat. clade (Takamatsu et al. 2015) from various hosts. Therefore, the current specimen is tentatively determined as E. trifoliorum s. lat. This is the first report of E. trifoliorum s. lat. on A. americana in Thailand.
8. Erysiphe sp. ex Indigofera dosua
Mycelium amphigenous, mainly hypophyllous, effuse or in patches, thin, evanescent, often covering the entire leaf surface; hyphal cells 3.5–6.5 μm long, hyaline, thin-walled, smooth; hyphal appressoria lobed, solitary or in opposite pairs; conidiophores 42.5–91 μm long, erect, arising ± centrally from upper surface of the mother cell; foot cells 20–60 × 7.5–9 μm, straight, cylindrical, followed by 1–2 cells shorter, forming conidia singly; conidia 25–38 × 16–20 μm, ellipsoid-ovoid, subcylindrical; germ tubes almost terminal, short to moderately long, with lobed appressoria (Pseudoidium type) (Fig. 8).
Material: On Indigofera dosua Buchanan-Hamilton ex D. Don (Fabaceae), THAILAND, Chiang Mai province, Suthep, 10 February 2014, J. Meeboon, MUMH3755.
Note: Based on conidiophore morphology and conidial germination patterns, this fungus belongs to the genus Pseudoidium, asexual morph of Erysiphe. This is the first report of Erysiphe on I. dosua in Thailand.
9. Erysiphe sp. ex Indigofera linnaei
Mycelium amphigenous, mainly hypophyllous, thin, evanescent, persistent, effuse or in patches, often covering the entire leaf surface; hyphal cells 3.5–5 μm wide; hyphal appressoria lobed, solitary or in opposite pairs; conidiophores 42–82 μm long, erect, arising ± centrally from upper surface of the mother cell; foot cells 18–35 × 8–10 μm, usually sinuous to almost straight, followed by 1–2 cells shorter or occasionally about the same length, forming conidia singly; conidia 28–40 × 15–20 μm, ellipsoid-ovoid to sub-cylindrical; germ tubes almost terminal, moderately long, with lobed appressoria (Pseudoidium type) (Fig. 9).
Material: On Indigofera linnaei Ali (Fabaceae), THAILAND, Chiang Mai province, Mae Rim, 20 January 2014, J. Meeboon, MUMH1746.
Note: Yen (1966) described anamorphic characteristics of Oidium indigoferae on Indigofera hirsute in Asia (Singapore). This species is also reported in Australia and Africa (Ghana, South Africa) (Braun 1987). The teleomorphic state of this fungus was first described by Shin (1988) on I. kirilowii in Korea, and named a new species Microsphaera indigoferae H.D. Shin & Y.J. La [= Erysiphe indigoferae (H.D. Shin & Y.J. La) U. Braun & S. Takam]. This species is also reported in Asia (Indonesia, Japan, China, incl. Taiwan, India, Singapore), Africa (Ghana, India, Kenya, Kongo, Mauritius, Niger, Sierra Leone, South Africa, Tanzania, Zambia, Zimbabwe) and Australia (Shin 2000; Braun and Cook 2012). Based on conidiophore morphology and conidial germination patterns, this fungus belongs to the genus Pseudoidium, asexual morph of Erysiphe. This is the first report of Erysiphe sp. on I. linnaei in Thailand.
10. Erysiphe sp. ex Pueraria wallichii
Mycelium epiphyllous, thin, persistent, in white patches or effuse, sometimes covering entire leaves; hyphae 3–5 μm wide, branched, septate, hyaline, thin-walled, smooth; hyphal appressoria lobed, usually solitary; conidiophores up to about 100 μm long, erect, arising from the upper surface of mother cells; foot cells 30–50 × 6–8 μm, straight, occasionally slightly flexuous, cylindrical, followed by 1–2 usually shorter cells, following cells occasionally about as long as the foot cell or rarely longer, forming conidia singly; conidia 25–40 × 18–20 μm, ellipsoid to cylindrical; germ tubes subterminal, short, with lobed appressoria (Pseudoidium type) (Fig. 10).
Material: On Pueraria wallichii DC. (Fabaceae), THAILAND, Chiang Mai province, Suthep, 10 February 2014, J. Meeboon, MUMH6636.
Note: Erysiphe puerariae R.Y. Zheng & G.Q. Chen is the only Erysiphe species found on Pueraria (Braun and Cook 2012). However, we could not compare the morphological characteristics of the current specimen with E. puerariae due to lacking of asexual morph of this species. This is the first report of Erysiphe sp. on P. wallichii in Thailand.
11. Erysiphe sp. ex Clitoria ternatea
Mycelium epiphyllous, effuse, evanescent to persistent, often following veins, sometimes covering the entire leaf surface; hyphae 5–6 μm wide, hyaline, branched; hyphal appressoria moderately lobed; conidiophores 81–143 μm long, erect, arising from the upper surface of mother cells; foot cells 28–62 × 7.5–10 μm, cylindrical, straight to slightly flexuous, followed by 1–3 usually shorter cells, occasionally by a cell of about the same length, forming conidia singly; conidia 30–51 × 17.5–21 μm, ellipsoid-ovoid (Fig. 11).
Material: On Clitoria ternatea L. (Fabaceae), THAILAND, Chiang Mai province, Suthep, 10 February 2014, J. Meeboon, MUMH3819.
Note: Pseudoidium clitoriae (Narayanas. & K. Ramakr.) U. Braun & R.T.A. Cook was reported as Pseudoidium on Clitoria spp. (Braun and Cook 2012). The present specimen differs from Ps. clitoriae (Braun and Cook 2012) by having longer conidiophores, larger foot cells and larger conidia. This is the first report of Erysiphe sp. on Clitoria ternatea in Thailand.
12. Erysiphe sp. ex Mucuna bracteata
Mycelium amphigenous, mainly hypophyllous, white, effuse or in dense patches, persistent or ± evanescent; hyphae 3–4.5 μm wide; hyphal appressoria lobed, solitary or in opposite pairs; conidiophores 70–150 μm long, erect from top of mother cell; foot cells 35–76 × 6–10 μm, cylindrical, straight to slender, sometimes flexuous, followed by 1–2 shorter cells, forming conidia singly; conidia 30–50 × 13.5–20 μm, ellipsoid-ovoid (Fig. 12).
Material: On Mucuna bracteata A.DC. (Fabaceae), THAILAND, Chiang Mai province, Mae Rim, 2 April 2013, J. Meeboon, MUMH6647.
Note: Based on conidiophore morphology and conidial germination patterns, this fungus belongs to the genus Pseudoidium, asexual morph of Erysiphe. This is the first report of Erysiphe on M. bracteata in the world.
13. Erysiphe baliensis Siahaan & S. Takam. ex Desmodium triflorum
Mycelium amphigenous, persistent or evanescent, effuse or in thin, irregular patches; hyphal cells 4–7 μm wide; hyphal appressoria lobed, solitary, occasionally in opposite pairs; conidiophores 65–128 μm long, erect, arising mostly centrally or somewhat laterally from top of mother cell; foot cells 18–66 × 6.5–9 μm, cylindrical, straight, curved or somewhat flexuous-sinuous, followed by 0–3 cells, usually shorter, sometimes about as long as the foot cell, forming conidia singly; conidia 30–43 × 15–19 μm, ellipsoid-ovoid to doliiform; germ tubes ± terminal, short to moderately long, with lobed appressoria (Pseudoidium type) (Fig. 13).
Material: On Desmodium triflorum (L.) DC. (Fabaceae), THAILAND, Chiang Mai province, Mae Rim, 3 April 2013, J. Meeboon, MUMH2605, accession number: LC163916.
Note: Erysiphe diffusa (Cooke & Peck) U. Braun & S. Takam., and E. glycines F.L. Tai have been known on Desmodium (Braun and Cook 2012). The molecular phylogenetic analysis based on ITS sequence data showed that this sequence identical to E. baliensis Siahaan & S. Takam. on Gliricidia sepium and Wisteria japonica (Fig. 19). This is the first report of E. baliensis on D. triflorum in the world.
Fagaceae
14. Erysiphe sp. ex Lithocarpus lindleyanus
Mycelium amphigenous, mainly epiphyllous, persistent to evanescent, effuse or in patches; hyphae 3–6 μm wide, thin-walled, smooth, hyaline to yellowish; hyphal appressoria lobed; conidiophores 70–141 μm long, erect, arising from top of mother cell; foot cells 22–55 × 6.5–9 μm, curved, followed by 1–2 shorter cells, forming conidia singly; conidia 28–45 × 14–20 μm, ellipsoid or cylindrical (Fig. 14).
Material: THAILAND, Chiang Mai province, Suthep, on Lithocarpus lindleyanus (Wall.) A. Camus (Fagaceae), 9 February 2014, J. Meeboon, MUMH3730.
Note: Based on the morphological examination, the current specimen belongs to Pseudoidium, asexual morph of Erysiphe. Erysiphe sikkimensis Chona, J.N. Kapoor & H.S. Gill is the only Erysiphe species other than E. sect. Californiomyces recorded on Lithocarpus spp. However, DNA sequencing was failed. This is the first report of Erysiphe sp. on L. lindleyanus in Thailand.
15. Erysiphe sp. ex Lithocarpus vestitus
Mycelium amphigenous, mainly hypophyllous, white, persistent, irregularly effuse, forming patches, sometimes covering the entire surface of the leaves; hyphal appressoria lobed to multilobed, solitary or in opposite pairs; conidiophores 55–100 μm long, arising from superficial hyphae, terminal on the mother cells; foot cells 32–65 × 5–7 μm, short, cylindrical, slightly curved, followed by 1–2 cells, shorter or about as long as the foot cell, forming conidia singly; conidia 20–40 × 12–18 μm, ellipsoid-doliiform; germ tubes terminal or subterminal, short to moderately long, with lobed appressoria (Pseudoidium type) (Fig. 15)
Material: On Lithocarpus vestitus (Hickel & A.Camus) Chun (Fagaceae), THAILAND, Chiang Mai province, Suthep, 9 February 2014, J. Meeboon, MUMH4994.
Note: Three species of Erysiphe and two species of Cystotheca have been reported on Lithocarpus (Braun and Cook 2012), but none of them found on L. vestitus. The current specimen belongs to Pseudoidium, asexual morph of Erysiphe. This is the first report of Erysiphe on L. vestitus in Thailand.
Hydrangeaceae
16. Pseudoidium hortensiae (Jørst.) U. Braun & R.T.A. Cook ex Hydrangea macrophylla
Mycelium amphigenous, evanescent or almost persistent, effuse, forming thin white and irregular patches; hyphae hyaline, branched, septate, hyphal cells 4–5.5 μm wide; hyphal appressoria multilobed, 5–25 μm diam., numerous, solitary or often in opposite pairs, sometimes in sequences of up to four pairs per cell; conidiophores 40–99 μm long, erect, present on both leaf surfaces, arising ± centrally from the upper surface of mother cells; foot cells 27–44 × 7.5–12.5 μm, straight, occasionally slightly curved, cylindrical, usually followed by 1–2 shorter cells or a single longer cell, forming conidia singly, rarely in short false chains; conidia 28–42 × 19–23 μm, ellipsoid-ovoid to cylindrical; germ tubes at an end, short to moderately long, with lobed appressoria (Pseudoidium type) (Fig. 16).
Material: On Hydrangea macrophylla (Thunb.) Ser. (Hydrangeaceae), THAILAND, Chiang Mai province, Suthep, 8 January 2007, J. Meeboon, MUMH1832.
Note: The morphological characteristics of this specimen are identical to Ps. hortensiae (Braun and Cook 2012). This is the first report of Ps. hortensiae on Hydrangea macrophylla in Thailand.
Lamiaceae
17. Erysiphe sp. ex Microtoena insuavis
Mycelium amphigenous, white, effuse or in patches, persistent; hyphae 3.5–7.5 μm wide, branched, septate; hyphal appressoria nipple-shaped to moderately lobed, mostly solitary; conidiophores 40–70 μm long, erect, arising from the upper surface of mother cells; foot cells 12–30 × 10–12.5 μm, cylindrical, straight, followed by 0–1 shorter cells, by a longer cell or by a cell of about the same length, forming conidia singly; conidia 25–38.5 × 19.5–22.5 μm, ovoid-doliiform to subcylindrical; germ tubes ± terminal, with lobed appressoria (Pseudoidium type) (Fig. 17).
Material: On Microtoena insuavis (Hance) Prain ex Briq. (Lamiaceae), THAILAND, Chiang Mai province, Suthep, 8 January 2007, J. Meeboon, MUMH1757.
Note: Powdery mildews have been recorded on numerous species of Lamiaceae, but not on M. insuavis. This study is the first record of powdery mildew on M. insuavis in the world. Microtoena is distributed in the area from the Himalaya Mountains to Western China, and it is also found in northern Thailand. Based on conidiophore morphology and conidial germination patterns, this fungus belongs to the genus Pseudoidium, asexual morph of Erysiphe.
18. Erysiphe sp. ex Ocimum sanctum
Mycelium amphigenous, effuse, evanescent, occasionally ± persistent; hyphae 4–7 μm wide; hyphal appressoria lobed, solitary or in opposite pairs; conidiophores 72–110 μm long, erect, on top of mother cell; foot cells 23–55 × 7–10 μm, straight or slightly curved, cylindrical, followed by 1–2 mostly shorter cells, with a basal septum at the branching point of the mycelium, forming conidia singly; conidia 28–36 × 8–22 μm, ellipsoid-ovoid to cylindrical; germ tubes short to moderately long, with lobed appressoria (Pseudoidium type) (Fig. 18 and 19).
Material: On Ocimum sanctum L. (Lamiaceae), THAILAND, Chiang Rai province, 8 December 2013, J. Meeboon, MUMH3753.
Note: This is the first study of Pseudoidium on Ocimum sanctum in Thailand. In Braun and Cook (2012), Oidium ocimi-sancti Puzari, A.K. Sarbhoy, N. Ahmad & D.K. Argawal is the only powdery mildew species found on Ocimum sanctum. However, the status of this species is unclear (Braun and Cook 2012). Further analysis is necessary to determine the identity of this specimen.
References
Amano (Hirata) K (1986) Host Range and Geographical Distribution of the Powdery Mildew Fungi. J Sci Soc Press, Tokyo
Braun U (1982) Morphological studies in the genus Oidium (III). Zentralblatt für Mikrobiologie 137:314–324
Braun U (1987) A monograph of the Erysiphales (powdery mildews). Beihefte zur Nova Hedwigia 89:1–700
Braun U, Cook RTA (2012) Taxonomic manual of the Erysiphales (powdery mildews). CBS Biodivers. Ser. No. 11. CBS–KNAW Fungal Biodivers. Centre, Utrecht
Divarangkoon R, Meeboon J, Monkhung S, To-anun C, Takamatsu S (2011) Two new species of Erysiphe (Erysiphales, Ascomycota) from Thailand. Mycosphere 2:231–238
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinf 5:113
Falloon RB, Sutherland PW, Hallett IC (1989) Water, fungicides and host resistance affect development of Erysiphe pisi on pea leaves; an electron microscope study. Agron. Soc. of N. Z., Proc. of the National Symp. and Workshop on Grain Legumes held at Lincoln College, December 11–12, p. 121
Farr DF, Rossman AY (2016) Fungal Databases, Syst. Mycol. and Microbiol. Lab., ARS, USDA. Available at: http://nt.ars-grin.gov/fungaldatabases/. Accessed on 3 June 2016
Hidayat I, Meeboon J, Takamatsu S (2014) First report of Pseudoidium aff. neolycopersici in Indonesia. Australas Plant Pathol Soc 9:1–3
Hirata T, Takamatsu S (1996) Nucleotide diversity of rDNA internal transcribed spacers extracted from conidia and cleistothecia of several powdery mildew fungi. Mycoscience 37:283–288
Meeboon J, Takamatsu S (2015) Erysiphe takamatsui, a powdery mildew of lotus: rediscovery of teleomorph after 40 years, morphology and phylogeny. Mycoscience 56:159–167
Meeboon J, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand II. Erysiphe. Trop. Plant. Pathol. 41:357–369
Meeboon J, Hidayat I, Kramadibrata K, Nurcahyanto D, Siahaan SAS, Takamatsu S (2012a) Cystotheca tjibodensis (Erysiphaceae, Ascomycota): rediscovery in Java after 90 years and first finding of anamorph. Mycoscience 53:386–390
Meeboon J, Hidayat I, Takamatsu S (2012b) Erysiphe javanica sp. nov., a new tropical powdery mildew from Indonesia. Mycotaxon 120:189–194
Meeboon J, Hidayat I, Takamatsu S (2012c) Pseudoidium javanicum, a new species of powdery mildew on Acalypha spp. from Indonesia. Mycoscience 54:183–187
Meeboon J, Hidayat I, Takamatsu S (2012d) Setoidium castanopsidis, a new species of anamorphic Cystotheca (Ascomycota, Erysiphales) from Indonesia. Mycoscience 54:274–278
Meeboon J, Divarangkoon R, Takamatsu S (2013) Two new species of Erysiphe sect. Uncinula (Erysiphales): Erysiphe fernandoae and E. michikoae. Mycoscience 54:2–7
Meeboon J, Hidayat I, Takamatsu S (2016) Notes on powdery mildews (Erysiphales) in Thailand I. Podosphaera sect. Sphaerotheca. Plant Pathol Quar 6:142–174
Monkhung S, To-anun C, Takamatsu S (2011) Molecular approach to clarify taxonomy of powdery mildew on Chilli plants caused by Oidiopsis sicula in Thailand. J Agric Technol 7:1801–1808
Monkhung S, To-anun C, Takamatsu S (2013) Molecular and morphological characterization of Phyllactinia cassiae-fistulae (Erysiphaceae; Ascomycota) from Thailand. Afr J Biotechnol 12:109–114
Shin HD (1988) Erysiphaceae of Korea. Thesis, Dept. of Agric. Biol., Graduate Sch. of Seoul National Univ
Shin HD (2000) Erysiphaceae of Korea. National Inst. of Agric. Sci. and Technol. Suwon, Korea
Shin HD, La YJ (1993) Morphology of edge lines of chained immature conidia on conidiophores in powdery mildew fungi and their taxonomic significance. Mycotaxon 66:445–451
Siahaan SAS, Hidayat I, Kramadibrata K, Meeboon J, Takamatsu S (2015) Phyllactinia poinsettiae sp. nov.: a new species of powdery mildew on poinsettia from Indonesia. Mycoscience 56:580–583
Siahaan SAS, Kramadibrata K, Hidayat I, Meeboon J, Takamatsu S (2016a) Erysiphe baliensis and E. sidae, two new species of anamorphic Erysiphe (powdery mildew) from Indonesia. Mycoscience 57:35–41
Siahaan SAS, Kramadibrata K, Hidayat I, Meeboon J, Takamatsu S (2016b) Bauhinia purpurea, Durio zibethinus, and Nephelium lappaceum: additional hosts of the asexual morph of Erysiphe quercicola. Mycoscience 57:375–383
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer, Sunderland, MA.
Takamatsu S, Kano Y (2001) PCR primers useful for nucleotide sequences of rDNA of the powdery mildew fungi. Mycoscience 42:135–139
Takamatsu S, Ito H, Shiroya Y, Kiss L, Heluta V (2015) First comprehensive phylogenetic analysis of the genus Erysiphe (Erysiphales, Erysiphaceae) I. The Microsphaera lineage. Mycologia 107:475–489
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729
To–anun C, Limkaisang S, Fangfuk W, Sato Y, Braun U, Takamatsu S (2003) A new species of Brasiliomyces (Erysiphaceae) on Dalbergia cultrata var. cultrata from Thailand. Mycoscience 44:447–451
To–anun C, Kom–un S, Sunawan A, Fangfuk W, Sato Y, Takamatsu S (2005) A new subgenus, Microidium, of Oidium (Erysiphaceae) on Phyllanthus spp. Mycoscience 46:1–8
Walsh SP, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR–based typing from forensic material. Biotechniques 10:506–513
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Acad. Press, San Diego, pp 315–322
Acknowledgements
This work was financially supported in part by a Grant-in-Aid for Scientific Research (No. 16 K07613 and 16 F16097) from the Japan Society for the Promotion of Science to ST; and The JSPS postdoctoral fellowship to JM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Meike Piepenbring
Rights and permissions
About this article
Cite this article
Meeboon, J., Takamatsu, S. Notes on powdery mildews (Erysiphales) in Thailand III. Erysiphe species on Fabaceae, Fagaceae, Hydrangeaceae and Lamiaceae. Trop. plant pathol. 42, 239–249 (2017). https://doi.org/10.1007/s40858-017-0137-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-017-0137-5