Abstract
In this research work, the pressure leaching of chalcopyrite concentrate with oxygen was studied. Experiments were carried out under conditions of the solid–liquid ratio of 0.1, agitation speed of 750 rpm, and sulfuric acid concentration of 2 M in a stainless-steel autoclave system under 0.6 to 1 MPa oxygen gas. Central composite design was used to design experiments, and three factors of temperature, time, and oxygen pressure were selected as the design variables. A linear model for %Cu and a quadratic model for %Fe were proposed by Minitab Software. The results showed that temperature is the most effective factor in chalcopyrite leaching. Under optimal conditions, 93.08% of copper with the aim of maximum dissolution was extracted. The kinetic study illustrated that the diffusion of the passive layer, produced during chalcopyrite leaching, controls the rate of the process. It can be represented by the \( 1 - \frac{2}{3}\alpha - (1 - \alpha )^{{\frac{2}{3}}} = k_{d} t \) equation. The activation energy was calculated to be 14.19 kJ mol−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper in the earth’s crust is most often found in the form of oxides, sulfides, carbonates, and native copper. But the most important sources of copper are copper sulfides, e.g., chalcopyrite (CuFeS2), bornite (Cu5FeS4), covellite (CuS), and chalcocite (Cu2S) [1]. One of the most abundant copper-bearing minerals is chalcopyrite (CuFeS2), which accounts for around 70% of the world’s copper reservoir. Chalcopyrite concentrate is produced in a mineral concentration plant using various methods such as flotation [2]. Although more than 80% of copper is produced by pyrometallurgical methods, environmental pollution has led many researchers to focus on hydrometallurgical routes [3]. Using the hydrometallurgical process for copper extraction is not only environmentally friendly but also suitable for low-grade sulfide ores [4]. Extensive research has been carried out in the field of hydrometallurgy for the dissolution of copper from chalcopyrite in various media, and researchers have proposed different leaching media. These media are classified, according to the type of lixiviant to sulfate, chloride, ammonia, and nitrate [5,6,7,8]. Many studies about using different oxidizing reagents such as ferric and cupric ions, hydrogen peroxide, bacteria, and oxygen have been carried out in sulfuric acid solution under the atmospheric or pressure leaching conditions[9,10,11,12,13]. According to several research works, the initial reaction rate decreases over time. Researchers argued that the formed passive layer on mineral surfaces prevents the progress of dissolution. There are three different hypotheses to explain the nature of this produced layer. Its ingredients include elemental sulfur, polysulfide, or iron compound that have been known by various researchers [9]. Hackl et al. [14] believed that the passive layer on chalcopyrite particles is a copper-rich polysulfide, formed in initial steps and controls the leaching process. Temperature and acidity are two influential factors in the formation of sulfate and sulfur so that increasing temperature and decreasing acidity are favorable conditions for sulfate formation. Pressure leaching not only provides favorable conditions for sulfate formation (high temperature) but also increases the leaching kinetics. Besides, this method has the potentiality to commercialize. So in recent years, the use of pressure leaching has been investigated for the treatment of sulfide minerals, and today, it is used for the treatment of zinc concentrate and gold ore [13]. Hence, its application to chalcopyrite concentrate is of interest for this research work.
Various chemical reactions have been proposed for leaching of chalcopyrite in sulfate solution with dissolved oxygen as the oxidant. Hiroyoshi [15] and Holliday [16] have suggested the following reactions for the leaching of chalcopyrite:
Yu et al. [17] studied the rate of chalcopyrite leaching in the sulfuric acid solution at the temperature range of 125–175 °C and the oxygen pressure range of 0.52 to 2.76 MPa.
They have noticed that slightly elemental sulfur is produced under these conditions and proposed the following reaction as the main reaction:
Vizsolyi et al. [18] dissolved chalcopyrite in a dilute sulfuric acid under conditions of 110° C and 3.4 MPa. They found that the elemental sulfur and hydrolyzed iron are produced during the leaching steps, and the major reaction is approximately as follows:
Pressure-oxidative leaching process, based on temperature, can be classified to low (< 100 °C), medium (140–180 °C), and high-temperature (> 200 °C)[19]. Depending on the purpose, chalcopyrite requires the effective parameters to be at their optimal level. Response surface methodology (RSM) is a set of mathematical and statistical techniques for constructing an empirical model in which several variables influence a response, and the aim is to optimize this response. One of the advantages of RSM is the smaller number of empirical tests to analyze multiple parameters and their interactions [20, 21]. This study consists of modeling and optimizing the effective factors of copper and iron dissolution as a response. Then, the kinetics of the leaching process and activation energy are determined. Table 1 shows the activation energy in different leaching mediums and temperature ranges.
2 Thermodynamics of Chalcopyrite Dissolution
Pourbaix diagrams are used to identify predominant species in equilibrium with respect to the oxidation potential and pH of the solution. However, Eh–pH diagrams show only the equilibrium and do not provide information about reaction kinetics [30]. Figure 1 shows the Pourbaix diagram of the Cu–Fe–S system which is plotted by the HSC software at the temperature of 140 °C. According to the diagrams, the dissolution of copper requires a pH of less than 3.1 and an oxidizing redox potential higher than 0.3 V, but to maintain iron ions in solution and prevent iron precipitation, low pH and the oxidizing redox potential above 0.8 V are required.
3 Materials and Procedure
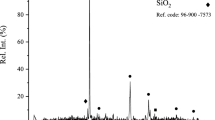
In this research, the chalcopyrite concentration from Sarcheshmeh copper reservoir in Iran was used as a feed for the pressure leaching experiments. The mineralogical composition of the chalcopyrite concentrate was determined by X-ray diffraction (XRD) analysis (Fig. 2). The elemental composition of chalcopyrite concentrate was determined by XRF, as shown in Table 2.
Merck’s sulfuric acid was used in leaching experiments. All solutions were made with distilled water. The grain size distribution of copper concentrate is presented in Fig. 3. The results show that the particle size of the chalcopyrite concentrate (d80) was 80% less than 90 µm. The experiments were carried out in a stainless-steel container in an autoclave equipped with a temperature controller, a variable speed stirrer, a barometer, and a heater. 25 g of concentrate sample was added to 250 ml of 2 M sulfuric acid solution in a stainless-steel container with a total volume of 500 ml to reach S/L = 0.1, and then, the vessel was put into the autoclave. The charged feed was heated under pressure of pure oxygen gas to the required temperature (100–180 C°). When the system reached the required temperature, stirring was started. The stirring rate was maintained at 750 rpm to ensure that the oxygen got dispersed and the reaction was not limited by mass transfer. After the tests, the autoclave was rapidly cooled with water, and the obtained solution was filtered. Concentrations of dissolved copper and iron in the solution were measured using atomic absorption spectrometry (AAS).
X-ray mapping by SEM–EDS for the before leaching sample in Fig. 4 shows how the elements were distributed in the chalcopyrite sample. The specific surface area of the chalcopyrite concentrate was determined to be 0.796 \( \frac{{\varvec{m}^{2} }}{\varvec{g}} \) by the BET method.
4 Design of Experiments
CCD is one of the RSM methods that is the most suitable design for fitting second-order polynomial equations [21]. In this design, the variables are studied at five levels (− α, − 1, 0, + 1, + α). The total number of experiments is as follows:
where N is the total number of experiments, k is the number of factors, and n is the number of replicates. Central composite design (CCD) was used to investigate the effect of significant factors (oxygen pressure, temperature, and time) and their interactions. These factors were studied at five different levels coded as − α, − 1, 0, + 1, and + α, in which the range of oxygen pressure, leaching temperature, and leaching time were from 0.6 to 1 MPa, 100 to 180 °C, and 100 to 240 min, respectively. The primary value and range of variables at levels of − 1, 0, and 1 were determined by trial and error, but the α levels were determined by the software. The number of α was 1.681. Dissolution of copper and iron was selected as the system response. The actual and coded values of the factors are summarized in Table 3. Minitab software was employed to design, analyse, and optimize the experiments.
The experimental results of the CCD model are obtained from Eq. 5 [31]:
where Y is the response variable; \( \beta_{0} \) is the intercept; \( \beta_{\text{i}} \), \( \beta_{{ij}}\;{\text{and }}\; \beta_{ii} \) are coefficients of the linear effect, double interactions; \( X_{i} ,X_{j} \) are the independent factors; and ɛ is error. Y represents the Cu or Fe dissolution.
5 Result and Discussion
These 20 tests based on CCD and the response of each test are shown in Table 4. The order of experiments has been arranged randomly.
6 Regression and Adequacy of the Model for Copper Dissolution
The results were analyzed by Minitab software. The proposed regression equation for the copper dissolution in uncoded units is as follows:
where X1, X2, and X3 are temperature, time, and pressure, respectively. The significance of each factor in the model was determined using F-value and p-value. If the F-value is higher than the critical value and the p-value is less than 0.05, it shows that the model is well-fitted to experimental data. The results of variance analysis are summarized in Table 4. The 95% confidence level (α = 0.05) was used to determine statistical significance. As shown in Table 5, the p-value less than 0.05 indicates that the model is significant, and there will be an acceptable performance in the prediction of response. The terms with p-values more than 0.05 are insignificant, and they can be omitted from the model. Hence, they were removed from the model. The obtained final equation for copper dissolution with significant terms is as follows:
The coefficient of regression (R2) for fitness of the model and adjusted R2 for confirmation of the model adequacy were used to evaluate the proposed model in terms of its proximity to the actual system. The obtained equation, in terms of actual factors, can predict the percentage of copper dissolution according to the given values of the specified range for each factor. Removing the nonsignificant terms of the model causes a change in the R2 values of the model. Table 5 shows their values before and after removing these terms. According to Table 6, although the R-square decreases slightly, the increase in pred-R2 makes the model more accurate than the previous one.
The normal probability plot shows the status of residuals relative to a straight line. If residuals follow this line, it indicates that they have a normal distribution. According to Fig. 5, the residuals follow a straight line illustrating the normal distribution of errors. The model’s P-value less than 0.05 and the Lack-of-Fit’s P-value more than 0.05 imply that the model is significant.
7 Regression and Adequacy of the Model for Iron Dissolution
The final equation obtained for iron dissolution by software with significant terms is as follows:
where \( X_{1} , \)\( X_{2} , \), and \( X_{3} \) are similar to the preceding equation. The summary of statistical analysis (Table 7) and adequacy of the model were justified by the analysis of variance (Table 8).
Based on Table 7, the R2 value of 0.9793 depicts the high fitness of the model. The high value of the adjusted R2 (0.9719) and the prediction R2 (0.9311) further prove the adequacy of the model. The normal probability of residuals is shown in Fig. 6, which represents the normal and independent distribution of errors.
8 Response Surface Analysis
Minitab software was used to develop 3D response surface plot. The 3D plots are graphical representation of the influence of parameters on the response surface and one of the useful ways to reveal optimal response conditions. The effect of variables on the response (%Cu and Fe) is shown in Fig. 7. In these plots, the effect of two factors on the response surface is presented, while the other one is fixed at the center point level. Figure 7a, b shows the interaction effect of temperature–time and temperature–oxygen pressure on the %Cu, indicating that temperature is the most important factor in the copper dissolution. Increasing time and pressure also have a positive effect on the response. The interaction between time and pressure is shown in Fig. 7c, indicating that the influence of these two variables on the response surface is alike. The interaction between temperature–time and temperature–pressure on iron dissolution are shown in Fig. 7c, e, respectively. Unlike copper, increasing temperature during the short leaching time first increases %Fe and then decreases. Figure 7f illustrates the effect of the interaction between pressure and time on iron dissolution. By comparing Fig. 7a, d, in a short time and high temperatures, copper dissolution is more than iron. Also, Fig. 7b, e represents the same effect of oxygen pressure on copper and iron extraction. Minitab software was used to determine optimum conditions. The optimum conditions are defined with the aim of maximum copper dissolution. Optimal conditions were determined at t = 287.7 min, P = 1.13 MPa, and T = 207 °C. Under these conditions, 93.08% of copper was extracted. The optimal conditions with the predicted and experimental percentages of copper and iron dissolution are shown in Table 9.
To recover remaining copper of leached chalcopyrite, methods exerted for low-grade concentrates such as heap leaching or heap bioleaching [32, 33] can be used. Iron is considered an impurity in PLS solution, but it can be removed from PLS by precipitation and SX process.
First, it may seem that the high agitation rate causes the passive layer to be separated from the surface of the spherical particles. But since the temperature of experiments is higher than the melting point of elemental sulfur (T > 115 °C), they cause this element to be melted on the surface of particles. Hence, the diffusion process will be hindered from this melted layer. Figure 8 shows X-ray mapping of leached residue under conditions of T = 180 °C, t = 4 h, and P = 1 MPa (Test # 2). As shown in Fig. 9, it can be seen that the elemental sulfur is formed and it adheres to the chalcopyrite particles. Although the temperature and pressure are at high levels, the formation of elemental sulfur prevents the dissolution process. The leaching residue, which was obtained under the above conditions, was characterized by XRD (Fig. 10). According to the XRD spectrum, α-sulfur is produced during chalcopyrite leaching. The BET surface area of the reacted sample is determined to be 0.55 \( \frac{{\varvec{m}^{2} }}{\varvec{g}} \). Based on the obtained results, the primary leaching equation is accomplished according to Eq (1).
9 Kinetics Study of the Process
Several research works have been carried out on the kinetics and mechanism of chalcopyrite leaching in different media with various oxidants. In the present work, the kinetics and mechanism of pressure leaching of chalcopyrite were investigated. A shrinking core model (SCM) was used to determine the leaching process kinetics. In this model, it is assumed that the reaction first begins from the external shell of the spherical solid particle, and then, the reaction zone (ash) moves toward the inner surface of the solid core. According to the model, it can be considered that the leaching process consists of three major steps. The slowest step controls the process kinetics: (1) mass transfer from the fluid layer around the solid core, (2) chemical reaction on the solid and liquid interface, and (3) diffusion through the ash layer. The equations for these steps are:
where kf, kr, and kd are kinetic constants, α is the fraction reacted, and t is the reaction time. To determine the mechanism of the process, the effect of temperature and oxygen pressure variables on the leaching process was investigated. Figure 11a, b shows the effect of temperature and pressure on copper dissolution, respectively. During the study of temperature effect, the pressure was maintained at 0.8 MPa (center point level), and during the study of pressure effect, the temperature was maintained at 140 °C.
After the tests, the correlation of different kinetics models was examined (the three above mentioned models) with the obtained data. The kinetic equations with their linear correlation coefficients and kinetic constant are presented in Table 10.
As can be seen in Table 9, model 3 is in close agreement with the data. It can be due to the formation of the elemental sulfur layer produced by reaction 1. Arrhenius equation shows the kinetic dependence of the dissolution process on temperature [34] that is shown as follows:
where k is the kinetic constant, A0, Ea, R, and T are pre-exponential factor, activation energy, the universal gas constant, and absolute temperature, respectively. By drawing the \( \ln k \) versus \( \frac{1000}{T}, \) the obtained line slope shows \( \frac{{ - E_{\text{a}} }}{R} \). It is represented in Fig. 12.
The activation energy obtained is 14.19 kJ.mol, which is less than 40 kJ/mol. It indicates that the diffusion mechanism from the produced layer controls the process. The formation of elemental sulfur illustrates that the main leaching equation is accomplished as shown in Fig. 13a. In addition, the presence of oxygen in the acidic solution causes conversion of Fe2+ to Fe3+ (Fig. 13b). It can contribute in the leaching process matching to Fig. 13c.
10 Conclusion
In this research, the pressure leaching of chalcopyrite concentrate was investigated in sulfuric acid solution. The experiments were designed by CCD to optimize the variables with the goal of maximum copper dissolution. The results are as follows.
-
1.
Temperature is the most important factor in the dissolution of copper, and increasing oxygen pressure has a positive effect of chalcopyrite leaching
-
2.
Under the optimal conditions, the maximum copper dissolution reaches 93.08%.
-
3.
Unexpectedly, according to FESEM-BSE image, the elemental sulfur is formed at high temperature (T = 180 °C) and hinder the leaching process which can be due to the high percentage of sulfur in the chalcopyrite sample.
-
4.
A linear model for %Cu and a quadratic model for %Fe have been proposed by Minitab software. Both models are well-fitted to experimental data that can be confirmed by the high R2, adj-R2, and pred-R2 values.
-
5.
The kinetics studies of the process shows that the diffusion of the produced layer control the dissolution process and the activation energy obtained is 14.19 kJ/mol.
References
Davenport W G, King M J, Schlesinger M E, and Biswas A K, Extractive Metallurgy of Copper, (4th ed), Pergamon (2002).
Baba A A, Ayinla K I, Adekola F A, Ghosh M K, Ayanda O S, Bale R B, Sheik A R, and Pradhan S R, Int J Min Eng Miner Process, 1 (2012) 1.
Li Y, Kawashima N, Li J, Chandra A, and Gerson A A, Adv Colloid Interface Sci, 197 (2013) 1.
Pradhan N, Nathsarma K C, Srinivasa Rao K, Sukla L B, Mishra B K, Miner Eng, 21 (2008) 355.
Koleini S M J, Aghazadeh V, and Sandström A, Miner Eng, 24 (2011) 381.
Chiluiza E L R, and Donoso P N, J Mex Chem Soc60 (2016) 238.
Guan Y C, and Han K N, Metall Mater Trans B28 (1997) 979.
Hernández P C, Taboada M E, Herreros O O, Graber T A, and Ghorbani Y, Minerals8 (2018) 238.
Hiroyoshi N, and Kuroiwa S, Hydrometallurgy74 (2004) 103.
Dutrizac J E, Can Metall Q28 (1989) 337.
Pinches A, Al-Jaid F O, Williams D J A, Hydrometallurgy2 (1976) 87.
Turan M D, and Altundoğan H S, Metall Mater Trans B44 (2013) 809.
McDonald R G, and Muir D M, Hydrometallurgy86 (2007) 191.
Hackl R P, Dreisinger D B, Peters E, King J A, HydrometaIlurgy39 (1995) 25.
Hiroyoshi N, Arai M, Miki H, Tsunekawa M, Hirajima T, Hydrometallurgy63 (2002) 257.
Holliday R I, and Richmond W R, J Electroanal Chem Interfacial Electrochem288 (1990) 83.
Yu P H, Hansen C K, and Wadsworth M E, Metall Trans4 (1973), 2137.
Vizsolyi H V, Warren I H, and Mackiw V N, Metals19 (1967) 52.
Han B, Altansukh B, Haga K, Takasaki Y, and Shibayama A, J Sustain Metall3 (2017) 528.
Montgomery D C, Design and Analysis of Experiments Response Surface Method and Designs, Wiley, New Jersey (2005).
Bezerra M A, Santelli R E, Oliveira E P, Villar L S, and Escaleira L A, Talanta76 (2008) 965.
Peters E, and Loewen F, Metall Trans4 (1973) 5.
Gok O, Anderson C G, Cicekli G, and Cocen E L, Physicochem Prob Min Process50 (2014).
Qiu T S, Nie G H, Wang J F, and Cui L F, Trans Nonferr Metals Soc China17 (2007) 418.
Padilla R, Pavez P, and Ruiz M C, Hydrometallurgy91 (2008) 113.
Cháidez J, Parga J, Valenzuela J, Carrillo R, and Almaguer I, Metals9 (2019) 189.
Adebayo AO, Ipinmoroti KO, Ajayi OO, Chem Biochem Eng Q17 (2003) 213.
Antonijević M M, Janković Z D, and Dimitrijević M D, Hydrometallurgy71 (2004) 329.
Aydogan S, Ucar G, and Canbazoglu M, Hydrometallurgy81 (2006) 45.
Garverick L, (ed) Corrosion in the petrochemical industry, ASM international (1994) p 77.
Turan M D, Arslanoğlu H, and Altundoğan H S, J Taiwan Inst Chem Eng (2015).
McBride D, Gebhardt J, Croft N, and Cross M, Minerals8 (2018) 9.
Panda S, Sanjay K, Sukla L B, Pradhan N, Subbaiah T, Mishra B K, Prasad M S R, and Ray S K, Hydrometallurgy125 (2012) 157.
Han B, Altansukh B, Haga K, Takasaki Y, and Shibayama A, Leaching and Kinetic Study on Pressure Oxidation of Chalcopyrite in H2SO4Solution and the Effect of Pyrite on Chalcopyrite Leaching, The Minerals, Metals & Materials Society (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mojtahedi, B., Rasouli, S. & Yoozbashizadeh, H. Pressure Leaching of Chalcopyrite Concentrate with Oxygen and Kinetic Study on the Process in Sulfuric Acid Solution. Trans Indian Inst Met 73, 975–987 (2020). https://doi.org/10.1007/s12666-020-01882-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01882-3