Abstract
Purpose of Review
The increasing use of engineered nanoparticles (ENPs) has led to growing concerns about their environmental impacts. It has become a focus for researchers to explore their detection, quantification, as well as their fate and transport, and hence their ecotoxicity. A review of recent findings sets a basis for current knowledge of ENP levels in the surface water environment and provides a perspective to understand their toxicity.
Recent Findings
Among the various mechanisms of toxicity that have been evidenced by recent research, an important mechanism that is shared by multiple ENPs is the generation of reactive oxygen species (ROS) and subsequent oxidative stress. Another common toxic effect is cell membrane damage from physical encounters or ENPs adsorbing onto the membrane. The ecotoxicity of ENPs is dependent on many factors; however, the ENP’s physiochemical characteristics and functional behavior are two main groups. Additionally, the chemical composition and charge of ENPs are greatly influencing their toxicity.
Summary
A critical overview of updated knowledge on the regulatory standards, environmental detection, and aquatic fate and ecotoxicity of ENPs with a special focus on the most environmentally affluent nanosized titanium dioxide (n-TiO2), cerium dioxide (n-CeO2), zinc oxide (n-ZnO), silicon dioxide (SiO2), silver (n-Ag), and carbon nanotubes (CNTs) is presented in this article. Among the ENPs reviewed, n-Ag and n-TiO2 are more studied and the most ecotoxic; n-Ag dissociates into cations (Ag +) causing significant harm to organisms and cells, while light and pH notably influence the toxicity of n-TiO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology provides methods for restructuring matter at the nanoscale (~1 to 100 nm), the purpose of which is the development of nanomaterials with fundamentally new properties and functions due to their small structure. Applications of engineered nanoparticles (ENPs) have rapidly expanded. For example, metallic ENPs’ global market value is expected to reach 30 billion USD in the nearest future [1, 2], while the annual production of titanium dioxide (n-TiO2), one of the most common ENPs, is expected to reach 2.5 million tons by 2025 [3]. The increasing production and use of ENPs in consumer products, evident from the increasing number of patents and scientific publications, are significantly correlated with the surge of ENPs found in the environment [4]. The escalated concentration of ENPs in the environment poses a concerning threat to the health of surface water ecosystems and, potentially, the treatment and quality of water and wastewater facilities. Relatively little is yet known about the amenability of ENPs to conventional treatment operations such as coagulation and filtration. The degree and kinetics of ENPs treatment fate in drinking water and wastewater systems are likely a function of the surface characteristics of carbonaceous adsorbents, metal coagulants, and/or filtration media. ENPs’ behavior in the environment is an emerging topic and has increasingly become the subject of studies over the past decade, trying to understand their unique physical, chemical, and biological properties in the environment [5, 6•]. The extremely small dimensions of ENPs allow them to penetrate and accumulate in both aquatic and terrestrial organisms, animals, and human beings [6•]. However, it is a difficult task to assign certain toxicity to ENPs in natural environments as their behavior in the environment is not fully explored.
The scientific community and regulatory agencies are constantly finding ways to characterize the hazard, exposure, and risk factors associated with ENPs. Although the importance and potential risk of ENPs have been acknowledged, relevant regulations for the control of ENPs in the environment are lacking due to the absence of a comprehensive and standardized risk analysis of ENPs [7]. The U.S. Environmental Protection Agency (EPA) requires product developers to review their nanomaterials before the production of any such materials for commercial use. One of the most common ways of conducting a chemical review of new material is by using environmental fate simulation models. However, environmental fate models developed for traditional contaminants, like pesticides or hydrophobic organic chemicals, are limited in their ability to simulate the environmental behavior of ENPs due to incomplete understanding and quantification of the processes governing ENPs distribution in the environment [8]. Acknowledging the lack of environmental fate models for ENPs, the material and environmental communities are embracing the development of new models or revising and testing existing model frames for simulating ENPs fate in the environment [9, 10]. Equally challenging is the lack of a standard technique and procedure to detect, quantify, and report the ENPs in the environment [5]. There is an immediate need to standardize procedures for detecting, characterizing, and categorizing ENPs’ ecotoxicity so that they can be appropriately regulated and treated.
While many ENPs are being developed and used, according to the World Health Organization, titanium dioxide (TiO2), cerium dioxide (CeO2), zinc oxide (ZnO), carbon nanotubes (CNTs) and silver (Ag) ENPs are among the top nine ENPs, by volume, commercially used in the market [11]. They are also among the nanomaterials that the EPA is studying to understand their implications on human and environmental health [12]. From the various studies on ENPs’ fate and toxicity, a basic understanding of how these NPs react with one another and other molecules in the environment and how they affect organisms is being formed. Several main toxicity mechanisms have been evidenced, and particular environmental factors are influential on the fate and toxicity of certain ENPs [13••, 14]. However, there exists a vital knowledge gap to build a complete idea of how ENPs act in the environment and interact with other chemicals and organisms. Analytical techniques and reporting of the relevant data related to experiments to fill in this gap should be standardized for better mutual understanding across the spectrum of research space globally.
Literature has reported multiple review papers with a primary focus on the findings on the environmental fate and transport of ENPs and their treatability mechanisms in the water environment [15–18]. To our knowledge, there is no significant publication that consolidated field-detected ENPs in multiple natural water environments, while such information is essential as ENPs concentration is expected to influence their toxicity. To fill in the gap, for the first time this review provides insight into literature-reported field-detected ENPs concentrations. Also, unlike previous literature which described the potential toxicity of ENPs, this review uniquely presents a critical comprehensive discussion on how environmental factors influence the toxicity of ENPs in humans and terrestrial and aquatic species. This review provides an in-depth understanding of current knowledge of the aquatic environmental occurrence, existing regulations, and toxicity of the ENPs, and hence future research thrusts to advance the knowledge for enhanced environmental management of the ENPs.
Environmental Detection and Regulatory Overview of ENPs
As is true in all other environments, the concentration of ENPs in aquatic environments is increasing. The ENPs’ concentration in freshwater is predicted to reach sixfold higher by the year 2050 [19•]. According to the Emerging Nanotechnologies Programs’ report, there are more than 1,600 manufacturer nanotechnology commercial products on the market [20]. As more products emerge and nanotechnology grows, it is expected that these ENPs will consequently end up in wastewater and natural water bodies. The main sources of ENPs entering aquatic ecosystems include: nanoparticle-containing products and the use of marine nanobiotechnologies to synthesize ENPs [21, 22], municipal and industrial wastewaters, and household processes, and the use of ENPs to purify wastewaters from pollutants [23], e.g., n-Ag is used to remediate wastewater because of its antibacterial properties and enhanced adsorption capacities [24]. It is generally understood that ENPs need to be measured in environmental conditions to obtain actual concentrations and therefore one can research their environmental risk at the environmental concentration. However, this is a difficult task primarily due to the lack of easy and affordable technology with low detection limits sensitive to expected trace concentrations of ENPs in the environment. Another major challenge is their inability to distinguish ENPs from other chemical forms (e.g., ions, dissolved) or from natural colloids. While it may not be comprehensive, Table 1 provides a summary of the real-field sample concentrations of ENPs in different water environments such that the information should provide a basis for setting realistic fate and transport and toxicity testing protocols for future research. As shown in Table 1, there are different NPs analyzed and they have wide and varying ranges of concentrations in different aquatic environments, i.e., rivers, rain waters, and wastewaters. Among the tested NPs, n-Ag is the most common across all aquatic environments with its concentration ranging from below the detection limit in Des Prairies river waters to 20.02 mg/L in Malaysian wastewaters. Not all reported concentrations are reported in weight-volume ratio, while some studies reported concentration as the number of particles per volume making direct comparison of detected ENPs concentration impossible. Also to note, not all ENPs have the same analytical technique for their detection and quantification.
There is significant published literature that discussed analytical techniques for ENPs in aquatic environments, with the predominant focus being methodological aspects for ENPs detection and fate characterization, often using controlled lab samples [15,16,17,18]. However, to our knowledge, no review literature identifies analytical techniques used in real-field sample analysis for ENPs’ detection and quantification. Traditional analytical and characterization methods of ENPs in the environment include dynamic light scattering, electron microscope, nanotracking analysis [25, 26], energy-dispersive X-ray spectroscopy [27], ultraviolet–visible spectroscopy [28] and X-ray absorption near-edge spectroscopy [29]. However, these techniques have high detection limits, so most are inadequately sensitive for detecting the actual environmental trace concentrations of ENPs [25, 26]. Single-particle inductively coupled plasma mass spectroscopy (SP-ICP-MS) is emerging as a promising analytical methodology to characterize and quantify size distribution, mass concentration, and particle concentration of metallic NPs in aquatic environments [30,31,32]. Cloud point extraction (CPE) is another emerging approach that separates metallic ENPs in an environmental sample as they co-exist with their bulk counterpart [33]. Notably, CPE is also ecofriendly and cost-effective with low toxicity and low solvent consumption [34]. Additionally, material flow analysis models are a type of ENP exposure models that are currently used to derive predicted environmental concentrations [35].
As indicated in Table 1, other analytical techniques used for detecting ENPs include inductively coupled plasma atomic emission spectroscopy (ICP-AES), electrothermal atomic absorption spectroscopy (ET-AAS), high resolution inductively coupled plasma mass spectroscopy (HR-ICP-MS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), hydrodynamic chromatography inductively coupled plasma mass spectroscopy (HDC-ICP-MS), hydrodynamic chromatography single-particle inductively coupled plasma mass spectroscopy (HDC-SP-ICP-MS), and inductively coupled plasma-optical emission spectrometry (ICP-OES). Despite how promising these new technologies seem, they are in their infancy, relatively, and often require expensive and complex instruments. One thing is certain, knowing the actual concentrations of ENPs in the environment is tantamount to understanding the risks and hazards of ENPs.
The presence of ENPs in water is an emerging concern for federal and state agencies. There are no clear regulatory standards that cover the spectrum of ENPs from their manufacturing to use to disposal creating uncertainty for not only nanopollution management but also future fundings, and research to develop new nanomaterial and relevant technologies [44]. The primary reason for that is due to the uncertainty of emerging nanomaterial properties and technologies that employ nanomaterials. However, progress has been made to develop scientific data to confirm the risk from emerging nanomaterial and products containing nanomaterial but such data are yet to be conclusive. Regulatory agencies are also having to catch up to update their standards to the current knowledge of scientific data. Countries and international organizations such as the Global Coalition for Regulatory Science Research, established in 2013 and comprising of regulatory bodies from ten countries including the United States of America and the European Union (EU), are trying to design guidelines and standards for toxicity evaluation and regulation plans; some of these standards being implemented are non-binding [7, 44, 45]. Here we discuss prevalent regulatory guidelines on ENPs that are not necessarily applicable to surface water quality standards.
The U.S. Food and Drug Administration (FDA) acknowledges that attributes of nanomaterial-enabled products may differ from the conventional products without nanomaterial, and thus recommends evaluations of the safety of FDA-regulated products that include nanomaterials or nanotechnology and should consider the unique properties and behaviors that nanomaterials may exhibit [46]. The FDA considered regulating nanoproducts under existing statutory authorities, which have established guidelines targeting the safety, effectiveness, and quality of the FDA-approved products containing ENP, following the specific legal standards applicable to each type of product under its jurisdiction but not based on nanomaterial themselves [46]. Most of the ENPs are labeled as “chemical substances" and categorized under the Toxic Substances Control Act (TSCA), subsequently subject to the requirements of the Act. Among these, CNTs are already required to be reported under Sect. 5 of TSCA. EPA also issued a one-time reporting rule, under TSCA Sect. 8(a), for the ENPs that are existing compounds. In 2016, to ensure the safety of the manufacturing and consumption of ENPs, EPA initiated an inclusive regulatory approach under TSCA including an information-gathering rule on new and existing nanomaterials and premanufacture notifications for new nanomaterials. While there exist no maximum contaminant level goals or maximum contaminant levels, ENPs could also be regulated under the Safe Drinking Water Act if they are exposed to drinking water or injected into a well. The ENPs could also be regulated under various other regulatory mechanisms including but not limited to the Comprehensive Environmental Response, Compensation Liability Act also known as the Superfund, Resource Conservation and Recovery Act, and Clean Water Act.
Furthermore, in 2007 the Europe Union established new legislation for managing chemicals called REACH (Regulation, Evaluation, Authorization, and Restriction of Chemicals) as well as the European Chemicals Agency (ECHA). The ECHA has been working to ensure consistency throughout the EU countries concerning chemicals and safety. ECHA offers a database where companies can report and store information on the properties of chemicals, as well as a website interface, where the general public can find information on the types of chemicals being produced in the EU. As of January 2020, “explicit legal requirements” under the REACH legislation apply to companies that manufacture and import nanoforms, and they are as follows: characterization of nanoforms, chemical safety assessment, registration information requirements, and downstream user obligations [47].
Environmental Transformation and Toxicity
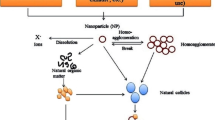
Due to the expected toxic nature of ENPs, their release into surface waters merits concern about how to control them in these aquatic environments. Control of ENPs in surface water systems is expected to be affected by their environmental transformational processes such as chemical and photochemical processes, physical and biological processes, and interactions with other surfaces and substances. The ENPs’ stability in a water environment is expected to be affected by several parameters such as ionic strength, water chemistry, water temperature, flow velocity, and the presence of natural organic matter (NOM), as well as particle properties such as surface charge, surface area, surface chemistry, and particle size [5, 48]. When compared to their bulk chemicals, ENPs show different physicochemical properties that, on the one hand, make them attractive for different applications but, on the other hand, equally make them much more complex to assess in terms of their fate and transport in environmental media and their treatment [49, 50]. Also, the ecological behavior of ENPs often differs from their bulk counterparts and the understanding of ENPs’ ability to bind and interact with biological systems is complex [13••]. Additionally, increasing evidence has suggested that ENPs rarely exist in a pristine condition, i.e., not exactly how they are manufactured; instead, they undergo many transformations in the environment [51,52,53]. Due to their nanoscale dimensions and a large surface area to volume ratio with a high adsorption capacity, ENPs are highly reactive with other chemicals such as heavy metals and NOM in the environment [54, 55].
ENPs’ ecotoxicity may be affected by their environmental transformation which also depends on water chemistry [56••]. For example, NOM is composed of hydrophobic and hydrophilic components and bears net negative charges due to the deprotonation of carboxyl groups [57]; therefore, NOM could theoretically mitigate the toxic effects of ENPs. As evidence has shown that positively charged ENPs and ions seem to cross cell membranes easier than negatively charged and neutral ENPs, and subsequently bind to negatively charged DNA causing damage [13••]. NOM-coating on ENPs should be a topic of study to better understand the toxicity of ENPs, because recent experiments have demonstrated equal importance of the ENPs’ core material and innate surface properties in the interactions of ENPs with the human cell [40, 58]. The chemical composition of ENPs is one of the most important factors when considering their potential risk and toxicity; it determines their dissolution, redox capability, ionization properties, and affinity to other molecules [59]. Moreover, the exact environmental concentrations of ENPs are very difficult to assess because most analytical techniques cannot detect such small particles. As stated previously, while it seems the main driving force for potential genotoxicity and propensity to trigger negative ecological toxic effects is the composition of the ENP, their reactivity (zeta-potential) and particle size are also other very important influencing factors [13••]. However, there are many parameters that influence the environmental fate and hence toxicity of ENPs, such as pH, light, the temperature of media, and exposure duration.
Human Health Toxicity
The bioaccumulative properties of ENPs result in their transfer through the food web, where they eventually make their way to the top of the food chain, into humans [60, 61]. The presence of ENPs in terrestrial and aquatic environments provides the opportunity for their entry into humans. The primary targets that get exposed to NPs in the natural ecosystem are microorganisms, including bacteria [62]; but these microorganisms are simply the first rung on the ladder that ENPs climb to move up the food chain eventually to humans. Nanoparticles can also be exposed to humans mostly by pulmonary inhalation, oral consumption, and skin contact [63,64,65,66], ENPs can even enter the human body intentionally, e.g., when ENPs are used for drug delivery purposes and administered orally, applied onto the skin, or injected. ENPs are capable of crossing the cell membrane and impacting DNA. The small size of ENPs allows them to penetrate through epithelial and endothelial barriers into the lymph and blood to be carried by the bloodstream and lymph stream to different organs and tissues in the body, such as the brain, heart, kidneys, liver, bone marrow, nervous system, and possibly the fetus in pregnant women [67, 68]. The ENPs can then be transported into cells by transcytosis mechanisms or simply diffuse across the cell membrane. Research on the toxic effects of ENPs on the body has shown that ENPs cause thrombosis by enhancing platelet aggregation [69], inflammation of the upper and lower respiratory tracts, neurodegenerative disorders, strokes, myocardial infraction, and other disorders [70,71,72]. Not only do ENPs enter organs and tissues, but they can also enter cell organelles, e.g., mitochondria and nuclei, where they can alter cell metabolism and cause DNA damage, and even cell death [73]. The genotoxicity mechanism mainly consists of two main categories—direct and indirect interactions of ENPs and DNA. In the case of direct interaction, ENPs interact with the chromosome during its interphase/mitotic phase and eventually bind with the DNA and thereby inhibits the process of replication or transcription resulting in chromosomal loss and/or chromosomal breakage. During the indirect interaction, the intermediates of NPs (from different environmental processes) induce genotoxicity by reactive oxygen species (ROS) generation and the release of toxic elements which interfere with the proteins that are essential for DNA replication, transcription, or repair [74, 75].
Terrestrial Ecotoxicity
Studies indicate that the concentration of nanoparticles is higher in soil compared to water or air [76, 77]. ENPs enter the soil and accumulate due to their weak migratory abilities in the soil media [13••]. ENPs have the capacity for beneficial effects on soils; one such benefit is ENPs have the potential to enhance nutrient storage in soils. One particular study by Zhao et al. (2021) investigated the effects of carbon nanoparticles (CNPs) on plant growth and nutrient use efficiency of corn (Zea mays). They found that CNPs affected corn growth in a dose-dependent manner, and at the optimal rate significantly enhanced plant height, biomass yield, nutrient uptake, and nutrient use efficiency. Interactions between ENPs and plants are of particular importance because plants, while they are primary producers and are responsible for converting solar energy into the organic matter [78], also serve as the potential pathways for the transport of ENPs through the food chain into the trophic-level consumers and represent the base of the food chain for many animals, including humans [79, 80]. Metal oxide NPs have been found to induce mainly the following toxic mechanisms in plants: production of ROS, which causes lipid peroxidation, DNA strand destruction, chlorophyll and carotenoid content reduction, photosynthetic rate reduction, plant biomass and soluble protein content reduction, and plant growth inhibition [81].
Aquatic Ecotoxicity
Marine ecosystems play a crucial role in maintaining biological diversity by contributing to the regulation of global heat distribution and providing large amounts of food up the trophic chain [82]. Therefore, the protection of aquatic environments from pollution and the toxicological evaluation of their state are among the most important fields of study [83]. ENPs in the water environment can easily find their way into organisms like phytoplankton or zooplankton through contact or intake, accumulate in benthic sediments and be swallowed by benthic organisms, and then further transfer through the food chain up to larger vertebrates [84]. Alternatively, ENPs can enter a body through epithelial barriers, such as gills, olfactory organs, and body walls, or simply with their food [85, 86]. As mentioned earlier, when ENPs get into an aquatic environment, they transform from interactions with other pollutants, acquire coatings of NOM, and adsorb a protein corona. These transformations alter their composition, possibly giving them a new biological identity and subsequently influencing their fate and toxicity [48]. In a review performed by Arvidsson et al. [87•], 80% (53 studies) of studies concluded that NOM reduced toxic effects, 15% of the studies determined no influence or unclear influence, while only 5% of studies reported that toxicity was increased, notably only for n-Ag. Thus, there is strong evidence that NOM reduces the toxic effect of ENPs in most cases. A possible explanation for this reduction is that the adsorbed NOM creates a “soft cushion” which physically hinders the ENPs from binding or getting into contact with organisms [88–90]. Other proposed explanations are that the NOM captures and pacifies ROS which might have otherwise caused damage to cellular pathways [91–93] or that the NOM forms complexes with dissolved ions released from ENPs, which would mitigate the harm caused by such ions [89, 92, 94,95,96,97].
In addition to the relationship between ENP properties and NOM assemblage or protein corona that determines how ENPs behave, many parameters of an aquatic environment influence the ecotoxicity of ENPs; pH and light are two factors that are important and pertinent when considering the fate and toxicity of ENPs [98–101]. Organisms such as microalgae and cyanobacteria can be used as helpful toxicological research species because they are important primary producers and are of basic significance for ecological balance, while their sensitivity to pollutants directly causes the general deterioration of many ecosystems [102]. Knowing how certain ENPs affect these species will ultimately help us understand the ecotoxicity of these ENPs and how certain ENPs will affect ecosystems. Table 2 synopsizes comprehensive literature on how ENPs affect certain species and ENPs’ toxicity toward organisms. The synopsis information presented tracks intrinsic properties of the ENP, exposure time and concentration, and environmental media properties. There is significantly more literature on TiO2 studies than any other ENP, probably in part because TiO2 is one of the most commonly used ENPs along with n-Ag, with its usage expected to reach 2.5 million tons by 2025 [3]. Lacking reported experimental parameters, e.g., temperature, pH, light conditions, exposure duration, etc., for most of the ENPs toxicity research reported limits the ability to synthesize and combine the research findings with other similar studies for drawing additional outcomes. If there was a standard for these toxicity studies and they were required to report detailed environmental factors, one could begin to identify how ENPs act in the environment and affect cells, organisms, etc. with higher confidence.
Table 2 also summarizes key findings of the toxicity research, especially the impact of environmental parameters on the ENPs’ toxicity. Among the reported literature, there were opposing findings on how salinity affects the toxicity of n-Ag. Johari et al. (2018) reported increasing salinity to increase microalgal growth inhibition, while Salari et al. (2012) reported the median lethal concentration (LC50) in brackish water to be significantly lower than that of freshwater, indicating that the toxicity of n-Ag is greatly influenced by other factors as well. Multiple studies showed that light increased the toxicity of n-TiO2, an expected result since TiO2 is photocatalytic. Another consistent factor that was found to affect the toxicity of n-TiO2 was pH, and toxicity mechanisms, such as ROS generation and the accumulation of n-TiO2, were seen to increase at lower pH values. The photochemical efficiency of photosynthesis and chlorophyll were shown to be significantly affected by n-TiO2 and seem to be common process and component that are affected by n-TiO2. However, chlorophyll content and antioxidant enzymatic activity did increase with co-exposure of n-TiO2 and phosphorus, emphasizing that interactions between ENPs and co-pollutants existing in the environment adding another level of complexity to ENPs’ ecotoxicity and demand further research. The bioaccumulation and toxicity of n-ZnO seem to be dose and exposure dependent; the higher the dose or longer the exposure, the more toxic the effects observed. From the literature, n-SiO2 is evident to cause low toxicity, and the severity of toxicity appears to be dependent on particle size. Smaller and single-walled carbon nanotubes (SWCNTs) are shown to be more toxic than larger or multi-walled carbon nanotubes (MWCNTs), while CNT conjugates seem to be the least toxic. Similar summarization of observations for n-CeO2 is not possible since there are only two studies and no trending results reported in the literature for the ENP.
Predominant mechanisms of toxicity in the selected ENPs are illustrated in Fig. 1. The generation of ROS and oxidative stress is a toxic mechanism of great significance for all selected ENPs, while cell membrane damage is not noted for n-TiO2. Although DNA damage was also noted for CeO2, it is predominantly recoded as a toxic mechanism in n-Ag, n-SiO2, and CNTs. An inflammatory response is reported as a common toxic mechanism of n-SiO2 and CNTs. While the reported literature provides a basic insight into the selected ENPs toxicity, the most important qualifying condition for the toxicity testing for the actual situation was not met by these studies. For example, n-TiO2 was studied predominantly in the milligram range concentration (Table 2), while its field-detected concentrations are in μg/L range (Table 1).
Toxicity of Individual Types of ENPs
n-TiO2
As it has been stated previously, n-TiO2 is among the most commonly studied nanomaterials. Because of their high refractive index, brightness, and resistance to discoloration, n-TiO2 is broadly used as pigments. Approximately 70% of the total TiO2 production is used as pigments in paints, varnishes, enamels, plastics, paper, fibers, and foods. But a substantial portion of n-TiO2 is used in cosmetics and pharmaceutical products, such as sunscreens, toothpastes, shampoos, deodorants, shaving creams, and as food additives [124]. Additionally, n-TiO2 is used as an antimicrobial agent and a catalyst in air and water purification [102].
While it has been observed that different ENPs exhibit different toxic properties and different mechanisms, various studies show that n-TiO2 causes ROS generation and/or accumulation. Jugan et al. [125] found that n-TiO2 caused an early accumulation of ROS in human cells via photocatalysis which resulted in alteration of mitochondrial membrane potential. Moreover, light—as one might expect because of the photocatalytic properties of TiO2—and pH are evidenced to affect the toxicity of n-TiO2. In a study of Mytilus galloprovincialis, Black Sea mussel, a higher toxicity was observed in the case of the natural photoperiod, i.e., dark and light, compared to total darkness. Greater proportion of underdeveloped larvae was observed as compared with the complete darkness, especially with 4 and 8 mg/L n-TiO2 [101].
The acidification of the oceans could possibly increase ecotoxicity of metal ENPs, n-TiO2 in particular, as the pH of the environment is an important factor that influences toxicity and susceptibility to n-TiO2. Studies have shown that lower pH seems to increase the ecotoxic effects of n-TiO2 more so than greater concentrations do. Experiments were performed with the mussel Mytilus coruscus [98, 126,127,128] and three bivalve species [99]. A worse hemocyte response was observed, when M. coruscus was exposed to 2.5 or 10 mg/L n-TiO2 in marine water at pH 7.3 for 14 days, including an increase in ROS and decrease in phagocytosis, esterase activity, and lysome content. Additionally, a longer recovery period was necessary after exposure to the maximal n-TiO2 concentration at lower pH. Furthermore, lower pH increased ammonia excretion and decreased clearance rate, breathing frequency, and the growth potential as compared with exposure at normal pH (8.1). Studies also showed clams (Tegillarca granosa, Meretrix meretrix, and Cyclina sinesis) accumulated TiO2 in far greater amounts when exposed to 100 μg/L n-TiO2 at lower pH values [99]. This could also be attributed to where clams mostly exist (on the floor of oceans and shelves of rock formations), as n-TiO2 sink and accumulate to the bottom sediments, they may pose greater threat to benthic species.
The environmental risk of n-TiO2 toward marine microbial species seems to be low; however, there is potential for adverse effects in hotspots of contamination [129]. The Gram-negative bacterium Vibrio fischeri was not affected negatively at all by n-TiO2, even at concentrations of up to 20 g/L [110]. Experiments with Phaeodactylum tricornutum have shown that ROS are generated, in the presence of TiO2, to damage cell membranes. Again, light influenced toxicity and a greater toxic effect on cells was observed in the case of UV-A exposure as opposed to visible light exposure. Furthermore, the EC50 decreased from 132.0 ± 7.0 mg/L on visible light exposure to 1.98 ± 0.09 mg/L on UV-A exposure [100, 102]. The biological effects of n-TiO2 were studied using marine microalga Isochrysis galbana, and while there was not an observed effect on algal cell size and reproduction, algal chlorophyll was affected and photosynthesis decreased consequently [109]. Again, photochemical efficiency was dramatically decreased, i.e., photosystem II—the first protein complex in photosynthesis—was inhibited, in the alga Microcystis aeruginosa when exposed to various concentrations of n-TiO2 (0.1, 1, 10, 50 and 100 mg/L), thus inhibiting photosynthetic activity [14]. The ecologically significant cyanobacterium, Prochlorococcus, suffers short-term (72 h) adverse effects following exposure to n-TiO2, but then populations seem to recover, mainly due to hetero-aggregation with agglomerated n-TiO2 and subsequent sinking out of the water column. No other metabolic stresses were observed, suggesting the cell declination was due to physical interactions between ENPs and cyanobacteria [129]. Once the n-TiO2 aggregate and sink to the floor, they are no longer a toxic threat to organisms in the water but do pose a threat to benthic organisms where the agglomerated n-TiO2 rests.
As for n-TiO2 in the presence of other ENPs or pollutants, Matouke et al. [111] showed the increasing toxic effect of n-TiO2 in the presence of phosphorous on microalga Chlorella ellipsoides. The optical density, cholorphylls a and b, total chlorophyll, and antioxidant enzymatic activity were substantially changed on exposure to the combined compounds, as well as the biomass greatly decreased. However, these observations are not enlightening on all combined compounds with n-TiO2. In a different combination, the effects could be less toxic or even beneficial. Still so little is known about the ecotoxicity of n-TiO2 in the presence of other compounds.
It appears that n-TiO2, because of its photocatalytic properties that make it an ideal substance in commercial products, causes damage to photosynthetic processes in the plant cells. Additionally, light has been observed to increase these toxic effects, again perhaps because of n-TiO2’s innate properties. The accumulation of ROS and n-TiO2 is reportedly another one of the toxic effects of this ENP; however, this may be due to bivalve mollusks being a common experimental subject. This phylum of animals typically inhabits the sandy or muddy bottoms or attaches themselves to rocks in both freshwater and the sea, which makes them a target for sunk n-TiO2. n-TiO2 are prone to sedimentation, mainly due to hetero-aggregation with agglomerated n-TiO2, and they sink out of the water column [129] and accumulate on the floor. This makes their effects on benthic organisms of special concern [55].
The majority of literature available on the toxicity of ENPs cover n-TiO2, which may be the cause of n-TiO2 being one of the more seemingly toxic NPs. However, the scope of n-TiO2’s toxicity appears to be photocatalytic processes in plants and algae and also ROS and n-TiO2 accumulation in mollusks, or benthic animals. Little, if any, evidence has been published suggesting the hazards of n-TiO2 to humans.
n-Ag
Silver ENPs are used as components of consumer goods and have applications in agriculture as agents with a broad range of high bactericidal, fungicidal, and antiviral activities [130]. These metallic ENPs are utilized in the production of textiles, laundry products, household appliances, water purification systems, dyes, personal care products, food storage containers, and food additives [131]. Silver NPs, due to their small size, are capable of penetrating biological membranes into cells and causing toxicity at various levels [102]. Their properties like shape and aggregation also influence entry and accumulation inside cell organelles including mitochondria [132]. The mechanisms of n-Ag toxicity are still yet to be fully understood, but it is thought that both n-Ag and Ag+ ions released from ENP cores exert effects by facilitating membrane impairment, ROS generation, protein oxidation and denaturation, mitochondrial dysfunction, DNA damage, and inhibition of cell proliferations [133]. Silver NPs undergo oxidative dissolution in water, as well as biological tissues and cell culture media, wherein Ag+ ions are released [134]. Positively charged ENPs, such as n-Ag, can easily enter cells and cross through lipid bilayers through simple diffusion and therefore are proven to be more toxic than neutral or negatively charged ions [135]. ENPs made from silver have been reported to be the most ecotoxic nanoparticle because they affect numerous aquatic organisms [136].
Hyalella azteca, a freshwater crustacean, is highly responsive to pollution and has been a reliable tool used in standard ecotoxicity assays to determine sediment and water quality [137,138,139,140,141]. Silver is highly toxic to this species and H. azteca are among the first group of organisms to disappear after exposure to n-Ag in contaminated waters [142, 143]. Many studies have explored the hypothesis that n-Ag has surface charge-related toxicity, and it has been discovered that, indeed, surface functionalization has a major role in the toxicity of n-Ag [144].
In experiments exposing H. azteca to n-Ag, citrate-coated n-Ag, and polyvinylpyrrolidone (PVP)-coated n-Ag, it was discovered that the PVP-coated n-Ag was the least toxic, and thus a neutral non-ionic coating of an ENP can reduce its toxicity. It has been shown that gills have negatively charged proteins with a high affinity for charged particles [84]. Thus, higher surface charged citrate-Ag may have enhanced the uptake of Ag+ by the gills of the amphipods [145]. Moreover, PVP, unlike citrate, is a non-ionic polymer [146], making PVP-Ag less attractive to the negatively charged gill proteins to cause toxic effects. Samrot et al. [147] also observed damage to organs such as gills, eyes, and intestine of Danio rerio after exposing them to n-Ag. Gills, with their negatively charged proteins, seem to be a reoccurring site of n-Ag accumulation and/or toxic effects. The ionic nature of n-Ag greatly influences its toxicity, largely making negatively charged proteins or atoms their binding target and exerting toxic effects from there.
Another factor that has been observed to influence the toxicity of n-Ag is salinity; however, how salinity affects the toxicity of n-Ag has yet to be elucidated. The toxicity of n-Ag toward the marine alga Dunaliella salina was experimentally studied at different water salinity levels (35, 70, and 140 g/L). The degree of microalgal growth inhibition was found to increase with the increasing salinity [105]. However, an opposite effect was observed when Salari et al. [106] studied the acute toxicity of n-Ag in rainbow trout, Oncorhynchus mykiss, and found the LC50 in brackish water was 12 times lower than that found in freshwater in a 96-h experiment. How salinity affects the toxicity of n-Ag is unclear, but it has been determined to influence toxic effects. The abundance of Cl− anions in an aquatic environment with high salinity might bind to the Ag+ cations making them neutral, otherwise disarming them from causing damage.
As stated before, n-Ag is reported to be a highly ecotoxic ENP, but to whom or what? Perhaps because n-Ag does not dissociate into cations in soil media, it is less harmful to plants (Table 2). Although oxidative dissolution of silver can occur in biological tissues and solutions and while it has been evidenced that n-Ag can be toxic to humans, the literature that demonstrates its precise toxic effects on humans is lacking. Nevertheless, studies have confirmed that n-Ag can be transferred through the trophic chain and subsequently exert its toxic effects [148].
n-ZnO
Zinc oxide ENPs are the second nanomaterial most commonly used in certain fields after n-TiO2. Zinc oxide NPs are widely used to produce pigments, semiconductors, rubber solar cells, chemical fibers, sunscreen creams, and food additives [149,150,151]. Another potential application is designing n-ZnO-containing paint additives to prevent the formation of biofilms on marine ship hulls, as n-ZnO has shown high antibacterial activity, even against antibiotic-resistant bacteria [152, 153].
Research suggests, like other metallic ENPs, that the toxic effects vary according to the chemical properties of n-ZnO, different soil media or water chemistry and the form of n-ZnO [154]. It has been reported that n-ZnO has strong toxic effects on both bacterial and mammalian cells [155]. Cell membrane damage and increased oxidative stress have been seen to be the most common toxic effects of Zn-based ENPs on various mammalian cell lines [156]. Studies have shown a higher level of toxicity of n-ZnO compared to other common ENPs; this higher toxicity has been associated with its dissolution into Zn2+, in contrast to insoluble n-TiO2 and the non-toxic degradation products of n-SiO2 [157,158,159]. But to directly contradict this theory, a study on the early development of sea urchins by Manzo et al. [113] found n-ZnO to be more toxic than the ionic zinc (Zn2+) and marine water increased the toxic effect of n-ZnO because surface interactions occurred between n-ZnO and the medium, and NP aggregates would form. Perhaps the difference in findings can be explained by the water salinity, but without certain experimental parameters listed and standards for these toxicity studies, it is difficult to know what the toxic effects can be attributed to, e.g., ionic form or aggregates caused by salinity. Indeed, water salinity does increase toxic effects of n-ZnO, as well as forms aggregates, so the accumulation of n-ZnO in marine organisms is an important topic related to the toxicity of n-ZnO [115]. Experiments with mollusks, worms, and oysters showed evidence of bioaccumulation of n-ZnO, indicating that n-ZnO accumulation leads to early degradation of mitochondria and causes oxidative stress [115, 116].
Certain studies indicate that the adverse effects of n-ZnO can be decreased by altering its surface chemistry. One study by Ramasamy et al. [160] aimed to lower the toxicity of n-ZnO by coating it with a silica layer (SiO2) and found that the silica-coated n-ZnO exhibited reduced enzyme leakage and oxidative stress compared to the bare n-ZnO. More research needs to be conducted to understand the toxic effects. For example, what are the effects of light? One would expect the light to increase the toxicity of n-ZnO because of its photocatalytic properties like n-TiO2, but there is very limited literature on the topic. Despite n-TiO2 and n-ZnO’s similarities, their difference— the insolubility of n-TiO2 and the dissolution of n-ZnO—seems to produce a difference in toxicity toward microorganisms suggesting that the toxic effects of n-ZnO on microbes are due to its Zn2+ ions.
n-SiO2
Silicon dioxide ENPs are widely used in consumer products, from ceramics and glass to medicine, cosmetics and food additives; their application has even extended to biomedical [161, 162]. More than 1 million tons per year are manufactured and imported to Europe alone [47], and more than 4 million tons globally are consumed each year [163], presumably due to their benefits, low toxicity and cost, and ease of production [117]. With the new and growing potential for n-SiO2, especially in medical applications where n-SiO2 could be introduced into the human body intentionally, the increasing potential for exposure has raised concerns for safety and adverse health effects. Furthermore, n-SiO2 are ultimately released into aquatic environments during production and through use and wastewater, which has led to an environmental concentration of n-SiO2 in surface waters that is predicted to be in a range of 0.12 and 2.6 μg/L [40, 164]. Manufactured n-SiO2 consists of either crystalline or amorphous silica. The crystalline structure of silica is a repeating network of SiO2 tetrahedral units, while the amorphous structure is a randomly distributed network of tetrahedral units [165].
When considering the inhalation of particles, the critical cells involved in this process are the macrophages and epithelial cells of the bronchi and alveoli. These cells respond by releasing inflammatory mediators locally and inducing phagocytic/endocytic uptake of the particles [166]. A review by Fruijtier-Pölloth [165] concluded that there was no bioaccumulation of n-SiO2 and it all disappeared within a short period from living organisms by physiological excretion methods, and the smaller-sized ENPs were excreted faster. Human health effects associated with exposure to crystalline silica (0.5–10 μm) have been widely studied; exposure to crystalline silica induces silicosis, a progressive fibrotic lung disease, and is also associated with lung cancer, emphysema, and pulmonary tuberculosis among workers who have been exposed occupationally [167]. The toxicological potential of silica has been linked to its crystallinity, while natural amorphous silica has generally been considered less harmful and clearing more rapidly from the lung in vivo [168]. However, recent studies have shown that amorphous silica can be as reactive as crystalline particles [169] and adsorbs to cellular surfaces and affects membrane structures and integrity [165]. A study by Murugadoss et al. [170] demonstrated that n-SiO2 induces oxidative stress and mediated apoptosis, cytotoxicity, genotoxicity, and pro-inflammatory responses with significant effects being observed only at or above the concentration of 25 mg/L (25 μg/mL) and in a size- and concentration-dependent pattern. ROS-mediated toxicity is believed to be an important mechanism of toxicity related to n-SiO2 [171]; however, one study indicated that n-SiO2 induced DNA damage without the generation of ROS, and thus other mechanisms such as direct DNA damage might be a toxic effect of n-SiO2 [74].
The ecotoxicity of n-SiO2 has been linked to the number of hydroxyl groups on their surface [172], and the amount of protonated silanol groups (Si–O-H) on the ENP surface seems to be an important influential factor for toxic effects. The low toxicity of n-SiO2 is partly derived from these unique surface silanol groups, which can either form hydrogen bonds or dissociate into SiO−, which electrostatically interact with biomolecules and can disrupt the cytomembrane, lysosome, and mitochondria [173]. Cell membrane proteins can also become denatured by the proton-donating silanol group which leads to damage [119]. In water, SiO2 has silanol groups, but at the nanoscale there is a higher density of these groups making n-SiO2 more reactive and prone to environmental modification [174].
In aquatic environments, there are multiple influential factors such as composition, size, charge and media exposure that affect the ultimate toxicity of n-SiO2 [175]. Aggregation of n-SiO2 has frequently been reported, and while the size is an important factor affecting toxicity, it has been shown that smaller SiO2 NPs are more toxic [119, 176, 177]. Capping agents are key parameters for toxicity as well, in a study by Dumitrescu et al. [178] where zebrafish were exposed to n-SiO2, there was dose-dependent mortality and other developmental toxic effects, but no toxic effects were observed with glycine functionalized n-SiO2. Different responses were also observed according to the functionalization of n-SiO2 in experiments with zebrafish and Oncorhynchus mykiss cell lines [118, 119]. Little research has been done considering other environmental parameters like light, pH, and temperature, so how these factors affect the toxicity of n-SiO2 is still to be understood.
CNTs
CNTs are a major building block used in nanotechnology and have unique chemical and physical properties as a result of their nanostructure. While CNTs are being manufactured, the most common catalysts for their synthesis are Co, Fe, Ni, and Mo, which can result in residual metal impurities of the CNTs and subsequently influence toxicity. CNTs can also be functionalized or coated to improve their dispersion or achieve certain functions [179]. The most influential factors affecting toxicity are functionalization and composition of the CNTs, length and diameter, the specific surface area ratio, and their propensity to form agglomerates and aggregates, with the most confirmed aggravators affecting lung cells and tissues being composition or impurities and CNT diameter [180]. Pulmonary tissue exposed to CNTs through inhalation has not been shown to cause mesothelioma, but it does lead to the formation of granulomas [181], lung inflammation and fibrotic responses [182]. Gernand and Casman [180] found that larger aggregates of CNTs caused less damage to cells than the smallest aggregates, but were more difficult to clear from the lungs, requiring a longer post-exposure time to recover to normal levels. This finding is inconsistent with an in vitro study that found increasing aggregate size leads to greater damage to the cell membrane [183], but corroborates the findings of an in vivo study from Muller et al. [184] where larger aggregates increased neutrophil count and reduced lactate dehydrogenase (LDH) release. Literature indicates that the tendencies of CNT toxicity larger CNTs are more toxic to microbes and bacteria or when the CNT is interacting with the outside of the organism, while smaller CNTs are more toxic to lung cells and tissues or when the CNT is inside the organism.
One finding from Gernand and Casman’s [180] study that contradicts the heavily proven observation that increased length of CNTs increases toxicity in microbes and bacteria, was that increased CNT length markedly decreased the observed toxicity, except for the count of total proteins in the bronchoalveolar lavage fluid. So perhaps this highly observed “increased length increases toxicity” result is only true in microorganisms, where the CNTs can cause physical damage. It is hypothesized that longer lengths increase the chance of CNTs aggregating and tangling, therefore reducing their characteristics of fibers in the lung and subsequently their toxicity. The longer diameter of CNTs has been shown to consistently increase toxicity [180]. This is consistent with the hypothesis that stiffer nanotubes produce greater cell damage in the lungs and are more resistant to the body’s natural process of clearing particles [185, 186], which also allows for a plausible alternative for the difference between single-walled (SWCNTs) and multi-walled CNTs (MWCNTs). Pulmonary toxicity is also greatly increased by the number of metallic impurities on the CNTs. Among the metals tested, including chromium, nickel, aluminum, iron, copper, and cobalt, the metal impurity that negatively affected toxicity the most was cobalt [180].
Like many carbon structures, CNTs have demonstrated antimicrobial properties which are mostly attributed to physical membrane damage mechanisms [187–189]. The length of CNTs is a critical factor for antimicrobial activity, and a study by Yang et al. [190] confirmed that the longer the CNT, the more powerful the antimicrobial properties are because contact with the bacterial membrane has greater potential at longer lengths. Research by Kang et al. [191] showed CNTs accumulated on the membrane of the bacteria Escherichia coli causing damage to main structures and ultimately abolishing the bacteria. The functional groups on CNTs are also an important influential factor for the antimicrobial properties and ultimate toxicity. Functionalized CNT with biologic groups, such as poly-lysine, have shown to cause insignificant damage to the human body and the environment [192, 193]. It has also been demonstrated that functionalized CNT with cationic amino acids have significant antibacterial activity against various bacteria, such as E. coli, Staphylococcus aureus, and Salmonella typhimurium, suggesting positively charged groups could be more damaging to bacterial membranes [187, 188].
n-CeO2
Cerium dioxide ENPs, like TiO2, are photocatalytic when exposed to certain wavelengths of light, and generates ROS including hydroxyl radicals, peroxide, and superoxide [194, 195]. While the generation of ROS has been strongly tied to n-TiO2 toxicity, there is significantly less evidence that has demonstrated a definitive link between ROS and toxicity for n-CeO2; this should be determined by seeing if the toxic effects occur because of UV light-facilitated photocatalytic ROS generation or simply because of n-CeO2 [196, 197]. It is important to consider the effect of illumination on toxicity with a semiconducting material like n-CeO2, so again, if researchers took more care in explicitly noting specific experimental parameters, it might be easier to understand the cause of toxic mechanisms or certain phenomena.
One mechanism of toxicity that has been numerously reported is the sorption of CeO2 to the surface of various organisms resulting in localized exposure of ROS [198, 199], to cell membranes causing membrane damage [199,200,201], to bacteria and algae resulting in their sedimentation, which may lead to nutrient or light inhibition [202, 203], and to the outer and inner membranes of higher trophic organisms resulting in a correlation with toxicity [204, 205]. There is much evidence that confirms the main interaction that causes n-CeO2 toxicity in various environments, e.g., human body or aquatic environment, is the adsorption of n-CeO2 onto a particular, target surface, e.g., cell membrane or organism. It has also been proposed that the toxicity of n-CeO2 is caused by ROS generation and/or oxidative stress [202, 206] and cell membrane damage [207, 208]. On the other hand, some studies propose n-CeO2 to act as an antioxidant and radical scavenger [159, 194, 209, 210]. But just like opposing findings in the studies of other ENPs, studies on n-CeO2 reported results that are often contradictory. There have been different toxic mechanisms proposed, as well as a finding that n-CeO2 is toxic to some organisms and non-toxic to others [211].
In solutions, the hydrolysis of CeO2 occurs and is followed by the polymerization and precipitation of gelatinous hydrated cerium (IV) oxide. The dissolution of CeO2 has been noted to be suppressed by the phosphate ligand, while dissolution is increased at lower pH values. Phosphate ligands are strongly adsorbed onto n-CeO2, quickly forming cerium phosphate which is insoluble and immediately precipitates once formed. Furthermore, the phosphate ligand seems to be likely involved in inhibiting any oxidative stress-related toxicity effects [212].
Conclusion
There is still much to be understood about the environmental fate and transport of ENPs and their toxicity. Although it is certainly an area of research that is of concern, until we have a standard for these studies, our understanding will always be inferior and incomplete. It is important to standardize studies in a way so that all environmental and experimental parameters are noted, so one can begin to comprehend the effects of ENPs and how certain environmental factors influence those effects (e.g., does a change in pH change the toxicity of n-TiO2?). Confirmation is yet to be obtained on the physicochemical properties, environmental behavior, and toxicological effects of the ENPs as one has to recognize the fact that these ENPs may behave differently than they do in their original product, which they typically do not exist as in natural ecosystems. Or they may behave differently depending on the chemistry of the environment (e.g., salinity or pH), which may explain the opposing findings with n-CeO2 toxicity. Once we can better understand how ENPs interact within and exist in the environment, only then will we be able to fully realize the risks of these ENPs and can take measures to protect the environment and ourselves. Usually, ENPs in the environment are not by themselves but with other chemicals and contaminants. These co-contaminant(s), some of which are emerging such as microplastics, could have a synergistic or antagonistic effect on the environment including toxicity. The effect of ENPs combined with these co-contaminant(s) should be elucidated to be more realistic. Also, all ENPs have impurities and for most studies, it is unclear whether toxicity is from ENPs or the impurities or both. This issue should be investigated to understand truly the impact of ENPs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fu Y, et al. The effect of pH on the sorption of gold nanoparticles on illite. Acta Geochimica. 2020;39:172–80.
Fréchette-Viens L, Hadioui M, Wilkinson KJ. Quantification of ZnO nanoparticles and other Zn containing colloids in natural waters using a high sensitivity single particle ICP-MS. Talanta. 2019;200:156–62.
Wu Y, et al. A key moment for TiO 2: Prenatal exposure to TiO 2 nanoparticles may inhibit the development of offspring. Ecotoxicol Environ Safety. 2020.
Hendren CO, et al. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol. 2011;45(7):2562–9.
Bathi JR, et al. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci Total Environ. 2021;793: 148560.
• Suman TY, Li WG, Pei DS. Chapter 5 - Toxicity of metal oxide nanoparticles, in Nanotoxicity, S. Rajendran, et al., Editors. 2020:107–23 Elsevier. This chapter describes the toxicity of metal-oxide nanoparticles in key species, and explains the mechanisms of toxicity for each.
Allan J, et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul Toxicol Pharmacol. 2021;122: 104885.
Garner KL, Suh S, Keller AA. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ Sci Technol. 2017;51(10):5541–51.
Williams RJ, et al. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Current Opinion in Environmental Sustainability. 2019;36:105–15.
Toropova AP, Toropov AA. Nanomaterials: Quasi-SMILES as a flexible basis for regulation and environmental risk assessment. Sci Total Environ. 2022;823: 153747.
WHO. Guidelines on protecting workers from potential risks of manufactured nanomaterials. 2017.
Research on Nanomaterials. 2021 [cited 2021; Available from: https://www.epa.gov/chemical-research/research-nanomaterials.
•• Cypriyana PJ, et al. Overview on the toxicity of nanoparticles, it’s mechanism, models used in toxicity studies and disposal methods—A review. Biocataly Agricult Biotechnol. 2021;36(102117). This review discussed how the induction of ROS is initiated by different mechanisms depending on various factors, emphasizing the many and varying factors and combinations of such that leads to a complex phenomenon of toxicity.
Wu D, et al. Effects of titanium dioxide nanoparticles on Microcystis aeruginosa and microcystins production and release. J Hazard Mater. 2019;377:1–7.
Simonet BM, Valcárcel M. Monitoring nanoparticles in the environment. Anal Bioanal Chem. 2008;393(1):17.
Hassellöv M, et al. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology. 2008;17(5):344–61.
De la Calle I, Menta M, Séby F. Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim Acta, Part B. 2016;125:66–96.
Mansor M, et al. Application of Single-Particle ICP-MS to Determine the Mass Distribution and Number Concentrations of Environmental Nanoparticles and Colloids. Environ Sci Technol Lett. 2021;8(7):589–95.
• Giese B, et al. Risks, Release and concentrations of engineered nanomaterial in the environment. Sci Rep. 2018;8(1):1565. This article conveys the difficulty that exists when trying to measure environmental concentrations of ENPs; since existing concentrations are extremely low, the current technology is not reliable at reading such measurements so predictive modeling tends to be used.
Vance ME, et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–80.
Patil MP, Kim GD. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf B Biointerfaces. 2018;172:487–95.
Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles:Mechanism, application and toxicity. J Clean Prod. 2020;272(7).
Hlongwane GN, et al. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci Total Environ. 2019;656:808–33.
Ganguly K, et al. Silver nanoparticles for wastewater treatment. 2021:385–401.
Gallego-Urrea JA, Tuoriniemi J, Hassellöv M. Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. TrAC Trends Anal Chem. 2011;30(3):473–83.
von der Kammer F, et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem. 2012;31(1):32–49.
Liu J-F, et al. Cloud point extraction as an advantageous preconcentration approach for analysis of trace silver nanoparticles in environmental waters. Anal Chem. 2009;81(15):6496–502.
Waissi-Leinonen GC, et al. Fullerenes(nC60) affect the growth and development of the sediment-dwelling invertebrate Chironomus riparius larvae. Environ Pollut. 2015;206:17–23.
Ma Y, et al. Xylem and phloem based transport of CeO 2 Nanoparticles in hydroponic cucumber plants. Environ Sci Technol. 2017;51(9):5215–21.
Yang Y, et al. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci Total Environ. 2016;563–564:996–1007.
Donovan AR, et al. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere. 2016;144:148–53.
Kińska K, et al. Study of the uptake and bioaccumulation of palladium nanoparticles by Sinapis albausing single particle ICP-MS. Science fo the Total Environment. 2018;615:1078–85.
Yang Y, et al. Separation and determination of silver nanoparticle in environmental water and the UV-induced photochemical transformations study of AgNPs by cloud point extraction combined ICP-MS. Talanta. 2016;161:342–9.
Samaddar P, Sen K. Cloud point extraction: A sustainable method of elemental preconcentration and speciation. J Ind Eng Chem. 2014;20(4):1209–19.
Suhendra E, et al. A review on the environmental fate models for predicting the distribution of engineered nanomaterials in surface waters. Int J Mol Sci. 2020;21(12).
Proulx K, Hadioui M, Wilkinson KJ. Separation, detection and characterization of nanomaterials in municipal wastewaters using hydrodynamic chromatography coupled to ICPMS and single particle ICPMS. Anal Bioanal Chem. 2016;408(19):5147–55.
Syafiuddin A, et al. Silver Nanoparticles in the Water Environment in Malaysia: Inspection, characterization, removal, modeling, and future perspective. Sci Rep. 2018;8(1):986.
Wimmer A, et al. Sampling and pre-treatment effects on the quantification of (nano)silver and selected trace elements in surface water - Application in a Dutch case study. Sci Total Environ. 2019;663:154–61.
Markus AA, et al. Determination of metal-based nanoparticles in the river Dommel in the Netherlands via ultrafiltration HR-ICP-MS and SEM. Sci Total Environ. 2018;631–632:485–95.
Wang Y, Nowack B. Environmental risk assessment of engineered nano-SiO. Environ Toxicol Chem. 2018;37(5):1387–95.
Choi S, et al. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO. Sci Total Environ. 2018;625:1321–9.
Rand LN, et al. Quantifying Nanoparticle Associated Ti, Ce, Au, and Pd Occurrence in 35 U.S. Surface Waters. ACS ES&T Water. 2021;1(10):2242–50.
Sanchís J, et al. Nanoparticle tracking analysis characterisation and parts-per-quadrillion determination of fullerenes in river samples from Barcelona catchment area. Anal Bioanal Chem. 2015;407(15):4261–75.
Subhan MA, Subhan T. Chapter 5 - Safety and global regulations for application of nanomaterials, in Nanomaterials Recycling. M. Rai and T.A. Nguyen, Editors. 2022:Elsevier p 83–107.
Faghih Akhlaghi M, Daeihamed M, Jafari SM. Chapter 7 - Regulatory principles on food nano-particles legislated by North and South American countries, in Safety and Regulatory Issues of Nanoencapsulated Food Ingredients. S.M. Jafari, Editor. Acad Press. 2021:239–50.
FDA’s Approach to Regulation of Nanotechnology Products. 2018. [cited 2021; Available from: https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products.
European Chemicals Agency. 2020. [cited 2022 Feb 12]; Available from: https://echa.europa.eu.
Biswas JK, Sarkar D. Nanopollution in the Aquatic Environment and Ecotoxicity: No Nano Issue! Current Pollution Reports. 2019;5(1):4–7.
Caballero-Guzman A, Nowack B. A critical review of engineered nanomaterial release data: Are current data useful for material flow modeling? Environ Pollut. 2016;213:502–17.
Meesters JA, et al. Multimedia modeling of engineered nanoparticles with SimpleBox4nano: model definition and evaluation. Environ Sci Technol. 2014;48(10):5726–36.
Pulido-Reyes G, et al. Bio-nano interface and environment: A critical review. Environ Toxicol Chem. 2017;36(12):3181–93.
Pradhan N, et al. Antiamyloidogenic Chemical/Biochemical-Based Designed Nanoparticle as Artificial Chaperone for Efficient Inhibition of Protein Aggregation. Biomacromol. 2018;19(6):1721–31.
Lowry GV, et al. Transformations of nanomaterials in the environment. Environ Sci Technol. 2012;46(13):6893–9.
Wang Y, et al. Probabilistic modeling of the flows and environmental risks of nano-silica. Sci Total Environ. 2016;545–546:67–76.
Abdel-Latif HMR, et al. Environmental transformation of n-TiO. Ecotoxicol Environ Saf. 2020;200: 110776.
•• Turan NB, et al. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf Environ Prot. 2019;130:238–49. This review described factors affecting ENPs’ toxicity in aquatic environments by categorizing the factors into three main groups: (1) ENPs’ physiochemical traits, (2) interaction with co-pollutants found in the aqueous media, (3) functional behavior of ENPs.
Ritchie JD, Perdue EM. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim Cosmochim Acta. 2003;67:85–96.
Bai X, et al. Regulation of Cell Uptake and Cytotoxicity by Nanoparticle Core under the Controlled Shape, Size, and Surface Chemistries. ACS Nano. 2020;14(1):289–302.
Liu Z, Malinowski CR, Sepúlveda MS. Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere. 2022;291(Pt 2): 132941.
Ferry JL, et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat Nanotechnol. 2009;4(7):441–4.
Lopez-Chaves C, et al. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine. 2018;14(1):1–12.
Niazi JH, Gu MB. Toxicity of metallic nanoparticles in microorganisms a review. Springer Netherlands. 2009:193–206.
Cai R, et al. Corona of Thorns: The Surface Chemistry-Mediated Protein Corona Perturbs the Recognition and Immune Response of Macrophages. ACS Appl Mater Interfaces. 2020;12(2):1997–2008.
Cai X, et al. Molecular Mechanisms, Characterization Methods, and Utilities of Nanoparticle Biotransformation in Nanosafety Assessments. Small. 2020;16(36): e1907663.
Misra SK, et al. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ. 2012;438:225–32.
Setyawati MI, Zhao Z, Ng KW. Transformation of Nanomaterials and Its Implications in Gut Nanotoxicology. Small. 2020;16(36): e2001246.
Dukhin SS, Labib ME. Convective diffusion of nanoparticles from the epithelial barrier toward regional lymph nodes. Adv Colloid Interface Sci. 2013;199–200:23–43.
Oberdörster G, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8.
Radomski A, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146(6):882–93.
Madl AK, et al. Nanoparticles, lung injury, and the role of oxidant stress. Annu Rev Physiol. 2014;76:447–65.
Lucchini RG, et al. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2012;33(4):838–41.
Zhu MT, et al. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol Sci. 2009;107(2):342–51.
Barua S, Mitragotri S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today. 2014;9(2):223–43.
Magdolenova Z, et al. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8(3):233–78.
Mahaye N, et al. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat Res Rev Mutat Res. 2017;773:134–60.
Gottschalk F, et al. Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, Fullerenes) for different regions. Environ Sci Technol. 2009;43(24):9216–22.
Yi Z, et al. How to distinguish natural versus engineered nanomaterials: insights from the analysis of TiO2 and CeO2 in soils. Environ Chem Lett. 2020;18(1):215–27.
McKee MS, Filser J. Impacts of metal-based engineered nanomaterials on soil communities. 2016:3.
Maurer-Jones MA, et al. Toxicity of engineered nanoparticles in the environment. Anal Chem. 2013;85(6):3036–49.
Ma C, et al. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol. 2015;49(12):7109–22.
Zhu Y, et al. Recent advances in the biotoxicity of metal oxide nanoparticles: Impacts on plants, animals and microorganisms. Chemosphere. 2019;237: 124403.
van Hoof L, et al. Food from the ocean; towards a research agenda for sustainable use of our oceans’ natural resources. Mar Policy. 2019;105:44–51.
Roma J, et al. Engineered metal nanoparticles in the marine environment: A review of the effects on marine fauna. Mar Environ Res. 2020;161: 105110.
Kleiven M, et al. Route of exposure has a major impact on uptake of silver nanoparticles in Atlantic salmon (Salmo salar). Environ Toxicol Chem. 2018;37(11):2895–903.
Baun A, et al. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17(5):387–95.
Djearamane S, et al. A Review on bio-distribution and toxicity of silver, titanium dioxide and zinc oxide nanoparticles in aquatic environment. Pollut Res. 2016;35(4):701–12.
• Arvidsson R, Hansen SF, Baun A. Influence of natural organic matter on the aquatic ecotoxicity of engineered nanoparticles: Recommendations for environmental risk assessment. NanoImpact. 2020;20(100263). This article explored how NOM in an aquatic environment is typically adsorbed to the NPs influencing their toxicity while many studies confirmed that NOM decreases the toxicity of metal and metal-oxide ENPs.
Chen J, et al. Effect of natural organic matter on toxicity and reactivity of nano-scale zero-valent iron. Water Res. 2011;45(5):1995–2001.
Li M, et al. Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media. Environ Sci Technol. 2011;45(2):755–61.
Liu Y, et al. Interpreting the effects of natural organic matter on antimicrobial activity of Ag. Water Res. 2018;145:12–20.
Gao J, et al. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles. Chemosphere. 2012;89(1):96–101.
He M, et al. Influence of Interaction Between α-Fe. Bull Environ Contam Toxicol. 2017;99(6):719–27.
Lawrence JR, et al. Complex organic corona formation on carbon nanotubes reduces microbial toxicity by suppressing reactive oxygen species production. Environ Sci Nano. 2016;3:181–9.
Angel BM, et al. The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere. 2013;93(2):359–65.
Cáceres-Vélez PR, et al. Humic acid attenuation of silver nanoparticle toxicity by ion complexation and the formation of a Ag. J Hazard Mater. 2018;353:173–81.
Kteeba SM, et al. Exposure to ZnO nanoparticles alters neuronal and vascular development in zebrafish: Acute and transgenerational effects mitigated with dissolved organic matter. Environ Pollut. 2018;242(Pt A):433–48.
Noventa S, et al. Dissolution and bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: an in vivo study with oyster embryos. Nanotoxicology. 2018;12(1):63–78.
Huang X, et al. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO. Aquat Toxicol. 2016;180:1–10.
Shi W, et al. Ocean acidification increases the accumulation of titanium dioxide nanoparticles (nTiO. Sci Rep. 2019;9(1):3516.
Sendra M, et al. Toxicity of TiO, in nanoparticle or bulk form to freshwater and marine microalgae under visible light and UV-A radiation. Environ Pollut. 2017;227:39–48.
Libralato G, et al. Embryotoxicity of TiO2 nanoparticles to Mytilus galloprovincialis (Lmk). Mar Environ Res. 2013;92:71–8.
Vasyukova A, et al. Toxic Effect of metal-based nanomaterials on representatives of marine ecosystems: A review. Nanobiotechnology Reports. 2021;16:138–54.
Kaveh R, et al. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol. 2013;47(18):10637–44.
García-Sánchez S, Bernales I, Cristobal S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics. 2015;16:341.
Johari SA, et al. Influence of salinity on the toxicity of silver nanoparticles (AgNPs) and silver nitrate (AgNO. Chemosphere. 2018;209:156–62.
Salari JH, Kalbassi MR, Johari SA. Chronic effect of waterborne colloidal silver nanoparticles on plasma biochemistry and hematology of rainbow trout (Oncorhynchus mykiss). Iran J Health Environ. 2012:5.
Ringwood AH, et al. The effects of silver nanoparticles on oyster embryos. Mar Environ Res. 2010;69(Suppl):S49-51.
Vijayaraj V, et al. Transfer and Ecotoxicity of Titanium Dioxide Nanoparticles in Terrestrial and Aquatic Ecosystems: A Microcosm Study. Environ Sci Technol. 2018;52(21):12757–64.
Hu J, et al. Effect of TiO. J Environ Sci (China). 2018;66:208–15.
Heinlaan M, et al. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71(7):1308–16.
Matouke MM, Elewa DT, Abdullahi K. Binary effect of titanium dioxide nanoparticles (nTio. Aquat Toxicol. 2018;198:40–8.
Aravantinou AF, et al. Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol Environ Saf. 2015;114:109–16.
Manzo S, et al. Embryotoxicity and spermiotoxicity of nanosized ZnO for Mediterranean sea urchin Paracentrotus lividus. J Hazard Mater. 2013;254–255:1–9.
Suman TY, Radhika Rajasree SR, Kirubagaran R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol Environ Saf. 2015;113:23–30.
Buffet PE, et al. Fate of isotopically labeled zinc oxide nanoparticles in sediment and effects on two endobenthic species, the clam Scrobicularia plana and the ragworm Hediste diversicolor. Ecotoxicol Environ Saf. 2012;84:191–8.
Trevisan R, et al. Gills are an initial target of zinc oxide nanoparticles in oysters Crassostrea gigas, leading to mitochondrial disruption and oxidative stress. Aquat Toxicol. 2014;153:27–38.
Kim Y, et al. Physiological and Behavioral Effects of SiO. Micromachines (Basel). 2021;12(9).
Paatero I, et al. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci Rep. 2017;7(1):8423.
Book F, et al. Ecotoxicity screening of seven different types of commercial silica nanoparticles using cellular and organismic assays: importance of surface and size. NanoImpact. 2019;13:100–11.
Serag MF, et al. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano. 2011;5(1):493–9.
Sekar G, et al. Multiple spectroscopic studies on the interaction of BSA with pristine CNTs and their toxicity against Donax faba. J Lumin. 2016;170:141–9.
Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25.
Birbaum K, et al. No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol. 2010;44(22):8718–23.
Guseva Canu I, et al. Weight of epidemiological evidence for titanium dioxide risk assessment: current state and further needs. J Expo Sci Environ Epidemiol. 2020;30(3):430–5.
Jugan ML, et al. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology. 2012;6(5):501–13.
Hu M, et al. CO. Sci Rep. 2017;7:40015.
Kong H, et al. Nano-TiO. Chemosphere. 2019;237: 124561.
Shang Y, et al. Specific dynamic action of mussels exposed to TiO. Chemosphere. 2020;241: 125104.
Dedman CJ, et al. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ Sci Nano. 2021;8(5):1236–55.
Tortella GR, et al. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater. 2020;390: 121974.
He X, Deng H, Hwang HM. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27(1):1–21.
Akter M, et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J Adv Res. 2018;9:1–16.
Liao C, Li Y, Tjong SC. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int J Mol Sci. 2019:20(2).
Loza K, Epple M. Silver nanoparticles in complex media: an easy procedure to discriminate between metallic silver nanoparticles, reprecipitated silver chloride, and dissolved silver species. RSC Adv. 2018;8(43):24386–91.
Liu Y, et al. Intracellular dynamics of cationic and anionic polystyrene nanoparticles without direct interaction with mitotic spindle and chromosomes. Biomaterials. 2011;32(32):8291–303.
Geary SM, Morris AS, Salem AK. Assessing the effect of engineered nanomaterials on the environment and human health. J Allergy Clin Immunol. 2016;138(2):405–8.
Boros, BV, Ostafe V. Evaluation of ecotoxicology assessment methods of nanomaterials and their effects. Nanomaterials (Basel). 2020;10(4).
Anderson BS, et al. Relative toxicity of bifenthrin to Hyalella azteca in 10 day versus 28 day exposures. Integr Environ Assess Manag. 2015;11(2):319–28.
Oviedo-Gómez DG, et al. Diclofenac-enriched artificial sediment induces oxidative stress in Hyalella azteca. Environ Toxicol Pharmacol. 2010;29(1):39–43.
Wang N, et al. Acute and chronic toxicity of aluminum to a unionid mussel (Lampsilis siliquoidea) and an amphipod (Hyalella azteca) in water-only exposures. Environ Toxicol Chem. 2018;37(1):61–9.
Hartz KEH, et al. Survey of bioaccessible pyrethroid insecticides and sediment toxicity in urban streams of the northeast United States. Environ Pollut. 2019:254.
Berry WJ, et al. Predicting toxicity of sediments spiked with silver. Environ Toxicolog Chem. 1999:40–48.
Burton, A.G., Sediment Toxicity Assessment. Chelsea. MI: Lewis Publishers Inc; 1992.
Wu F, Harper BJ, Harper SL. Differential dissolution and toxicity of surface functionalized silver nanoparticles in small-scale microcosms: impacts of community complexity. Environ Sci Nano. 2017;4:359–72.
Xu JY, Zhang H. Long-term effects of sliver nanoparticles on the abundance and activity of soil microbiome. J Environ Sci (China). 2018;69:3–4.
Asadishad B, et al. Effect of gold nanoparticles on extracellular nutrient-cycling enzyme activity and bacterial community in soil slurries: role of nanoparticle size and surface coating. Environ Sci Nano. 2017;4(4):907–18.
Samrot AV, et al. Evaluation of nanotoxicity of Araucaria heterophylla gum derived green synthesized silver nanoparticles on Eudrilus eugeniae and Danio rerio. J Clus Sci. 2019:1017–24.
Wang J, Wang WX. Low bioavailability of silver nanoparticles presents trophic toxicity to marine medaka (Oryzias melastigma). Environ Sci Technol. 2014;48(14):8152–61.
Shetti NP, et al. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens Bioelectron. 2019;141: 111417.
Tang KS. The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci. 2019:239.
Weldegebrieal GK. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg Chem Commun. 2020:120.
Mahamuni-Badiger PP, et al. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater Sci Eng C Mater Biol Appl. 2020;108: 110319.
El-saied HA, Ibrahim AM. Effective fabrication and characterization of eco-friendly nano chitosan capped zinc oxide nanoparticles for effective marine fouling inhibition. J Environ Chem Eng. 2020;8(4):103949.
Mwaanga P, et al. Investigating the toxicity of Cu, CuO and ZnO nanoparticles on earthworms in urban soils. Journal of Pollution Effects and Control. 2017;195(3):32–6.
Huang Z, et al. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir. 2008;24(8):4140–4.
Shao W, et al. Carbon nanotubes for use in medicine: Potentials and limitations. IntechOpen. 2013;13:285–311.
Farcal L, et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE. 2015;10(5): e0127174.
Ivask A, et al. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology. 2014;8(Suppl 1):57–71.
Xia T, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–34.
Ramasamy M, et al. Role of surface modification in zinc oxide nanoparticles and its toxicity assessment toward human dermal fibroblast cells. Int J Nanomedicine. 2014;9:3707–18.
Selvarajan V, Obuobi S, Ee PLR. Silica Nanoparticles-A Versatile Tool for the Treatment of Bacterial Infections. Front Chem. 2020;8:602.
Yang X, et al. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nat Commun. 2019;10(1):1620.
Chemical Economics Handbook: Silicates and Silicas. 2021.
Wang Z, et al. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ Sci Nano. 2016;3(2):240.
Fruijtier-Pölloth C. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology. 2012;294(2–3):61–79.
Breznan D, et al. Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology. 2017;11(2):223–35.
Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379(9830):2008–18.
Arts JH, et al. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem Toxicol. 2007;45(10):1856–67.
Turci F, et al. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: the role of crystallinity and surface disorder. Part Fibre Toxicol. 2016;13(1):32.
Murugadoss S, et al. Toxicology of silica nanoparticles: an update. Arch Toxicol. 2017;91(9):2967–3010.
Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013: 942916.
Book F, Backhaus T. Aquatic ecotoxicity of manufactured silica nanoparticles: A systematic review and meta-analysis. Sci Total Environ. 2022;806(Pt 4): 150893.
Tarn D, et al. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46(3):792–801.
Ambrosone A, et al. Impact of Amorphous SiO2 Nanoparticles on a Living Organism: Morphological, Behavioral, and Molecular Biology Implications. Front Bioeng Biotechnol. 2014;2:37.
Ale A, et al. Ecotoxicity of silica nanoparticles in aquatic organisms: An updated review. Environ Toxicol Pharmacol. 2021;87: 103689.
Vo NT, et al. Cytotoxicity evaluation of silica nanoparticles using fish cell lines. In Vitro Cell Dev Biol Anim. 2014;50(5):427–38.
Zhu B, et al. The fate and oxidative stress of different sized SiO2 nanoparticles in zebrafish ( Danio rerio ) larvae. Chemosphere. 2019;225:705–12.
Dumitrescu E, et al. Developmental toxicity of glycine-coated silica nanoparticles in embryonic zebrafish. Environ Pollut. 2017;229:439–47.
Aschberger K, et al. Review of carbon nanotubes toxicity and exposure–appraisal of human health risk assessment based on open literature. Crit Rev Toxicol. 2010;40(9):759–90.
Gernand JM, Casman EA. A meta-analysis of carbon nanotube pulmonary toxicity studies–how physical dimensions and impurities affect the toxicity of carbon nanotubes. Risk Anal. 2014;34(3):583–97.
Varga C, Szendi K. Carbon nanotubes induce granulomas but not mesotheliomas. In Vivo. 2010;24(2):153–6.
Muller J, et al. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110(2):442–8.
Wick P, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168(2):121–31.
Muller J, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–31.
Kostarelos K. The long and short of carbon nanotube toxicity. Nat Biotechnol. 2008;26(7):774–6.
Donaldson K, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22.
Amiri A, et al. Efficient method for functionalization of carbon nanotubes by lysine and improved antimicrobial activity and water-dispersion. Mater Lett. 2012;72:153–6.
Zardini HZ, et al. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf B Biointerfaces. 2012;92:196–202.
Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9(6):674–9.
Yang C, et al. Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir. 2010;26(20):16013–9.
Kang S, et al. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23(17):8670–3.
Murugan E, Vimala G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J Colloid Interface Sci. 2011;357(2):354–65.
Yan L, et al. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3(2):362–82.
Bennett SW, Keller AA. Comparative photoactivity of CeO2, gamma-Fe2O3, TiO2and ZnO in various aqueous systems. Appl Catal B. 2011;102:600–7.
Li F, et al. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat Toxicol. 2015;158:1–13.
Miller RJ, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE. 2012;7(1): e30321.
Angel BM, Vallotton P, Apte SC. On the mechanism of nanoparticulate CeO2 toxicity to freshwater algae. Aquat Toxicol. 2015;168:90–7.
Booth A, et al. Freshwater dispersion stability of PAA-stabilised cerium oxide nanoparticles and toxicity towards Pseudokirchneriella subcapitata. Sci Total Environ. 2015;505:596–605.
Kuang Y, et al. Comparison study on the antibacterial activity of nano- or bulk-cerium oxide. J Nanosci Nanotechnol. 2011;11(5):4103–8.
He X, et al. Changing exposure media can reverse the cytotoxicity of ceria nanoparticles for Escherichia coli. Nanotoxicology. 2012;6(3):233–40.
Rodea-Palomares I, et al. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol Sci. 2011;119(1):135–45.
Fang X, et al. Stresses exerted by ZnO, CeO2 and anatase TiO2 nanoparticles on the Nitrosomonas europaea. J Colloid Interface Sci. 2010;348(2):329–34.
Navarro E, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17(5):372–86.
Artells E, et al. Exposure to cerium dioxide nanoparticles differently affect swimming performance and survival in two daphnid species. PLoS ONE. 2013;8(8): e71260.
Arnold MC, et al. Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch Environ Contam Toxicol. 2013;65(2):224–33.
Babu KS, et al. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int J Nanomedicine. 2014;9:5515–31.
Kim IS, Baek M, Choi SJ. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol. 2010;10(5):3453–8.
Krishnamoorthy K, et al. Surface chemistry of cerium oxide nanocubes: toxicity against pathogenic bacteria and their mechanistic study. J Ind Eng Chem. 2014;20:3515–7.
Cassee FR, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41(3):213–29.
Xue Y, et al. Direct evidence of hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem Part C. 2011;115:4433–8.
Leung YH, et al. Toxicity of CeO2 nanoparticles - the effect of nanoparticle properties. J Photochem Photobiol B. 2015;145:48–59.
Pauluhn J. Fate of inhaled Nano-CeO2 revisited: Predicting the unpredictable. Regul Toxicol Pharmacol. 2018;97:63–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biology and Pollution
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bathi, J.R., Wright, L. & Khan, E. Critical Review of Engineered Nanoparticles: Environmental Concentrations and Toxicity. Curr Pollution Rep 8, 498–518 (2022). https://doi.org/10.1007/s40726-022-00237-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-022-00237-4