Abstract
The ecotoxicity of α-Fe2O3 nanoparticles (NPs) and its interaction with a typical natural organic matter (NOM), fulvic acid (FA) on the physiological responses of Synechococcus sp. PCC7942 was studied. α-Fe2O3 NPs inhibited the algae growth at concentration higher than 10 mg L−1 and induced oxidative stress, indicated by enhanced antioxidant enzymes activities, elevated protein and sugar content. FA could efficiently recover cell growth and reduce antioxidant enzyme activities which induced by α-Fe2O3 NPs, indicating the toxicity of NPs was alleviated in the presence of FA. α-Fe2O3 NPs could form large aggregates coating on cell surface and inhibit cell growth. FTIR spectra verified FA interacted with α-Fe2O3 NPs through carboxyl groups, partly replaced the binding sites of α-Fe2O3 NPs on algal cell walls, thus reduced NPs aggregates coating on cell surface. This favors reducing the oxidative stress caused by direct contact and increasing light availability, thus mitigate NPs toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Nano ferric oxide (nano-Fe2O3) has been widely used in magnetic storage, medicine, chemical industries and water purification (Mohapatra and Anand 2010), and is inevitably brought to the aquatic environment. Nano-Fe2O3, has been found to cause significant structural changes in the epithelium and inhibit Staphylococcus aureus growth (Tran et al. 2010; Kalive et al. 2012). As the key primary producers, algae play an ecological important role in most aquatic ecosystem and have been used as the model organism for toxicity assessment of toxicants and nanomaterials (Ji et al. 2011). Therefore, more attention has been paid to the effect of nanoparticles (NPs) on algae. Only a few studies reported the toxicity of nano-Fe2O3 on aquatic organisms. Demir et al. (2015) reported the toxicity of α-Fe2O3 and γ-Fe2O3 on Nannochloropsis sp. and Isochrysis sp. appeared at a low concentration of 1 mg L−1. An opposite observation of higher toxicity of α-Fe2O3 than γ-Fe2O3 to Chlorella pyrenonedosa was reported in Lei’s work (Lei et al. 2016), with the IC50 of ~ 71.0 and ~ 132.0 mg L−1, respectively. Unfortunately, only morphological changes and cell growth were presented in the above works and still little is known about the physiological and biochemical changes of algae in response to nano-Fe2O3.
In aquatic environment, nano-Fe2O3 might adsorb pollutants and interact with other nanoparticles, and consequently alter their toxicity. Natural water environment always contain widely-distributed dissolved natural organic matter (NOM), which is a complex organic molecule and has aroused widespread attention due to its unique properties and high concentration (near 100 mg L−1 in fresh water) (Kim et al. 2012). The interaction between NPs and NOM may result in the formation or breakage of aggregates, which is mainly determined by their surface properties (Stumm and Morgan 2012). It is reported that NOM could decrease the attachment of the NPs to the bacteria and alleviate the nanotoxicity (Fabrega et al. 2009; Li et al. 2010). However, the influence of NOM is likely to be dependent on NP-type and test-species. NOM could enhance the toxicity of nano-CuO by increasing Cu2+ concentration, membrane permeability and internalization (Wang et al. 2011).
To understand the toxicity of the nanoparticles in the aquatic ecosystem to algae, it is essential to study their interaction with NOM, due to its common existence in the aquatic environment. Therefore, Synechococcus sp. PCC7942 was selected as the representative model for the indicative organisms in this work to study the effect of nano-Fe2O3 in the aquatic environment. In addition, the interaction of nano-Fe2O3 with a representative NOM, fulvic acid (FA) was selected for investigating the effect of its interaction with α-Fe2O3 NPs on the physiological responses of Synechococcus sp. PCC7942 (here after presented as Synechococcus).
Materials and Methods
The freshwater strain, Synechococcus sp. PCC7942, was obtained from Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. Algal cells were pre-grown in 500 ml erlenmeyer flasks containing 300 ml of BG11 medium with initial cell density of 107 cells mL−1. The cultures were grown at 28 ± 0.5°C and illuminated from two sides at a light intensity of 40 μE m−2 s−1 under a dark/light cycle of 12/12 h for 48 h. Nano ferric oxide (α-Fe2O3, CAS: 1309-37-1, purity > 99.5%), with an average particle size of 30 nm was used to prepare nanoparticle suspensions. Fresh nano Fe2O3 stock suspensions (300 mg L−1) were prepared by sonication for 60 min. Before the inoculation of algal cells, different concentrations of the sterilized suspension of test substances were added into the growth media to the final concentrations for α-Fe2O3 NPs of 0.3, 1, 3.3, 10, 30 mg L−1. Measured concentrations of Fe in water were 1.84, 6.11, 19.87, 66.12, 186.77 µM respectively. Fe concentrations in medium were performed according to ISO 6351-1985 (E), using an atomic absorption spectrometer instrument (AAnalyst 700, Perkin Elmer). Detection limits for Fe was 3 nM. The fulvic acid (CAS: 1415-93-6, purity > 90%) stock solution (FA) of 1.2 g L−1 was prepared and stirring for 24 h at 25 ± 1°C to equilibrate before use. A wide range of α-Fe2O3 NPs concentration (0.3–150 mg L−1) was tested for the impact on Synechococcus growth in our previous experiment (data not shown), among which 10 mg L−1 of α-Fe2O3 NPs was found to impose the maximum repression on cell growth of Synechococcus. Therefore, a range of 0.3–30 mg L−1 was tested in this work for understanding the impact of α-Fe2O3 NPs, and 10 mg L−1 of α-Fe2O3 NPs applied to study the effect of FA on the toxicity of α-Fe2O3 NPs. The common tested FA concentration ranges from 0.01 to 100 mg L−1 (Palomino and Stoll 2013; Ahmad et al. 2016), thus three concentrations (10, 20, 40 mg L−1) of FA were added to the medium with and without 10 mg L−1 of α-Fe2O3 NPs. The cultures without addition of α-Fe2O3 NPs and FA were set as the control groups. The cultures were exposed to α-Fe2O3 NPs and FA for 48 h before determination of physiological and biological changes. Assays were performed by triplicate.
Chlorophyll a content (Chl a) were measured spectrometrically by methanol extraction (Lichtenthaler and Wellburn 1983). The total sugar content was measured by phenol–sulfuric acid method (Kaplan et al. 1987). The protein content was determined by bicinchonininc acid method using the total protein assay kit (Nanjing Jiancheng Biology Engineering Institute, China). Algae cells of approximately 0.4 g fresh weight were resuspended in 5 ml of 0.1 M phosphate buffer (pH 7.4) and broken by sonication (Scientz Biotechnology Co. Ltd, Ningbo, China). The supernatant was collected for measurement of total soluble protein, catalase (CAT), peroxidase (POD), superoxide dismutase (SOD). The CAT and POD activities were measured by assay kits (Nanjing Jiancheng Biology Engineering Institute, China). The SOD activity was determined according to the method of Mishra (Mishra et al. 1993). The activity of SOD was defined as the quantity of SOD required to inhibit 50% the photochemical reduction of nitroblue of tetrazolium (NBT). The accuracy of measurements was as follows: 20 µg/mL for protein; CAT, POD and SOD was 0.0001, 0.0005 and 0.001 U/mL respectively.
The particle size and shape of α-Fe2O3 nanoparticles were characterized by Transmission electron microscopy (TEM, Tecnai G2 F30 S-TWIN, FEI Company, US). 10 mg L−1 of α-Fe2O3 NPs was immersed in acetone solution and sonicated for 15 min at 750 W in a ultrasonic processor. The dispersed particles were then deposited onto the lacey-carbon-coated copper grid and subsequently submitted to TEM imaging. After exposure to α-Fe2O3 NPs for 48 h, the surface of algal cells were observed by Field emission Scanning electron microscopy (SEM, Hitachi S-4800, Japan). Aliquots of 10 mL algal suspensions under treatments with 10 mg L−1 α-Fe2O3 NPs and with both α-Fe2O3 NPs (10 mg L−1) and FA (10 mg·L−1) were withdrawn and cells were harvested at 1000 rpm for 3 min. The pellets were then chemically fixed for ~ 24 h using 4% (w/v) glutaraldehyde. Then the samples were subjected to a dehydration procedure by graded series of ethanol before air-drying. Finally, the sample films were coated with gold and loaded for SEM analysis. Each sample was analyzed for at least 15 view fields at different magnifications. The sizes of NPs aggregates and their coverage area on cell surface were roughly estimated and compare between treatments.
Fourier transform infrared (FTIR) sample preparation was carried out as described by D’Souza et al. (2008). 2 mg of dry algal powder was sampled and mixed thoroughly with 2.5 mg of dry potassium bromide (KBr). The mixed powder was tableted to obtain a clear press disc before subjecting to FTIR scans. All IR spectra were recorded at 25 ± 1°C in the mid infrared range (4000–400 cm−1) by Nicolet IR200 FTIR Spectrometer (Thermo Scientific Instruments Groups, US).
All samples were evaluated in three replicates. Results from different treatments were treated using one way ANOVA with Tukey’s multiple comparisons test. The mean values and standard deviations were measured for each treatment. Differences were determined to be significance at a probability of 5% (p < 0.05). IBM SPSS Statistics 20 software was used for statistical analysis.
Results and Discussion
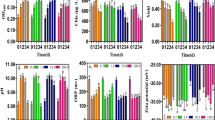
The TEM images (Fig. 1) showed the α-Fe2O3 NPs in different sizes ranging from 12 to 67 nm. Nearly all the particles exhibited spherical shape. The effects of α-Fe2O3 NPs on the cell density of Synechococcus sp. PCC7942 are showed in Fig. 2a. Compared to the control group, a slight inhibition on cell growth was observed in cultures with 10 and 30 mg L−1 of α-Fe2O3 NPs. Cultures treated with low concentration of α-Fe2O3 NPs (0.3, 1 and 3.3 mg L−1) presented similar cell density as the control group. Similar trend was observed in changes of Chl a content, with a reduction in Chl a content at 10 and 30 mg L−1 of α-Fe2O3 NPs (Fig. 2b). Significant increases in total soluble sugar (Fig. 2b), protein content (Fig. 3a) and antioxidant enzyme activities (Fig. 3b–d) were observed in cultures treated with 3.3, 10 and 30 mg L−1 of α-Fe2O3 NPs (p < 0.05) after 48 h exposure. Compared to the control group, the highest total soluble sugar and protein content was recorded at 30 mg L−1 of α-Fe2O3 NPs, which was increased by 47% and 1.35 fold respectively. Activities of CAT, POD and SOD increased with the increasing α-Fe2O3 NPs concentrations. The maximum activities of CAT, POD and SOD were obtained at 30 mg L−1 of α-Fe2O3 NPs after 48 h exposure (Fig. 3b–d).
The common toxicity mechanism of the NPs involves the generation of (reactive oxygen species) ROS, leading to oxidative damage to protein and other macromolecules, and final cellular organelles injury (Wang et al. 2011). Enhancement of CAT, POD and SOD activities is the most important way to scavenge ROS. The increase of protein content (Deng et al. 2012), sugar content (Mohamed 2008) is usually considered as an active defense mechanism to prevent algae cells from damaging by abiotic stress, via protecting against oxidative stress and scavenging the free radicals in response to nanoparticles. Our results showed that 3.3 mg L−1 of Fe2O3 NPs was able to stimulate protein, soluble sugar accumulation, as well as the antioxidant enzymes activities, while the inhibiting concentration for cell growth was 10 mg L−1, suggesting that α-Fe2O3 NPs would induce toxicity to algae when its concentration reaches 10 mg L−1 in the aquatic environment. Above results also indicated that antioxidant enzymes, along with accumulation of protein and sugar might serve as the key barrier to scavenge the burst of ROS generation induced by α-Fe2O3 NPs even at low concentration (3.3 mg L−1). However, this defense response was limited and insufficient to diminish the toxic effect induced by increasing concentration of nanoparticles (> 10 mg L−1), thus inhibition on cell growth was observed under higher concentration of α-Fe2O3 NPs (> 10 mg L−1) treatments. Under such condition, excessive ROS which was not depleted promptly would induce nanotoxicity to algae.
As shown in Fig. 4a, the addition of FA at all the tested concentrations increased the cell density of Synechococcus sp. PCC7942 treated with 10 mg L−1 of α-Fe2O3 NPs. Treatments with different concentrations of FA showed negligible differences in cell density, comparing with the normal growth cultures without nanoparticles and FA. Similarly, the Chl a content (Fig. 4b) in cultures treated with 10 mg L−1 of α-Fe2O3 NPs was restored to normal level (without α-Fe2O3 NPs) when exposed to FA. Insignificant differences in the total soluble sugar (Fig. 4b) and protein content (Fig. 5a) among untreated cultures and the cultures exposed to both α-Fe2O3 NPs and different concentration of FA were observed. Cultures with α-Fe2O3 NPs alone (10 mg L−1) produced significant higher soluble sugar, compared to the group with FA. As shown in Fig. 5b–d, the activities of CAT, POD and SOD in all the treatments with FA decreased to the same level as in the untreated group. There was no significant differences in the activities of antioxidant enzymes of the treatments among different FA concentrations.
After addition of FA, nanoparticle-treated algal cells also exhibited unrepressed growth behavior as untreated algal cells (Fig. 4a). Meanwhile, the content of biochemical component such as Chl a (Fig. 4b), total soluble sugar (Fig. 4b) and total soluble protein (Fig. 5a) returned to the basal level as the normal cultures. Therefore, FA was supposed to alleviate nanotoxicity of α-Fe2O3 NPs to Synechococcus sp. PCC7942 in this study. NOM might act as an antioxidant and react with reactive oxygen species (Fabrega et al. 2009). FA is found to inhibit ROS (O·2−, ·OH, 1O2) generation by WS2, MoS2 and CdS NPs, especially almost completely represses 1O2 generation (Maurer-Jones et al. 2013). Significant reduction in the activity of antioxidant enzymes in cultures under FA treatment was observed (Fig. 5b–d). This might be attributed to the reduction of ROS generation under FA treatments, possibly due to the direct reaction with ROS, which in turn reduced the activity of scavenging enzymes.
SEM images (Fig. 6) showed the attachment of α-Fe2O3 nanoparticles onto the cell walls of Synechococcus sp. PCC7942 after interaction. Intact and spherical cells were observed under non-treated condition (Fig. 6a). Winkled and abnormal cells can be seen clearly after incubation of algal cells in α-Fe2O3 NPs suspension (Fig. 6c). Large aggregates of nanoparticles wrapping the algal cells were observed under α-Fe2O3 NPs treatment (Fig. 6b). The aggregates of nanoparticles were smaller and entrapped less surface on the cell walls in the presence of FA (Fig. 6d, f), where cells maintained their normal spherical shapes as the in the control condition.
Scanning electron micrographs of a control, (b, c, e) Synechococcus cells exposed to 10 mg L−1 α-Fe2O3 NPs particles. d, f SEM images of algal cells treated with 10 mg L−1 of α-Fe2O3 NPs particles + FA (40 mg L−1). The red arrows indicate the α-Fe2O3 NPs aggregates adhered to the cell walls, while the yellow arrows indicate the distorted cells under α-Fe2O3 NPs treatment
The hypothetical active sites involved in the interaction between algal cells and α-Fe2O3 NPs in the presence of FA were determined by FTIR analysis. Typical bands of FTIR spectra such as –OH of carbohydrates and/or –NH of proteins (3400 cm−1), –CH, –CH3 and –CH2 stretching vibrations (2960, 2925 and 2851 cm−1) were marked on Fig. 7. Typical bands for α-Fe2O3 NPs are ranged in the region of 536–570 and 460–480 cm−1 (Demir et al. 2015), which are attributed to the Fe–O vibration. After the algal cells exposed to α-Fe2O3 NPs, a clear band at 454 cm−1 appeared, which indicates the typical peak of α-Fe2O3 nano-powders, suggesting the direct adsorption of α-Fe2O3 NPs to algal cells. Interestingly, this peak disappeared after FA treatment, implying the interaction of FA with α-Fe2O3 NPs might more or less remove surface coating formed by α-Fe2O3 NPs on algal cell wall. The transmittance at band 1384 cm−1, which is indicative of stretching vibrations of C–O or COO− groups, decreased after exposure to α-Fe2O3 NPs indicated the possible interaction with carboxyl groups on cell surface. The typical FTIR peak of algal samples at 1384 cm−1 lowered, indicating the interacted substance, FA could bind to the α-Fe2O3 NPs directly and partly replace the surface coatings of nanoparticles (Zhou et al. 2016). The peak at 1076 cm−1 is corresponded to C–O stretching vibration in carbohydrate derivatives. Zhou (2016) observed the disappearance of the 1076 cm−1 peak after the algal exopolymeric substances (EPS) coated by NPs, indicating the interaction of the direct involvement of carboxylic groups in the EPS-NPs interaction. The absorbance peak area (in Fig. 7) which was calculated by integration lowered after the exposure of nano–algal mixture to FA, suggesting the direct involvement of carboxylic groups of FA in the interaction of FA-NPs-cells. However, it’s difficult to clarify whether this interaction be dominantly FA-NPs or FA-cells based on current data. It is deduced that the involvement of carboxylic groups in the interaction of FA-NPs-cells, which might assist the desorption of NPs from algal cells.
SEM (Fig. 6) and FTIR (Fig. 7) results provided direct evidence for surface coating by nanoparticles. Repression on growth rates in algal tests may be also caused by direct adhesion of α-Fe2O3 NPs to the cell surfaces, resulting in the occurrence of shading and agglomeration, which might cause insufficient illumination in the cultures and nutrition absorption, and finally lead to slowing down cellular division and deteriorating the algal viability (Schwab et al. 2011). The pH value at the point of zero charge (pHPZC) of Fe2O3 NPs is usually around 7–8.5 (Baalousha 2009; Palomino and Stoll 2013; Ahmad et al. 2016), indicating Fe2O3 NPs is zero-valent charged or negatively charged in culture medium (Berg et al. 2009; Auffan et al. 2011). In our preliminary experiment, the pH was found to drop slightly from 7.08 to 6.77 when the FA concentration increased from 0 to 40 mg L−1 in the presence of 10 mg L−1 α-Fe2O3 NPs. Since the above pH fluctuation locates in the range of reported pHPZC of Fe2O3 NPs (7–8.5), changes in the surface charge of NPs are supposed to be insignificant under such condition. Although the pHPZC values could be shifted to lower pH in the presence of high NOM (humic acid) concentration (> 50 mg L−1) (Baalousha 2009), the Fe2O3 NPs are inferred still negatively charged in an alkaline cell growth medium. FA was found to promote stabilizing and disaggregating Fe2O3 NPs when they are neutral or negative charged at pH equal to or higher than pHPZC (Palomino and Stoll 2013). However, the adsorbed FA could promote the aggregation of α-Fe2O3 NPs when the NPs surface is positively charged at a lower pH than pHPZC (Palomino and Stoll 2013). The electrostatic force between α-Fe2O3 NPs and algal cells acts as the driven force for enhancing the adsorption of nanoparticles to algal cell walls and promote the formation of large aggregates on cell surface (Lin et al. 2015), leading to physical disruption of cell membrane or reduction reactions on cell surface that induce oxidative stress (Li et al. 2010).
SEM and FTIR results also indicate partial removal of the surface coating of NPs from cell surface and less direct contact between NPs and algal cells in the presence of FA. NOM could influence the stabilizing potency to nanoparticles (Van Hoecke et al. 2011) and induced disaggregation of α-Fe2O3 NPs (Baalousha 2009). It has been demonstrated NOM is readily adsorb to colloidal particles and forms a surface coating on colloids and NPs, which enhances their stability via electrosteric stabilization mechanism (Baalousha 2009; Chen et al. 2011). Therefore, the presence of negatively charged FA, might disaggregate the α-Fe2O3 NPs aggregates by increasing the negative charge of nanoparticles, thus enhances the electrostatic repulsion between nanoparticles and algae cells (Chen et al. 2011). This resulted in the limiting direct contact between NPs and algal cells and preventing the encapsulation of NPs on cell surface, which benefits in alleviating the shading inhibition effect on cell growth (Van Hoecke et al. 2011). In addition, limiting the adhesion of α-Fe2O3 NPs might alleviate the direct physical injury to the algal cells (Lei et al. 2016). Moreover, the cellular uptake and intracellular ROS generation is related to close contact with the nanoparticles (Li et al. 2010). Limitation in direct contact between algal cells and NPs would significantly reduce the production of intracellular ROS, as indicated by the lower antioxidant enzymes activities in the presence of FA (Fig. 5b–d), therefore mitigate the nanotoxicity of α-Fe2O3 NPs.
The aggregation and stability of Fe2O3 NPs usually depends on concentration of NPs itself, particle sizes, the time of exposure, ionic strength, concentration of natural organic matter (NOM), and pH etc (Dickson et al. 2012; Farooq et al. 2013). Ahmad (2016) reported that aggregation formed at lower Fe2O3 NPs concentration (< 30 mg L−1) but disaggregation took place with increasing Fe2O3 NPs concentration (50–200 mg L−1), possibly due to the increasing electrostatic repulsive interaction between NPs. In this study, Fe2O3 NPs tended to form aggregate at the tested concentration of 10 mg L−1. When the NPs enter the aquatic environment, the condition is far more complicated than in lab condition. Any changes in such a system with respect to pH, ionic strength, pH and types of biomacromolecules may destabilize the situation. Increasing ionic strength increases aggregation of most NPs (Keller et al. 2010; El Badawy et al. 2012). NPs are found to be stabilized and their aggregation is limited at realistic NOM concentrations (1–30 mg of carbon L−1), while flocculation occurs at higher NOM concentrations (Maurer-Jones et al. 2013). Other biomolecules in aquatic environment, such as extracellular polymeric substance (bacterial secretion containing polysaccharides and proteins), cause an increase in nanoparticle aggregation rate (Nason et al. 2012). A sharp shift of pH from neutral or alkaline to acidic environment will cause FA adsorb on positive charged NPs at a lower pH than pHPZC and promote aggregation of α-Fe2O3 NPs (Palomino and Stoll 2013), leading to cellular surface coating and inducing ROS generation. Therefore, the aggregation and stability of NPs is a integration result of multiple environmental factors. Those factors should be taken into account when analyze the realistic fate of α-Fe2O3 NPs and its interaction with FA.
In summary, high concentration of α-Fe2O3 NPs (10 and 30 mg L−1) could induce nanotoxicity to Synechococcus sp. PCC7942. Formation of large aggregates by α-Fe2O3 NPs on cell walls induced oxidative stress to cells and limited cellular light availability, resulting in repression of cell growth. A representative NOM, FA was found to effectively restore the cell growth and alleviate oxidative stress imposed by α-Fe2O3 NPs by reducing the ROS generation. The partial desorption of NPs from cell walls in the presence of FA leads to the removal of surface coating formed by NPs aggregates, avoiding direct physical injury and alleviating the inhibition of shading effect on cell growth. As a consequence, limitation of direct contact between α-Fe2O3 NPs and algal walls was proposed as the main contribution to mitigating the toxicity. FA could be used as a special detoxification substance of alleviating toxicity of α-Fe2O3 NPs, and possibly other nanoparticles.
References
Ahmad NS, Radiman S, Yaacob WZW (2016) Aggregation and stability of Fe2O3: influence of humic acid concentration, Fe2O3 concentration and pH. In: Proceeding of the 4th international conference of fundamental and applied sciences 2016 (ICFAS2016), vol 1787
Auffan M, Flahaut E, Thill A, Mouchet F, Carriere M, Gauthier L, Achouak W, Rose J, Wiesner MR, Bottero J-Y (2011) Ecotoxicology: Nanoparticle reactivity and living organisms. In: Nanoethics and nanotoxicology, Springer, Berlin, pp 325–357
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci Total Environ 407(6):2093–2101
Berg JM, Romoser A, Banerjee N, Zebda R, Sayes CM (2009) The relationship between pH and zeta potential of ~ 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 3(4):276–283
Chen J, Xiu Z, Lowry GV, Alvarez PJJ (2011) Effect of natural organic matter on toxicity and reactivity of nano-scale zero-valent iron. Water Res 45(5):1995–2001
D’Souza L, Prabha Devi DS, Naik CG (2008) Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal Chem Insights 3:135
Demir V, Ates M, Arslan Z, Camas M, Celik F, Bogatu C, Can ŞS (2015) Influence of alpha and gamma-iron oxide nanoparticles on marine microalgae species. Bull Environ Contam Toxicol 95(6):752–757
Deng X, Gao K, Sun J (2012) Physiological and biochemical responses of Synechococcus sp. PCC7942 to Irgarol 1051 and diuron. Aquat Toxicol 122–123(0):113–119
Dickson D, Liu G, Li C, Tachiev G, Cai Y (2012) Dispersion and stability of bare hematite nanoparticles: effect of dispersion tools, nanoparticle concentration, humic acid and ionic strength. Sci Total Environ 419:170–177
El Badawy AM, Scheckel KG, Suidan M, Tolaymat T, 2012. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci Total Environ 429(Supplement C):325–331
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43(19):7285–7290
Farooq W, Lee Y-C, Han J-I, Darpito CH, Choi M, Yang J-W (2013) Efficient microalgae harvesting by organo-building blocks of nanoclays. Green Chem 15(3):749–755
Ji J, Long Z, Lin D (2011) Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem Eng J 170(2–3):525–530
Kalive M, Zhang W, Chen Y, Capco D (2012) Human intestinal epithelial cells exhibit a cellular response indicating a potential toxicity upon exposure to hematite nanoparticles. Cell Biol Toxicol 28(5):343–368
Kaplan D, Christiaen D, Arad SM (1987) Chelating properties of extracellular polysaccharides from Chlorella spp. Appl Environ Microbiol 53(12):2953–2956
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967
Kim K-T, Jang M-H, Kim J-Y, Xing B, Tanguay RL, Lee B-G, Kim SD (2012) Embryonic toxicity changes of organic nanomaterials in the presence of natural organic matter. Sci Total Environ 426(0):423–429
Lei C, Zhang L, Yang K, Zhu L, Lin D (2016) Toxicity of iron-based nanoparticles to green algae: effects of particle size, crystal phase, oxidation state and environmental aging. Environ Pollut 218:505–512
Li Z, Greden K, Alvarez PJJ, Gregory KB, Lowry GV (2010) Adsorbed polymer and NOM limits adhesion and toxicity of nano scale zerovalent iron to E. coli. Environ Sci Technol 44(9):3462–3467
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lin M, Tseng YH, Huang C-P (2015) Interactions between nano-TiO2 particles and algal cells at moderate particle concentration. Front Chem Sci Eng 9(2):242–257
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanoparticles in the environment. Anal Chem 85(6):3036–3049
Mishra NP, Mishra RK, Singhal GS (1993) Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol 102(3):903–910
Mohamed Z (2008) Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology 17(6):504–516
Mohapatra M, Anand S (2010) Synthesis and applications of nano-structured iron oxide/hydroxides. Int J Eng Sci Technol 2(8):127–146
Nason JA, McDowell SA, Callahan TW (2012) Effects of natural organic matter type and concentration on the aggregation of citrate-stabilized gold nanoparticles. J Environ Monitor 14(7):1885–1892
Palomino D, Stoll S (2013) Fulvic acids concentration and pH influence on the stability of hematite nanoparticles in aquatic systems. J Nanopart Res 15(2):1428
Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L, Knauer K (2011) Are carbon nanotube effects on green algae caused by shading and agglomeration? Environ Sci Technol 45(14):6136–6144
Stumm W, Morgan JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, Hoboken
Tran N, Mir A, Mallik D et al (2010) Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomed 5:277–283
Van Hoecke K, De Schamphelaere KAC, Van der Meeren P, Smagghe G, Janssen CR (2011) Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength. Environ Pollut 159(4):970–976
Wang Z, Li J, Zhao J, Xing B (2011) Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ Sci Technol 45(14):6032–6040
Zhou K, Hu Y, Zhang L, Yang K, Lin D, 2016. The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae. Sci Rep 6
Acknowledgements
The authors are grateful for the financial support from Natural Science Foundation of Jiangsu Province (BK20140713) and China Postdoctoral Science Foundation Funded Project (No. 2015M571764), Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization and Co-Innovation Center for Jiangsu Marine Bio-Industry Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, M., Chen, Y., Yan, Y. et al. Influence of Interaction Between α-Fe2O3 Nanoparticles and Dissolved Fulvic Acid on the Physiological Responses in Synechococcus sp. PCC7942. Bull Environ Contam Toxicol 99, 719–727 (2017). https://doi.org/10.1007/s00128-017-2199-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2199-y