Abstract
Purpose of Review
Phenolic wastewaters represent a serious health and environmental problem. The remediation of phenolic wastewaters using oxidoreductase enzymes has emerged as an attractive environmentally friendly treatment method. However, the loss of enzyme activity during the treatment remains a key limitation. Thus, the aim of this article is to review and assess the recent progress in utilizing surface active additives (i.e., polymers, biopolymers, surfactants, and biosurfactants) for the reduction of enzyme inhibition and, thus, the enhancement of enzymatic remediation of phenolic wastewaters.
Recent Findings
The reported effect of polymeric and surfactant additives on the enzymatic remediation of phenolic pollutants is mixed. Some studies reported significant enhancements while others demonstrated minimal or no gains. More seriously, it has been reported that these fossil-based additives might lead to a higher toxicity of the treated wastewaters. Bio-based (biopolymers and biosurfactants) additives might address this toxicity issue; however, the bio-based additives are not always as effective as the fossil-based ones.
Summary
Despite the beneficial effect, with some exceptions, of additives, the enhancement level varies widely, probably due to the variations in the reaction environment. Thus, to draw meaningful and reliable conclusions on which additive(s) is more promising, thorough studies under unified conditions are needed. Additionally, generation of secondary pollutions associated with the fossil-based additives urges the replacement of such additives with bio-based ones. However, the effectiveness of the bio-based additives is still not sufficiently documented, stressing the need for more in-depth studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol and its derivatives are widely used in (produced from) several chemical industries such as oil refining, petrochemicals, textiles, plastic, and resin manufacturing. The release of such pollutants to the environment might lead to the contamination of soil, surface water, or/and groundwater [1, 2••]. Such pollutants are hazardous to human health since most phenolic pollutants are toxic [2••, 3,4,5]. For example, it has been reported that the exposure to phenol could cause negative health effects such as muscle fatigue, skin rashes, and diarrhea [3]. Furthermore, metabolic disorders and abnormalities in human babies have been linked to the exposure to bisphenol A (BPA), which is a phenolic derivative [6, 7]. In addition to their toxicity, some phenolic pollutants might be carcinogenic, mutagenic, or teratogenic. For instance, Slaga et al. [8] reported that isomeric phenols derived from 3,4-benzopyrene are carcinogenic. Other scientists also reported that several phenols originated from 3,4-benzopyrene are mutagenic [9]. Additionally, phenol has been reported to cause serious teratogenic effects to the embryos of Bufo arenarum [10]. Besides their impacts on human and animal health, phenolic pollutants represent a serious environmental hazard. For instance, the exposure of willow trees to wastewater containing 1000 parts-per million (ppm) phenols caused the death of these trees [11]. Moreover, phenol was also reported to negatively alter the aquatic biota such as algae and other microorganisms [12].
Most phenolic pollutants are caused by discharging phenol-containing wastewaters to land and water bodies. The concentration of these pollutants in the discharged wastewaters could range from 10 to more than 17,000 ppm, depending on the industrial discharge source [13,14,15]. Besides the potentially high levels of phenolic pollutants in industrial wastewaters, huge quantities of phenolic wastewaters are also generated annually. It has been reported that approximately 10 million tons of phenols are yearly discharged from different industrial sources to the environment [16].
Owing to the negative impacts of phenolic pollutants on public health and the ecological system, the US Environmental Protection Agency (EPA) has considered phenols as priority contaminants [17]. The permissible level of phenols in industrial wastewater effluents is set to 5 ppm when these effluents are to be discharged into a public sewage system and even lower (1 ppm) if these effluents are to be discharged into inland water bodies [18]. Thus, phenolic wastewaters have to be efficiently treated in order to reduce phenol concentrations to the permissible levels before the wastewater discharge. Additionally, if the treated wastewater is to be deemed suitable for human consumption, it should contain no more than 1 parts-per billion (ppb) phenols according to EPA [19].
There are several traditional methods for treating phenolic wastewaters including adsorption [20, 21], distillation [22, 23], and extraction [24, 25]. Moreover, membrane separation [26, 27] and advanced oxidation processes [28,29,30,31] have emerged in the past few decades as alternative techniques for treating phenolic wastewaters. Additionally, phenolic wastewater treatment using enzymes, which are biocatalysts with several industrial applications [32,33,34], has been also proposed as a possible feasible and environmentally friendly alternative [35,36,37]. Nonetheless, enzyme deactivation during the remediation process is a great obstacle for the large scale applications of enzymatic remediation of phenolic wastewaters. Such enzyme deactivation might result from the free radical attack on the enzyme molecules and/or via the formation of inhibitory polymeric products [38,39,40]. The presence of organic and/or inorganic contaminants in the enzymatically treated phenolic wastewaters might also contribute to the enzyme inhibition [41].
Different hypotheses have been proposed to explain such deactivations. For instance, it has been speculated that enzyme molecules possibly interact irreversibly with the formed intermediates (e.g., phenoxyl radicals). This interaction induces a covalent bond between the enzyme and the oxidized radicals, leading to a loss in the enzyme activity [42•]. Another proposed mechanism is the binding of the enzyme molecules to the surface of charged microaggregates, which are formed during the biocatalytic reaction. As a result, a stagnant layer is formed around these microparticles [42•], causing a potential reduction in the enzyme activity in addition to diffusional limitations of phenolic substrates to the enzyme active site. In an attempt to eliminate or reduce enzyme deactivation caused by free radical attack and/or the formation of inhibitory polymeric products, researchers have utilized surface active additives.
Thus, the aim of this article is to review the recent developments in utilizing polymeric additives for the enhancement of phenolic pollutant removal from wastewaters. The effects of different polymeric additives will be presented and assessed. The impact of the molecular weight of PEG as the key polymeric additive on the phenol removal will also be evaluated. In addition to the effects of fossil-based polymeric additives, natural polymeric (i.e., biopolymers) additives will also be addressed. Furthermore, the effects of different chemically synthesized surfactants on the enzymatic removal of phenolic pollutants from wastewaters will also be reviewed. Moreover, the utilization of biosurfactants, which are derived from sustainable and environmentally friendly sources, for the enhancement of enzymatic removal of phenols from wastewaters will be presented and their performance will be compared to that of chemical surfactants.
Polymeric Additives
Enzymatic remediation of phenolic wastewaters in the presence of polymeric additives has been reported in a number of published studies (see Table 1). The most commonly used polymeric additive is polyethylene glycol (PEG), mainly due to its low cost [40] and effectiveness at low concentrations [56]. It has been reported that the addition of PEG at a concentration of 4 g/L has reduced the required amount of horseradish peroxidase (HRP) by 200-fold [39, 57]. Phenol removal enhancement in the presence of PEG (and other polymeric additives) has been attributed to the association of PEG with the polymeric products, preventing the enzyme molecules from being removed from the reaction medium via adsorption onto the polymeric products [4]. Another possible mechanism is the prevention of free radicals formed during the biocatalytic reaction from accessing and, thus, blocking the enzyme active site [4, 39, 49, 58]. Both mechanisms would result in the presence of a higher level of active enzyme molecules in the reaction medium, leading to a higher enzymatic degradation rate of phenolic pollutants. Since the formed free radicals and/or the polymeric products might vary according to the characteristics of the enzymatically treated phenol pollutant, the level of enzyme protection and, thus the removal enhancement, might depend on the type of the phenol pollutant being treated. Additionally, the enzyme source has been also reported to impact the extent and the rate of phenolic pollutant removal [53, 54].
Diao et al. [59] have added PEG to peroxidases obtained from different plant sources (i.e., Allium sativum, Ipomoea batatas, Raphanus sativus, and Sorghum bicolor). The researchers reported that the addition of PEG has largely increased the removal efficiency of various phenolic pollutants (i.e., gallic acid, ferulic acid, 4-hydroxybenzoic acid, pyrogallol, and 1,4-tyrosol) from wastewater samples, obtained from a leather processing plant, by about 82% in the presence of 5 mM hydrogen peroxide (H2O2), which is an essential electron acceptor cofactor for effective degradation of phenolic substrates by HRP. However, even low concentrations of H2O2 can lower the enzymatic reaction rate [60] while high H2O2 concentrations could render the peroxidase enzymes totally inactive [60, 61]. Nonetheless, Diao et al. [59] did not assess the effect of H2O2 on the enzymatic activity in the presence of PEG.
Yamada et al. [49] have also utilized PEG for the enhancemnt of BPA removal from a wastewater sample in the presence of 0.3 mM H2O2. The addition of PEG (0.1 g/L) has facilitated the aggregation of the products formed during the enzymatic reaction, resulting in the preservation of the enzyme activity and thus the complete removal of BPA within 2 h of treatment. The addition of the same level of PEG (0.1 g/L) to a reaction medium containing white radish peroxidase (from Raphanus sativus) has enhanced the removal of α-naphtholic from a synthetic wastewater sample by 2.7 folds [51]. Additionally, phenol removal using a peroxidase enzyme extracted from Brassica oleracea waste has been significantly improved in the presence of PEG from 35% to over 90% [55].
The addition of PEG to laccase-catalyzed reaction media has also resulted in a positive effect. For example, the amount of laccase required to achieve over 95% removal of 2,4-dichlorophenol (2,4-DCP) reduced to half in the presence of PEG [44]. Such a trend has been also reported in a recent study where the addition of PEG to laccase, obtained from Trametes versicolor, has reduced the required enzyme amount for the removal of BPA and its derivatives by 50-fold [43]. Such a huge reduction in the enzyme amount has been attributed to the PEG-driven protection of the enzyme against the entrapment of laccase molecules within the water-insoluble oligomer precipitates [43]. In line with this assertion, Kim and Nicell [46•] proposed that water molecules bind to PEG, leading to the formation of a relatively large hydrated volume. PEG molecules have the ability to fold and, thus, entrap more water molecules. The interaction of PEG with water molecules leads to the formation of a globular PEG structure, which is responsible for minimizing enzyme deactivation [14].
The beneficial effect of PEG, however, is dependant on its molecular weight. Kimura et al. [45] have observed that the removal of BPA by laccase in the presence of PEG increased with increasing the polymer molecular weight. Another study has also reported that the extent of BPA removal by laccase increased with increasing the molecular weight of PEG up to 10,000 g/mol, above which no further gain in BPA removal was obtained [46•]. The extent of phenol removal has been also enhanced with increasing the molecular weight of PEG from 4000 to 10,000 [55]. Additionally, the level of peroxidase protection, and thus the enzyme activity, in the presence of PEG-10,000 was higher than in the presence of PEG-4000 [55]. Such enhancement of enzyme protection and phenol removal by using higher molecular weight PEG might be attributed to the more efficient formation of water-insoluble oligomers upon the interaction of high molecular weight PEG with the free radicals formed during the enzymatic degradation of phenolic pollutants [49].
Balancing the required amount of PEG with the gain obtained from increasing its molecular weight, Kim and Nicell [46•] postulated that the optimal PEG molecular weight for the laccase-catalyzed removal of BPA is 3350 g/mol. Such an optimal PEG molecular weight has been also reported for the laccase-catalyzed removal of o-cresol, where less amount of PEG-3350 was required relative to higher molecular weights PEG in order to obtain the same level of o-cresol removal at the same enzyme concentration [14]. Based on these observations, it might be postulated that the dependance of PEG effectiveness on its molecular weight is due to the varied levels of PEG-product interactions, which are functions of the nature of the formed products. Such varied levels of PEG-product interactions could lead to different extents of enzyme protection. However, further research work is still required to elucidate, on a molecular level, the relationship between PEG effectiveness and its molecular weight.
Contrarily to the reported enhancement of enzyme protection and phenol removal with increasing the PEG molecular weight, there are some studies reporting the opposite trend. For example, PEG-3350 was found to be superior to PEG-6000 when these polymers were added to a wild-type peroxidase extract and used for the removal of 2,4-DCP from wastewater samples [54]. Similar observation was also reported by Savic et al. [48] where PEG-300 was more effective than PEG-3350 in terms of HRP stability improvement and phenol removal enhancement. It must be noted that the experimental conditions of the reported studies in the relevant literature vary widely. Accordingly, the contradicting observations regarding the effects of PEG addition and its molecular weight might be due to the influence of other variables during the enzymatic degradation of phenolic pollutants. This highlights the need for more in-depth studies, which must be designed carefully in order to eliminate the contributions of any other operational factors, while assessing the effects of the polymer molecular weight.
Despite the above reported benefits of adding PEG to the enzyme-catalyzed reaction media, there are some researchers reporting minimal or even no gain from the addition of PEG, irrespective of its molecular weight. For example, Kurnik et al. [62] reported that PEG addition had no significant effect on the removal of 2,4-DCP using a peroxidase enzyme produced from potato pulp. Similarly, the addition of varied concentration (10–100 mg/L) of PEG to peroxidase-catalyzed removal of 2,4-DCP from synthetic wastewater samples did not provide any positive results [53]. The enzymatic remediations of phenol and some benzenediols from synthetic wastewater samples using a fungal laccase in the presence and the absence of 200 mg/L of PEG were not significantly different [13]. Additionally, Steevensz et al. [47•] studied the removal of phenol from synthetic and refinery wastewater samples using a fungal laccase obtained from Trametes villosa and also using SBP extracted from seed hulls. For both enzymes, the addition of PEG was not effective in the remediation of phenol from the refinery wastewater sample, while it showed a slight reduction in the SBP amount in the case of the synthetic wastewater sample [47•]. It is obvious that enzymatic remediation is a complex process. Many factors related to the characteristics of the utilized phenolic pollutant(s), the enzyme, and the formed products might play significant roles. The concentrations of the pollutant, the enzyme, and the polymeric additives are also important parameters. Additionally, the reaction environment (temperature, pH, the presence of organic/inorganic components in the reaction medium) is likely to impact the rate and the extent of phenol removal whether the degradation is carried out in the presence or the absence of polymeric additives. These factors are not unified in the above studies, contributing to the contradicting conclusions on the effect of PEG addition. Thus, it is recommended to minimize variabilities between future studies if a consistent conclusion on the effect of PEG (or any other additives) is to be drawn.
Besides studying the effect of PEG on the enzymatic remediation of phenolic wastewaters, other polymeric additives have been also investigated. For instance, Steevensz et al. [14] studied the removal of cresols by laccase in the presence of PEG, PVP, and PEI, and concluded that PVP and PEI were less effective compared to PEG. Similar observation was reported by Kim and Nicell [46•] who found that PEG was more effective than polyvinyl alcohol and Ficoll in the enhancement of BPA removal and laccase protection. The superiority of PEG might be intuitively correlated to its flexible, brush-like structure; however, further studies are needed to provide more insights into other, and probably more, influential factors.
In addition to the above commercially available polymers, some researchers have prepared polymeric materials with specific characteristics and utilized them as additives for the enhancement of enzymatic remediation of phenolic wastewaters. In this regard, polyallylamine-conjugated thermo-responsive polymer (PNIPAAm-PAA) was synthesized and its effectiveness in enhancing the enzyme-catalyzed removal of a number of phenolic pollutants from synthetic wastewater samples was investigated [50•]. This polymer has enhanced the removal of phenolic pollutants, despite that the remediation rate showed dependency on the utilized enzyme. For instance, the rate of phenolic pollutant removal from synthetic wastewater samples using HRP in the presence of PNIPAAm-PAA was faster than that using tyrosinase. Owing to the faster removal rate of the phenolic pollutants from synthetic wastewater samples using HRP-PNIPAAm-PAA system, it was utilized for the treatment of real wastewater samples. Despite the complexity of the studied real wastewater samples, almost complete removal of phenolic pollutants was achieved using HRP-PNIPAAm-PAA [50•].
Although the addition of polymers, especially PEG, might enhance the enzymatic remediation of phenolic wastewaters, the added polymers might not be (easily) biodegradable, leading to a secondary pollution. Toxicity of the added polymers is also a serious concern. For instance, it has been reported that the treated wastewater samples became more toxic with the addition of PEG [46•]. Additionally, radioactivity analysis indicated that the presence of PEG in a phenol-catalyzed reaction medium increased the quantity of soluble products along with the total organic carbon of the effluent [39], requiring a further treatment before discharge. Such additional treatment will add to the overall process cost and might overweigh the benefit gained from the PEG enhancement of phenolic pollutant removal. Another concern with the utilization of polymeric additives is the negative environmental impact of polymer manufacturing processes. Such processes are among the sources of air and water pollutions. Furthermore, polymers are derived from fossil sources, which are unsustainable and their exploration, extraction, and processing cause serious pollutions. Thus, research work on utilizing more environmentally friendly additives for the enhancement of enzymatic remediation of phenolic wastewaters is of immediate need.
Biopolymeric Additives
Owing to the reported toxicity of PEG and the unsustainable routes of PEG (and other polymers) production, few researchers have investigated the feasibility of replacing the chemical-based with bio-based polymeric additives (see Table 2). Among these researchers, Bratkovskaja et al. [42•] have studied the peroxidase-catalyzed removal of 1-naphthol, 2-naphthol, and 4-hydroxybiphenyl using two different bioploymeric additives (bovine serum albumin (BSA) and human serum albumin (HAS)) and compared the efficacy of these biopolymers with those of chemically synthesized polymers (PEG and PEI). The isoelectric points of the peroxidase (obtained from Coprinus cinereus), BSA, HAS, and PEI are, respectively, 3.5–3.8 [42•], 4.7 [65, 66], 4.7 [67], and 10.6 [68]. Since the removal of the above phenolic pollutants were carried out at pH 5.5 [42•], the net charge on the enzyme, BSA, and HAS is negative while PEI is positively charged. Accordingly, the investigators proposed that the biopolymeric additives (BSA and HAS) suppress peroxidase deactivation, most likely, due to the binding of naphthoyl radicals (which are positively charged) to the biopolymeric additives instead of the enzyme while in the case of PEI both the polymer and naphthoyl radicals bind to the enzyme, rendering it less effective [42•].
The addition of other biopolymers (i.e., 10.08 vol% dextran and 0.41 vol% sodium alginate in 64 mM sodium acetate buffer) has been also investigated and reported to be beneficial [64]. These biopolymers have improved both the activity and stability of HRP, leading to an enhanced phenol degradation [64]. Carbohydrates (i.e., 18.25 vol% galactose and 0.35 vol% guar gum in 20 mM sodium phosphate buffer) were also utilized as additives for the HRP-catalyzed removal of phenol from wastewater samples [63••]. The presence of these additives (individually) lowered the HRP dose required to achieve the same phenol degradation extent. A positive synergy was also observed, where the combination of guar gum and galactose provided higher removal of phenol as a result of more effective protection of the enzyme activity and stability in the presence of such a combination [63••]. Further studies on the enzymatic remediation of different phenolic pollutants in the presence of other biopolymers and their mixtures are urgently needed in order to gain more insights into the effectiveness of these environmentally friendly additives.
Unlike polymeric additives, no increase in toxicity of the enzymatically treated phenolic wastewater in the presence of biopolymeric additives has been reported yet in the published literature. Contrarily, the enzymatic (using HRP) treatment of a wastewater sample containing phenol in the presence of chitosan biopolymer has resulted in a decrease in the toxicity of the treated wastewater [69]. Other researchers [70] reported a similar observation upon treating wastewater samples containing phenol and chlorophenols using mushroom tyrosinase in the presence of chitosan. Although the addition of chitosan to the enzyme-catalyzed phenol removal resulted in a decrease in the toxicity of the treated wastewaters, further studies are required to confirm that this is also the case for other biopolymers.
Chemical Surfactant Additives
Chemical surfactants have been also utilized for the enhancement of enzyme-catalyzed removal of phenolic pollutants from wastewater samples (see Table 3). The enhancement of phenol removal in the presence of surfactants might stem from the entrapment (encapsulation) of some phenolic molecules within the surfactant micelle [1, 2••]. Another possible mechanism is via the formation of surfactant-pollutant insoluble complexes [58]. In the first mechanism, the concentration of the added surfactant must be at or above the critical micelle concentration (CMC) while in the second mechanism, monomeric surfactant concentrations might be sufficient. In addition to surfactant-pollutant interactions, surfactants might also interact with the enzymatic reaction products/intermediates and, thus, reduce the interaction of such components with the enzyme molecules, leading to the suppression/minimization of the enzyme deactivation. However, undesirable surfactant-enzyme interactions, leading to a partial or a complete enzyme denaturing, might be encountered in some cases, particularly for systems containing ionic surfactants [47•, 58•].
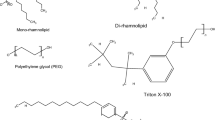
The interaction of the added surfactant with the phenolic pollutants and/or their products is likely affected by the characteristics of the surfactant molecules. Broadly, there are four classes of surfactants (anionic, cationic, zwitterionic, and nonionic). One of the most widely used surfactant for enhancing the enzyme-catalyzed removal of phenolic pollutants from wastewaters is Triton X-100, which is nonionic. For instance, Steevensz et al. [15] utilized Triton X-100 for the enhancement of phenol remedation from synthetic and real wastewater samples using SBP. The authors observed that the addition of Triton X-100 (125 to 645 mg/L) has reduced the required enzyme concentration for achieving more than 95% phenol removal from synthetic wastewater samples by more than 10-fold. The addition of Triton X-100 to real wastewater samples has also resulted in a remarkable increase in the phenol removal extent [15].
Ji et al. [38] have also utilized Triton X-100 for the enhancment of BPA removal from synthetic wastewater samples using laccase (obtained from Trametes versicolor) and reported an enhanced BPA degradation when the utilized Triton X-100 concentration was close to its CMC. However, above the CMC, the surfactant micelles entrapped some BPA molecules, shielding them from the contact with the enzyme, which has resulted in a lower BPA degradation. Despite the lower extent of BPA degradation in the micellar surfactant solutions, the enzyme stability was improved in both monomeric and micellar surfactant solutions [38]. In an effort to elucidate the mechanism of the improved enzyme stability in the presence of Triton X-100, Ji et al. [38] have conducted fluoresence studies and concluded that the interaction between the surfactant and the enzyme played a significant role in the folding and, thus, the stabilization of laccase. The binding of Triton X-100 molecules to laccase has contributed to the suppression of the enzyme deactivation caused by the free radicals and/or the polymeric reaction products [38]. In another study, Zhang et al. [1] used Triton X-100 at concentrations ranging from about 30 to 930 μM for the enhancement of phenol removal from a synthetic wastewater sample containing 50 ppm phenol using laccase, and reported that the highest phenol removal was obtained at 155 μM Triton X-100, which is below the CMC (310 μM) of this surfactant. Similar to the observation reported by Ji et al. [38], lower phenol removal extent was observed by Zhang et al. [1] in the presence of micellar Triton X-100 solutions. Such a decrease in the phenol removal in the presence of micellar Triton X-100 concentrations has been also attributed to the encapsulation of a fraction of phenol in the surfactant micelles.
In addition to Triton X-100, other surfactants have been also utilized for the enhancement of the enzymatic removal of phenolic pollutants from wastewaters. One of these surfactants is Dynol 604, which is acetylenic-based nonionic surfactant. Although the addition of Dynol 604 did not increase the initial degradation rate of phenolic pollutants (phenol, 1-naphthol, 2-naphthol, and 1-hydroxypyrene) by recombinant Coprinus cinereus peroxidase, the ultimate degradation extents of these phenolic pollutants have significantly increased [71]. For example, increasing the concentration of Dynol 604 from 1 to 10 ppm has doubled the extent of 1-naphthol removal. Additionally, no enzyme inhibition was observed in the presence of Dynol 604. Thus, the significant improvement of 1-naphthol removal upon the addition of an appropriate concentration of Dynol 604 might be correlated to the enzyme protection effect imparted by the surfactant molecules. Such a positive effect of Dynol 604 addition was also observed for the enzymatic removal of 2-naphthol [71]. However, in order to double the extent of 2-naphthol removal, Dynol 604 concentration has to be increased by 30-fold instead of 10-fold for the case of 1-naphthol. Additionally, marginal enhancement of 1-hydroxypyrene removal was observed even with increasing Dynol 604 from 20 to 70 ppm. The insignificant improvement of 1-hydroxypyrene removal with increasing Dynol 604 by almost 4-fold could, intuitively, be justified by the complexity of this phenolic pollutant. However, the
presence of Dynol 604 did not provide any enhancement for phenol removal regardless of the utilized concentration of the surfactant. Such null improvement in phenol removal with the addition of Dynol 604 suggests that the improved removal of 1-naphthol and 2-naphthol in the presence of this surfactant is not merely due to the protection of the enzyme against inhibition but rather through other (and probably more complex) mechanisms, which worth further investigations.
Another acetylenic-based nonionic surfactant that has been also proposed to boost enzyme-catalyzed removal of phenolic pollutants from wastewater is Surfynol 465. One of the proposed benefits of adding this surfactant to the enzymatically treated wastewater is the suppression of enzyme deactivation. Such benefit has been reported by Ruta and Juozas [72•] who observed that when this surfactant was added (in a dose manner) to the medium of peroxidase-catalyzed removal of 2-naphthol, the enzyme inhibition was completely eliminated. Such enzyme protection has improved the removal extent of 2-naphthol, which increased with increasing Surfynol 465 concentration. However, no further removal enhancement was observed above the surfactant CMC [72•].
Besides the above-mentioned nonionic surfactants, ionic surfactants have been also studied with the aim of revealing their potential for enhancing enzymatic removal of phenolic pollutants from wastewaters. For example, Chhaya and Gupte [73] studied the removal of BPA using laccase in the presence of reversed micelles of bis(2-ethylhexyl) sulfosuccinate sodium (AOT), which is an anionic surfactant, and reported a complete degradation of BPA within 2 h [73]. The authors also observed that the AOT reversed micellar solutions improved the stability and activity of laccase due to the effective shielding of the enzyme molecules by a water layer and a surfactant shell. The solubility of the substrate and the enzyme might also be improved in the reversed micellar solutions of AOT, allowing easier access of BPA molecules to the enzyme active site and, thus, the enhancement of BPA removal [73]. In addition to AOT, the HRP-catalyzed removal of phenol in the presence of another anionic surfactant, sodium dodecylbenzenesulfonate (SDBS), has been also enhanced [74]. For instance, adding 0.45 g of SDBS to the reaction medium (~ 50 mL) has resulted in an extensive polymerization of phenol (converted to phenylene and oxyphenylene), with more than 94% phenol conversion within 0.5 h relative to less than 5% in the absence of SDBS [74].
However, there are cases where the addition of ionic surfactants was not beneficial. For instance, the addition of sodium dodecyl sulfonate (SDS), which is an anionic surfactant with some similarities to SDBS, did not provide a significant improvement of phenol removal by laccase [2••]. Similar observation was also reported for the same system but with replacing the anionic surfactant (SDS) with the cationic surfactant, hexadecyltrimethylammonium bromide (CTAB) [2••]. These findings contradict those reported by Chhaya and Gupte [73] and Zhang et al. [1]. It is expected that phenol removal enhancment in the presence of a given surfactant is dependent on the surfactant-enzyme, surfactant-products/pollutants, and enzyme-products/pollutants interactions; these interactions might significantly vary with the variations in the reaction conditions (e.g., type of the phenolic pollutant, the utilized enzyme, medium temperature and pH, presence of salt ions or other additives/contaminants). Therefore, to draw a clear and reliable conclusion on which surfactant(s) are more effective, variations in the reaction conditions, in the presence of surfactants, have to be minimized.
Biosurfactant Additives
Despite that the addition of synthetic surfactants proved useful in some cases, these fossil-based materials are usually nonbiodegradable and might be toxic to aquatic life [75,76,77]. It was reported, for instance, that some chemical surfactants such as Triton X-100 and SDS are harmful to aquatic organisms and might pose long-term negative effects on marine creatures [72•]. To tackle the secondary pollution problems associated with the use of chemical surfactants, biosurfactants have been proposed as alternatives. Biosurfactants are biodegradable [78,79,80] and biocompatible [79, 81] and, thus, unlikely to pose environmental hazards. Additionally, biosurfactants are produced from sustainable sources and they are usually efficient even at low concentrations when compared to most chemical surfactants [78].
Biosurfactant molecules possess hydrophobic and hydrophilic moieties, making them amphiphilic compounds. The hydrophilic portion of the biosurfactant molecule can be alcohol, carboxylic acid, carbohydrate, cyclic peptide, phosphate, or amino acid while the hydrophobic moiety is based on long-chain or hydroxy fatty acids [82]. The presence of hydrophobic and hydrophilic moieties on every biosurfactant molecule promotes its self-assembly at fluid-fluid interfaces [83,84,85,86,87,88,89]. Additionally, this amphiphilic character leads to the formation of biosurfactant aggregates (i.e., micelles) in solutions when the biosurfactant concentration is equivalent or above its CMC. One of the appealing characteristics of biosurfactants is their relatively lower CMC compared to synthetic surfactants. The CMCs of biosurfactants are usually 10–40 times lower than those of common chemical surfactants [90]; lower CMC might be associated with the requirement of relatively less biosurfactant amount, which is an important economic factor. Furthermore, these bio-based surface active agents are usually effective even under extreme values of pH, temperature, and salinity [91,92,93], making them an attractive option as additives for the enhancement of enzymatic remediation of phenolic wastewaters.
Despite the attractiveness of biosurfactants, a limited number of studies have been published so far on their utilization for the enhancement of phenolic wastewater remediation using enzymes. One of these studies used rhamnolipid, which is an anionic glycolipid biosurfacatnt, and reported that the addition of this biosurfactant provided 60% enhancement of 2,4-DCP removal from wastewater samples using minced horseradish from Armoracia rusticana [94]. Interestingly, unlike chemical surfactants, micellar rhamnolipid concentration did not reduce the extent of 2,4-DCP removal [94]. In support of this observation, Liu et al. [2••] have reported more than 4-fold enhancement of phenol removal from wastewater samples using laccase in the presence of rhamnolipid biosurfactant. A higher concentration of the biosurfactant (3 times above the CMC) did not reduce the extent of phenol removal but rather a slight improvement relative to the premicellar solution was observed. Comparing this performance with those of CTAB and SDS reveals the superiority of the biosurfactant at both premicellar and micellar concentrations. These chemical surfactants were, indeed, detrimental to phenol removal regardless of their concentrations. This is in line with the statement presented by Otzen [95] that biosurfactants are less aggressive towards enzymes and, thus, they usually do not denature/destabilize the enzyme tertiary structure.
However, Ruta and Juozas [72•] reported a contradicting observation, where premicellar concentrations of rhamnolipid enhanced the removal of 2-naphthol using a peroxidase enzyme obtained from Coprinus cinereus, while micellar solutions resulted in a reduction in this phenolic pollutant remediation. Additionally, Ruta and Juozas [72•] observed that the rate of 2-naphthol removal in the presence of the biosurfactant was always lower than that in the presence of the nonionic chemical surfactant, Surfynol 465. It is unclear, however, whether such contradiction stems from the characteristics of the used biocatalyst/phenolic substrate or from other factors (e.g., experimental conditions). Regardless of the reason behind such contradiction, it is highly recommended to eliminate/minimize the operational variabilities between conducted studies in order to draw meaningful and reliable conclusions.
Besides the positive effect of bisourfactant addition (particularly at premicellar concentrations) on the enzymatic remediation of phenolic wastewaters, their positive effect on the biological utilization of phenolic substrates has been also demonstrated in some published studies. For example, Zhou et al. [80] reported that the addition of saponin and rhamnolipid to the fermentation medium of P. simplicissimum has led to a higher microbial consumption of phenol substrate and also to a higher activity of the laccase produced from the fermentation process. In another study [96], the addition of rhamnolipid to the growth medium of P. chrysosporium has improved the activity of the produced lignin peroxidase, CMCase and xylanase enzymes but inhibited the activity of manganese peroxidase. Contrarily, the addition of SDS has rendered these four enzymes almost inactive. Liu et al. [97] also studied the effect of adding rhanmolipid and Tween-80 to the fermentation medium of Trichoderma viride on the production of Avicelase, CMCase, and cellobiase enzymes. The researchers reported that despite the positive effective of both surface active agents on the activity of the produced enzymes, the biosurfactant was more effective. Similar observation was also reported by Jadhav et al. [98] who reported that the activities of lignin peroxidase and veratryl alcohol oxidase enzymes produced by Bacillus sp. VUS NCIM 5342 were improved when rhamnolipid was added to the growth medium. Such observations encourage further in-depth studies to fill in the huge gap with respect to the assessment of biosurfactants as potentially effective additives that pose no environmental hazards for enhancing the enzymatic treatment of phenolic wastewaters.
Conclusion
Polymeric additives, PEG in particular, have demonstrated a significant enhancement of the enzymatic remediation of phenolic wastewaters. However, a wide disagreement exists on which molecular weight of PEG is optimal. Additionally, some studies reported no benefit of adding PEG to the enzymatic reaction medium. Toxicity of the treated wastewater has increased in the presence of PEG, pinpointing to the need for utilizing more environmentally friendly and less/nontoxic additives such as biopolymers. Limited information, however, is available in the published literature in this regard, necessitating more research work on assessing the effectiveness of biopolymeric additives. Another alternative is chemical surfactants, which have demonstrated a remarkable effectiveness, even though not in all cases. However, as it is the case with polymeric additives, huge variations in the gained enhancement upon the addition of chemical surfactants have been found. Toxicity of chemical surfactants and their unsustainable and polluting routes of production are also of a great concern. Biosurfactants might resolve these issues; however, extensive work have to be carried out in order to get deep and clear insights into their performance and also to optimize their levels in the enzymatic reaction media.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zhang Y, Zeng Z, Zeng G, Liu X, Liu Z, Chen M, et al. Effect of Triton X-100 on the removal of aqueous phenol by laccase analyzed with a combined approach of experiments and molecular docking. Colloids Surfaces B Biointerfaces. 2012;97:7–12.
•• Liu ZF, Zeng GM, Zhong H, Yuan XZ, Fu HY, Zhou MF, et al. Effect of dirhamnolipid on the removal of phenol catalyzed by laccase in aqueous solution. World J Microbiol Biotechnol. 2012;28:175–81 This paper compares the effects of rahmnolipid, hexadecyltrimethylammonium bromide (CTAB), and sodium dodecyl sulfate (SDS) on the enzymatic degrdation rate of phenol using laccase.
Villegas LGC, Mashhadi N, Chen M, Mukherjee D, Taylor KE, Biswas N. A short review of techniques for phenol removal from wastewater. Curr Pollut Reports. 2016;2:157–67.
Kurnik K, Treder K, Skorupa-Kłaput M, Tretyn A, Tyburski J. Removal of phenol from synthetic and industrial wastewater by potato pulp peroxidases. Water Air Soil Pollut. 2015;226:254.
Liu Y, Zeng Z, Zeng G, Tang L, Pang Y, Li Z, et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour Technol. 2012;115:21–6.
vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8.
vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. JAMA. 2008;300:1353–5.
Slaga TJ, Bracken WM, Dresner S, Levin W, Yagi H, Jerina DM, et al. Skin tumor-initiating activities of the twelve isomerie phenols of benzo(a)pyrene1. Cancer Res. 1978;38:678–81.
Glatt HR, Oesch F. Phenolic benzo(a)pyrene metabolites are mutagens. Mutat Res Fundam Mol Mech Mutagen. 1976;36:379–83.
Paisio CE, Agostini E, González PS, Bertuzzi ML. Lethal and teratogenic effects of phenol on Bufo arenarum embryos. J Hazard Mater. 2009;167:64–8.
Pradeep NV, Anupama S, Navya K, Shalini HN, Idris M, Hampannavar US. Biological removal of phenol from wastewaters: a mini review. Appl Water Sci. 2015;5:105–12.
Babich H, Davis DL. Phenol: a review of environmental and health risks. Regul Toxicol Pharmacol. 1981;1:90–109.
Saha B, Taylor KE, Bewtra JK, Biswas N. Laccase-catalyzed removal of phenol and benzenediols from wastewater. J Hazard Toxic Radioact Waste. 2011;15:13–20.
Steevensz A, Al-Ansari MM, Taylor KE, Bewtra JK, Biswas N. Oxidative coupling of various aromatic phenols and anilines in water using a laccase from Trametes villosa and insights into the “PEG effect”. J Chem Technol Biotechnol. 2012;87:21–32.
Steevensz A, Madur S, Feng W, Taylor KE, Bewtra JK, Biswas N. Crude soybean hull peroxidase treatment of phenol in synthetic and real wastewater: enzyme economy enhanced by Triton X-100. Enzym Microb Technol. 2014;55:65–71.
Singh S, Mishra R, Sharma RS, Mishra V. Phenol remediation by peroxidase from an invasive mesquite: turning an environmental wound into wisdom. J Hazard Mater. 2017;334:201–11.
Chiong T, Lau SY, Khor EH, Danquah MK. Enzymatic approach to phenol removal from wastewater using peroxidases. OA Biotechnol. 2014;3:1–6.
Hussain A, Dubey SK, Kumar V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour Ind. 2015;11:81–90.
Kazemi P, Peydayesh M, Bandegi A, Mohammadi T, Bakhtiari O. Stability and extraction study of phenolic wastewater treatment by supported liquid membrane using tributyl phosphate and sesame oil as liquid membrane. Chem Eng Res Des. 2014;92:375–83.
Gao W, Fatehi P. Fly ash based adsorbent for treating bleaching effluent of kraft pulping process. Sep Purif Technol. 2018;195:60–9.
Li G, Xu Q, Jin X, Li R, Dharmarajan R, Chen Z. Enhanced adsorption and Fenton oxidation of 2,4-dichlorophenol in aqueous solution using organobentonite supported nZVI. Sep Purif Technol. 2018;197:401–6.
Crini G, Lichtfouse E. Wastewater treatment: an overview. Cham: Springer; 2018. p. 1–21.
Jaradat AQ, Gharaibeh S, Abu Irjei M. The application of solar distillation technique as a mean for olive mill wastewater management. Water Environ J. 2018;32:134–40.
González EJ, Díaz I, Gonzalez-Miquel M, Rodríguez M, Sueiras A. On the behavior of imidazolium versus pyrrolidinium ionic liquids as extractants of phenolic compounds from water: experimental and computational analysis. Sep Purif Technol. 2018;201:214–22.
Asrami MR, Saien J. Salting-out effect on extraction of phenol from aqueous solutions by [Hmim][NTf2] ionic liquid: experimental investigations and modeling. Sep Purif Technol. 2018;204:175–84.
Ouyang Z, Huang Z, Tang X, Xiong C, Tang M, Lu Y. A dually charged nanofiltration membrane by pH-responsive polydopamine for pharmaceuticals and personal care products removal. Sep Purif Technol. 2019;211:90–7.
Zhang Y, Yu W, Li R, Xu Y, Shen L, Lin H, et al. Novel conductive membranes breaking through the selectivity-permeability trade-off for Congo red removal. Sep Purif Technol. 2019;211:368–76.
Liu Z, Meng H, Zhang H, Cao J, Zhou K, Lian J. Highly efficient degradation of phenol wastewater by microwave induced H2O2-CuOx/GAC catalytic oxidation process. Sep Purif Technol. 2018;193:49–57.
Loos G, Scheers T, Van Eyck K, Van Schepdael A, Adams E, Van der Bruggen B, et al. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep Purif Technol. 2018;195:184–91.
Te LJC, Sopajaree K, Jitjanesuwan T, Lu MC. Application of visible light on copper-doped titanium dioxide catalyzing degradation of chlorophenols. Sep Purif Technol. 2018;191:233–43.
Nguyen DCT, Cho KY, Oh W-C. Mesoporous CuO-graphene coating of mesoporous TiO2 for enhanced visible-light photocatalytic activity of organic dyes. Sep Purif Technol. 2019;211:646–57.
Onaizi SA, He L, Middelberg APJ. Proteolytic cleaning of a surface-bound rubisco protein stain. Chem Eng Sci. 2009;64:3868–78.
Onaizi SA, He L, Middelberg APJ. Rapid screening of surfactant and biosurfactant surface cleaning performance. Colloids Surf B Biointerfaces. 2009;72:68–74.
Onaizi SA, He L, Middelberg APJ. The construction, fouling and enzymatic cleaning of a textile dye surface. J Colloid Interface Sci. 2010;351:203–9.
Upadhyay P, Shrivastava R, Agrawal PK. Bioprospecting and biotechnological applications of fungal laccase [Internet]. 3 Biotech. Springer; 2016. 1–12.
Wang F, Hu Y, Guo C, Huang W, Liu CZ. Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed. Bioresour Technol. 2012;110:120–4.
Mukherjee S, Basak B, Bhunia B, Dey A, Mondal B. Potential use of polyphenol oxidases (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater [Internet]. Rev Environ Sci Biotechnol. 2013;12:61–73.
Ji G, Zhang H, Huang F, Huang X. Effects of nonionic surfactant Triton X-100 on the laccase-catalyzed conversion of bisphenol A. J Environ Sci. 2009;21:1486–90.
Steevensz A, Villegas LGC, Feng W, Taylor KE, Bewtra JK, Biswas N. Soybean peroxidase for industrial wastewater treatment: a mini review. J Environ Eng Sci. 2014;9:181–6.
Feng W, Taylor KE, Biswas N, Bewtra JK. Soybean peroxidase trapped in product precipitate during phenol polymerization retains activity and may be recycled. J Chem Technol Biotechnol. 2013;88:1429–35.
Asif MB, Hai FI, Hou J, Price WE, Nghiem LD. Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi—a critical review. J Environ Manag. 2017;201:89–109.
• Bratkovskaja I, Vidziunaite R, Kulys J. Oxidation of phenolic compounds by peroxidase in the presence of soluble polymers. Biochem. 2004;69:985–92 This article reports the effects of some polymeric additives on the degradation rate of some phenolic pollutants.
Modaressi K, Taylor KE, Bewtra JK, Biswas N. Laccase-catalyzed removal of bisphenol-A from water: protective effect of PEG on enzyme activity. Water Res. 2005;39:4309–16.
Ghosh JP, Taylor KE, Bewtra JK, Biswas N. Laccase-catalyzed removal of 2,4-dimethylphenol from synthetic wastewater: effect of polyethylene glycol and dissolved oxygen. Chemosphere. 2008;71:1709–17.
Kimura Y, Takahashi A, Kashiwada A, Yamada K. Removal of bisphenol A and its derivatives from aqueous medium through laccase-catalyzed treatment enhanced by addition of polyethylene glycol. Environ Technol (United Kingdom). 2016;37:1733–44.
• Kim YJ, Nicell JA. Laccase-catalyzed oxidation of bisphenol A with the aid of additives. Process Biochem. 2006;41:1029–37 This article demonstrates the laccase protection effects of some polymeric additives during the removal of bisphenol A from wastewater samples.
• Steevensz A, Al-Ansari MM, Taylor KE, Bewtra JK, Biswas N. Comparison of soybean peroxidase with laccase in the removal of phenol from synthetic and refinery wastewater samples. J Chem Technol Biotechnol. 2009;84:761–9 This paper compares soybean with laccase in terms of phenol removal efficiency from both synthetic and real wastewater samples.
Savić SR, Stojmenović SM, Petronijević MŽ, Petronijević ŽB. Phenol removal from aqueous solutions by peroxidase extracted from horseradish. Appl Biochem Microbiol. 2014;50:214–8.
Yamada K, Ikeda N, Takano Y, Kashiwada A, Matsuda K, Hirata M. Determination of optimum process parameters for peroxidase-catalysed treatment of bisphenol A and application to the removal of bisphenol derivatives. Environ Technol. 2010;31:243–56.
• Saitoh T, Asano K, Hiraide M. Polyallylamine-conjugated thermo-responsive polymers for the rapid removal of phenolic compounds from water. React Funct Polym. 2012;72:317–22 This articles discusses the utilization of thermoresponsive polymers as additives to improve the enzymatic degradation of some phenolic pollutants.
Ashraf H, Husain Q. Removal of α-naphthol and other phenolic compounds from polluted water by white radish (Raphanus sativus) peroxidase in the presence of an additive, polyethylene glycol. Biotechnol Bioprocess Eng. 2009;14:536–42.
Chang Q, Huang J, Ding Y, Tang H. Catalytic oxidation of phenol and 2,4-dichlorophenol by using horseradish peroxidase immobilized on graphene oxide/Fe3O4. Molecules. 2016;21:1044.
González PS, Agostini E, Milrad SR. Comparison of the removal of 2,4-dichlorophenol and phenol from polluted water, by peroxidases from tomato hairy roots, and protective effect of polyethylene glycol. Chemosphere. 2008;70:982–9.
Angelini VA, Agostini E, Medina MI, González PS. Use of hairy roots extracts for 2,4-DCP removal and toxicity evaluation by Lactuca sativa test. Environ Sci Pollut Res. 2014;21:2531–9.
Deva AN, Arun C, Arthanareeswaran G, Sivashanmugam P. Extraction of peroxidase from waste Brassica oleracea used for the treatment of aqueous phenol in synthetic waste water. J Environ Chem Eng. 2014;2:1148–54.
D’Annibale A, Stazi SR, Petruccioli M. Effect of additives on enzyme-catalyzed polymerization of phenols and aromatic amines. Front Biosci (Sch Ed). 2012;(4):1249–65.
Nakamoto S, Machida N. Phenol removal from aqueous solutions by peroxidase-catalyzed reaction using additives. Water Res. 1992;26:49–54.
Torres JA, Chagas PMB, Silva MC, dos Santos CD, Corrêa AD. Evaluation of the protective effect of chemical additives in the oxidation of phenolic compounds catalysed by peroxidase. Environ Technol. 2016;37:1288–95.
Diao M, Ouédraogo N, Baba-Moussa L, Savadogo PW, N’Guessan AG, Bassolé IHN, et al. Biodepollution of wastewater containing phenolic compounds from leather industry by plant peroxidases. Biodegradation. 2011;22:389–96.
Chiong T, Lau SY, Khor EH, Danquah MK. Peroxidase extraction from jicama skin peels for phenol removal. IOP Conf Ser Earth Environ Sci. 2016;36:012048.
Ai J, Zhang W, Liao G, Xia H, Wang D. Immobilization of horseradish peroxidase enzymes on hydrous-titanium and application for phenol removal. RSC Adv. 2016;6:38117–23.
Kurnik K, Treder K, Twarużek M, Grajewski J, Tretyn A, Tyburski J. Potato pulp as the peroxidase source for 2,4-dichlorophenol removal. Waste Biomass Valoriz. 2017:1–11.
•• Kalaiarasan E, Palvannan T. Efficiency of carbohydrate additives on the stability of horseradish peroxidase (HRP): HRP-catalyzed removal of phenol and malachite green decolorization from wastewater. Clean Soil Air Water. 2015;43:846–56 This article reports the effect of biopolymeric additives (i.e., carbohydrates ) on the activity and stability of horseradish peroxidase during the enzymatic remediation of wastewater polluted with phenol and malachite green.
Kalaiarasan E, Palvannan T. Removal of phenols from acidic environment by horseradish peroxidase (HRP): aqueous thermostabilization of HRP by polysaccharide additives. J Taiwan Inst Chem Eng. 2014;45:625–34.
Li R, Wu Z, Wangb Y, Ding L, Wang Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol Reports. 2016;9:46–52.
Ge S, Kojio K, Takahara A, Kajiyama T. Bovine serum albumin adsorption onto immobilized organotrichlorosilane surface: influence of the phase separation on protein adsorption patterns. J Biomater Sci Polym Ed. 1998;9:131–50.
Vlasova IM, Saletsky AM. Study of the denaturation of human serum albumin by sodium dodecyl sulfate using the intrinsic fluorescence of albumin. J Appl Spectrosc. 2009;76:536–41.
Wang F, Liu P, Nie T, Wei H, Cui Z. Characterization of a polyamine microsphere and its adsorption for protein. Int J Mol Sci. 2013;14:17–29.
Wagner M, Nicell JA. Detoxification of phenolic solutions with horseradish peroxidase and hydrogen peroxide. Water Res. 2002;36:4041–52.
Ikehata K, Nicell JA. Color and toxicity removal following tyrosinase-catalyzed oxidation of phenols. Biotechnol Prog. 2000;16:533–40.
Kulys J, Ivanec-Goranina R. Peroxidase catalyzed phenolic compounds oxidation in presence of surfactant Dynol 604: a kinetic investigation. Enzym Microb Technol. 2009;44:368–72.
• Ruta IG, Juozas K. Effects of rhamnolipid biosurfactant JBR425 and synthetic surfactant Surfynol465 on the peroxidase-catalyzed oxidation of 2-naphthol. J Environ Sci (China). 2013;25:1431–40 This article compares the effectiveness of rhamnolipid and Surfynol 465 on the degradation rate of 2-naphthol using recombinant Coprinus cinereus peroxidase.
Chhaya U, Gupte A. Possible role of laccase from fusarium incarnatum UC-14 in bioremediation of bisphenol A using reverse micelles system. J Hazard Mater. 2013;254–255:149–56.
Zhang L, Zhao W, Ma Z, Nie G, Cui Y. Enzymatic polymerization of phenol catalyzed by horseradish peroxidase in aqueous micelle system. Eur Polym J. 2012;48:580–5.
El Zeftawy MAM, Mulligan CN. Use of rhamnolipid to remove heavy metals from wastewater by micellar-enhanced ultrafiltration (MEUF). Sep Purif Technol. 2011;77:120–7.
Yuan XZ, Meng YT, Zeng GM, Fang YY, Shi JG. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf A Physicochem Eng Asp. 2008;317:256–61.
Zouboulis AI, Matis KA, Lazaridis NK, Golyshin PN. The use of biosurfactants in flotation: application for the removal of metal ions. Miner Eng. 2003;16:1231–6.
Singh P, Jain R, Srivastava N, Borthakur A, Pal DB, Singh R, et al. Current and emerging trends in bioremediation of petrochemical waste: a review. Crit Rev Environ Sci Technol. 2017;47:155–201.
Vijayakuma S, Saravanan V. Biosurfactants-types, sources and applications. Res J Microbiol. 2015;10:181–92.
Zhou MF, Yuan XZ, Zhong H, Liu ZF, Li H, Jiang LL, et al. Effect of biosurfactants on laccase production and phenol biodegradation in solid-state fermentation. Appl Biochem Biotechnol. 2011;164:103–14.
Maikudi Usman M, Dadrasnia A, Tzin Lim K, Fahim Mahmud A, Ismail S. Application of biosurfactants in environmental biotechnology; remediation of oil and heavy metal. AIMS Bioeng. 2016;3:289–304.
Mulligan CN. Environmental applications for biosurfactants. Environ Pollut. 2005;133:183–98.
He L, Malcolm AS, Dimitrijev M, Onaizi SA, Shen HH, Holt SA, et al. Cooperative tuneable interactions between a designed peptide biosurfactant and positional isomers of SDOBS at the air-water interface. Langmuir. 2009;25:4021–6.
He L, Onaizi SA, Dimitrijev-Dwyer M, Malcolm AS, Shen H-H, Dong C, et al. Comparison of positional surfactant isomers for displacement of rubisco protein from the air–water interface. J Colloid Interface Sci. 2011;360:617–22.
Onaizi SA, Nasser MS, Twaiq FA. Micellization and interfacial behavior of a synthetic surfactant-biosurfactant mixture. Colloids Surf A Physicochem Eng Asp. 2012;415:388–93.
Onaizi SA, Nasser MS, Twaiq F. Adsorption and thermodynamics of biosurfactant, surfactin, monolayers at the air-buffered liquid interface. Colloid Polym Sci. 2014;292:1649–56.
Onaizi SA, Nasser MS, Al-Lagtah NMA. Self-assembly of a surfactin nanolayer at solid–liquid and air–liquid interfaces. Eur Biophys J. 2016;45:331–9.
Onaizi SA, Nasser MS, Al-Lagtah NMA. Benchmarking the self-assembly of surfactin biosurfactant at the liquid–air interface to those of synthetic surfactants. J Surfactant Deterg. 2016;19:645–52.
Onaizi SA. Dynamic surface tension and adsorption mechanism of surfactin biosurfactant at the air–water interface. Eur Biophys J. 2018;47:631–40.
Mehta SK, Sharma S, Mehta N, Cameotra SS. Biomimetic amphiphiles: properties and potential use. Adv Exp Med Biol. 2010;672:102–20.
Amani H, Sarrafzadeh MH, Haghighi M, Mehrnia MR. Comparative study of biosurfactant producing bacteria in MEOR applications. J Pet Sci Eng. 2010;75:209–14.
Abouseoud M, Yataghene A, Amrane A, Maachi R. Effect of pH and salinity on the emulsifying capacity and naphthalene solubility of a biosurfactant produced by Pseudomonas fluorescens. J Hazard Mater. 2010;180:131–6.
Xia W-J, Dong H-P, Yu L, Yu D-F. Comparative study of biosurfactant produced by microorganisms isolated from formation water of petroleum reservoir. Colloids Surf A Physicochem Eng Asp. 2011;392:124–30.
Tonegawa M, Dec J, Bollag J-M. Use of additives to enhance the removal of phenols from water treated with horseradish and hydrogen peroxide. J Environ Qual. 2003;32:1222.
Otzen DE. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta. 2017;1859:639–49.
Liu XL, Zeng GM, Tang L, Zhong H, Wang RY, Fu HY, Liu ZF, Huang H li, Zhang JC. Effects of dirhamnolipid and SDS on enzyme production from Phanerochaete chrysosporium in submerged fermentation. Process Biochem 2008;43:1300–1303.
Liu J, Yuan X, Zeng G, Shi J, Chen S. Effect of biosurfactant on cellulase and xylanase production by Trichoderma viride in solid substrate fermentation. Process Biochem. 2006;41:2347–51.
Jadhav M, Kalme S, Tamboli D, Govindwar S. Rhamnolipid from Pseudomonas desmolyticum NCIM-2112 and its role in the degradation of Brown 3REL. J Basic Microbiol. 2011;51:385–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Water Pollution
Rights and permissions
About this article
Cite this article
Alshabib, M., Onaizi, S.A. Effects of Surface Active Additives on the Enzymatic Treatment of Phenol and Its Derivatives: a Mini Review. Curr Pollution Rep 5, 52–65 (2019). https://doi.org/10.1007/s40726-019-00105-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-019-00105-8