Abstract
In this study, the effects of three surfactants, i.e. the anionic biosurfactant dirhamnolipid (diRL), the cationic surfactant hexadecyltrimethyl ammonium bromide (CTAB), and the anionic surfactant sodium dodecyl sulfate (SDS), on the removal of phenol catalyzed by laccase were studied first. CTAB and SDS were detrimental, while diRL improved phenol removal and was selected for detailed research. The biosurfactant increased the activity of laccase and the removal of phenol with the increase of diRL concentrations from 10.6 to 318 μM. DiRL at 318 μM improved the removal when the initial concentrations of phenol were from 50 to 400 mg/l. In particular, the removal of phenol with 318 μM diRL was 4.3–6.4 folds that of the controls within 24 h when the initial concentration of phenol was 400 mg/l. The presence of diRL at 318 μM also caused the complete removal (above 98%) of phenol at concentrations from 50 to 400 mg/l after 24 h. The enhancement of phenol removal was over a wide range of pH and temperatures, and the highest removal efficiency was obtained at pH 6.0 and 50°C. The results suggest that diRL had potential application in the enhancement of phenols removal catalyzed by laccase in water treatment or remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol-polluted waters are widely produced as wastes of several industrial and agricultural activities (Gianfreda et al. 2003; Liu et al. 2010; Qiu and Huang 2010). Phenolic compounds and their derivatives are considered as serious pollutants because even at low concentrations they are toxic, carcinogenic, mutagenic and teratogenic. If released into the environment, they may accumulate in ground water, surface water, or soil, thus representing an issue of great environmental concern (Coniglio et al. 2008; Gianfreda et al. 2003; Tang et al. 2008; Varma and Gaikwad 2010).
In recent years, an enzyme-catalyzed process has attracted more and more attentions for the treatment of aqueous phenols, mostly using laccases, peroxidases and tyrosinases (Modaressi et al. 2005). Laccase (EC 1.10.3.2) is one of the best known multicopper enzymes and catalyzes the oxidation of a variety of aromatic compounds, in particular phenolic substrates, coupled to the reduction of molecular oxygen to water (Cañas et al. 2007; Canfora et al. 2008; Qiu and Huang 2010). However, some actions, such as the interactions of phenoxyl radicals with the active center of enzymes and the adsorption of enzymes onto the polymer products of phenol formed during the reaction, can lead to the enzyme inactivation during the oxidation of phenols (Ji et al. 2009; Kulys and Ivanec-Goranina 2009; Modaressi et al. 2005; Sakurai et al. 2003). Several studies have shown that the addition of surfactants can prevent enzyme inhibitions (Ji et al. 2009; Kulys and Ivanec-Goranina 2009; Liu et al. 2008). In addition, the interactions between enzyme and surfactant may induce a change in the conformation and/or active site of the enzyme, thereby increasing the enzyme’s activity and stability (Karbassi et al. 2003; Yang et al. 2007). Previous studies also have reported that the surfactant micelles can adsorb the relative hydrophobic phenol and reduce the concentrations of free phenol in aqueous solution (Adamczak et al. 1999; Zeng et al. 2008), which may influence the removal of phenol catalyzed by enzymes. However, these investigations mainly focus on chemical additives.
Recently, biosurfactants have gained more and more attentions because of their special characteristics such as biodegradability, low toxicity and ecological compatibility. One of the most extensively studied biosurfactants is rhamnolipids (Liu et al. 2008; Liu et al. 2010; Noordman et al. 2000), of which the dirhamnolipid (2-O-α-L-rhamnopyranosyl-α-L-rhamnopyranosyl-β-hydroxyalkanoyl-β-hydroxyalkanoate, diRL) is the major component (Noordman et al. 2000). Liu et al. (2008) found that diRL has stimulative effects on the activities of carboxymethyl cellulose enzyme (CMCase), xylanase and lignin peroxidase from Phanerochaete chrysosporium in submerged fermentation. In our previous investigation, we also found that the presence of biosurfactant rhamnolipid can enhance the activity of laccase from Penicillium simplicissimum and the removal of phenol in solid-state fermentation (Zhou et al. 2011). The objective of this study was to investigate the effect of the biosurfactant diRL on phenol removal catalyzed by laccase over the reaction in various water environments. Various parameters, such as surfactant type, biosurfactant concentration, phenol concentration, pH value and temperature, will be tested. The investigation may provide useful information about the influence mechanisms of the reactions.
Materials and methods
Materials and reagents
Laccase (EC 1.10.3.2, 23.1 U/mg) produced by Trametes versicolor was from Fluka. DiRL was produced from Pseudomonas aeruginosa and extracted and purified as described by Zhong et al. (2008). Hexadecyltrimethyl ammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) (analytical grade) were from Kermal Chemicals (Tianjin, China). Phenol (purity > 99%) was obtained from Tianjin University Chemical Experimental Factory (Tianjin, China). Other reagents were of analytical grade. Ultra pure water with an initial resistivity of 18.2 MΩ/cm produced by Labconco Water Pro PS (Kansas, USA) was used throughout the experiment unless otherwise mentioned.
Britton-Robinson buffer was chosen to control the pH during the experiments since it is an effective buffer from pH 3 to 9 (Xu 1997). Stock solutions of laccase (10 mg/ml) were stored at 4°C and allowed to equilibrate to 25°C prior to use in experiments.
Experimental protocol
The batch experiments were conducted with the modified methods of Kurniawati and Nicell (2008) in 40 ml borosilicate glass vials with 5.0 ml mixture. Before reaction, the buffer containing phenol and surfactant was saturated with oxygen by vigorously stirring the mixture for 15 min. Laccase was added at last to start the reaction with the initial concentration of 0.05 mg/ml (1.155 U/ml). The vials were incubated statically in a water bath set at the desired temperature. The blank medium without the enzyme was placed under the same condition to evaluate the volatilization and spontaneous conversion of phenol. Control experiments, using medium without surfactants, were also performed. All the experiments were performed at least in triplicate, and the standard deviation was lower than 5%.
Chemical analysis
For phenol determination, samples were withdrawn from the vial and diluted (1:9, v/v) with 10% acetic acid to reduce the pH to 2, which immediately stopped enzymatic reactions in the samples (Kurniawati and Nicell 2008). The samples were then filtered through 0.45 μm membrane filter. The residual phenol contents in the medium were analyzed by high performance liquid chromatography (HPLC) using an HP1100 HPLC (Agilent Technologies, California, USA) system with a C18 column (150 mm × 4.6 mm, 5 μm, Agilent). Acetonitrile/water (70:30, v/v) was used as the mobile phase at a flow rate of 1.0 ml/min. Absorbance was measured at 280 nm, and data analysis was done using the Agilent GPC software.
The activity of laccase was measured at 420 nm using a UV–Visible spectrophotometer (model UV-2550; Shimadzu company, Tokyo, Japan) with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) as a color-generating substrate, where the rate of color formation was proportional to enzyme activity (Canfora et al. 2008). One unit of enzyme activity (U) was defined as the amount of enzyme that converted 1 μM of ABTS to ABTS+ per minute in the test system. The biosurfactant, ABTS and laccase were added in order into oxygen-saturated Britton-Robinson buffer. The enzyme activity was tested in an assay mixture containing 0.25 mg/l laccase, 2.0 mM ABTS and various concentrations of biosurfactants at pH 4.5 and 25°C.
Results and discussion
Effect of surfactant type
This investigation studied the effects of three surfactants, i.e. the anionic biosurfactant diRL, the cationic CTAB, and the anionic SDS, on the removal of phenol catalyzed by laccase. The surfactants were used at the initial concentrations of 0.5 and 3.0 critical micelle concentrations (CMCs). The CMCs of diRL, CTAB and SDS are 106, 1,000 and 8,000 μM, respectively (Yuan et al. 2007; Zhong et al. 2008). There were some dark brown precipitates formed in the reaction medium containing laccase. It was found that the precipitates increased with time. However, the color changed slightly in the blank medium without laccase. As shown in Fig. 1, diRL at both concentrations increased the removal efficiency of phenol. However, both CTAB and SDS inhibited the removal of phenol. The results indicate that the effect of surfactants on the removal of phenol was related closely to surfactant type. Previous studies also investigated the effects of additives on the removal of phenol catalyzed by enzymes. Ji et al. (2009) found that the addition of Trtion X-100 can improve the removal of bisphenol A with laccase. Tonegawa et al. (2003) reported that biosurfactant rhamnolipid, as well as PEG, Tween 20 and Trtion X-100, can enhance the removal of 2,4-dichlorophenol with minced horseradish. Sakurai et al. (2003) found that the addition of Trition X-100, Trition X-405, Tween 20 and PEG (300) have similar enhancement on phenol removal with Copirnus cinereus peroxidase, while Span, SDS, DTAB have less improvement than that of Triton X-100.
Effects of three surfactants on phenol removal. Conditions: 50 mg/l phenol, 0.05 mg/ml (1.155 U/ml) laccase and surfactant in Britton-Robinson buffer at pH 6 and 25°C after 6 h reaction. The critical micelle concentrations (CMCs) of diRL, CTAB and SDS are 106, 1,000 and 8,000 μM, respectively. The results are the means of three determinations ± standard deviation
Various diRL concentrations
DiRL improved phenol removal and was selected for detailed research. Figure 2 shows that the removal efficiency of phenol increased with the increasing concentrations of diRL from 10.6 to 318 μM. The results suggest that the effect of diRL was related closely to its concentration. Tonegawa et al. (2003) also found that the removal of phenol, 2,4-dichlorophenol, and 4-chlorophenol using horseradish increased when the concentration of PEG increased from 0 to 0.1 g/l. Several investigations reported that the removal of phenols may decrease when the concentration of additives increased further. Ji et al. (2009) reported that the conversion of bisphenol A increased with the concentration of Trition X-100 from 50 to 100 mg/l, then decreased when the concentration of Trtion X-100 exceeded 100 mg/l (about 0.67 CMC). Huang et al. (2003) also found that at lower concentration Tween 80 had a slightly synergetic effect on the VA-enhanced LiP-catalyzed oxidation of phenols, while at higher concentration the surfactant decreased the initial rate. In this study, however, diRL still increased the removal of phenol when its concentration was as high as 3.0 CMC. We postulated that these differences were mainly due to the different characteristics of these surfactants, such as the properties of micelle forms.
Effects of diRL on phenol removal and laccase activity as a function of biosurfactant concentration. Conditions for phenol removal: 50 mg/l phenol, 0.05 mg/ml (1.155 U/ml) laccase, and diRL in Britton-Robinson buffer at pH 6 and 25°C after 6 h reaction. Conditions for laccase activity: 2.0 mM ABTS, 0.25 mg/l laccase, and diRL in Britton-Robinson buffer at pH 4.5 and 25°C. The results are the means of three determinations ± standard deviation
The effect of diRL on laccase activity was also studied to further investigate the function of rhamnolipids in the phenol removal reaction. As shown in Fig. 2, the trends of the laccase activity and the removal efficiency of phenol were similar as diRL concentration increased. Especially at 3.0 CMC, diRL increased laccase activity by approximately 19%. The results indicate that diRL had positive effect on the activity of laccase, which was probably a reason for rhamnolipids to enhance phenol removal. The interactions between enzyme and surfactant may induce a change in the conformation and/or active site of the enzyme, thereby affecting the enzyme’s activity and stability and/or the enzyme-substrate binding constant (Abuin et al. 2005; Karbassi et al. 2003; Yang et al. 2007). Such interactions may involve the electrostatic interactions between the surfactant head group and the charged amino acid residues of the enzyme as well as the hydrophobic interactions between the alkyl chains of surfactants and the hydrophobic amino acid residues of the enzyme (Abuin et al. 2005; Karbassi et al. 2003; Yang et al. 2007). Previous studies also showed that the addition of surfactants can increase the activity of laccase. For example, Zhou et al. (2011) found that the addition of rhamnolipid and tea saponin can enhance the activity of laccase in solid-state fermentation. Ji et al. (2009) found that the addition of Triton X-100 causes hydrophobic amino acid residues (Tyr, Trp, etc.) of laccase to exposure to a less polar environment or bury into the inner part of the conformation of laccase, which is beneficial for stabilizing of laccase and the removal of bisphenol A.
Various phenol concentrations and time course reaction
The presence of 318 μM diRL increased the removal efficiency at various phenol concentrations during the first 24 h (Fig. 3). In addition, the higher the initial phenol concentration was, the more obvious the effect of the biosurfactant exhibited. In particular, the removal of phenol with diRL was 4.3–6.4 folds that of the controls within 24 h when the initial concentration of phenol was 400 mg/l. The removal efficiency in the controls was 89.6%, 66.7%, 31.0%, and 15.2% after 24 h when the phenol concentrations were 50, 100, 200, and 400 mg/l, respectively. However, the removal efficiency of phenol at concentrations from 50 to 400 mg/l was above 98% (complete removal) in the presence of 318 μM diRL after 24 h. The similar phenomena also occurred in previous investigations. For example, Tonegawa et al. (2003) found that the concentration of 2,4-dichlorophenol, ranging from 1 to 9 μM, had no significant effect on the removal of 2,4-dichlorophenol (90–95%) in the presence of PEG, while the removal decreased dramatically with increasing 2,4-dichlorophenol concentration in the control samples without PEG. The time course of phenol removal in the presence or absence of 318 μM diRL was also studied (Fig. 3). The removal reaction got slower in the controls as time went on. In particular, the reaction almost stopped after 10 h with 200 and 400 mg/l of phenol. However, the addition of diRL prolonged enzyme activity and increased the removal efficiency.
Effects of diRL on the removal of phenol at various concentrations. Conditions: phenol and 0.05 mg/ml (1.155 U/ml) laccase in Britton-Robinson buffer at pH 6 and 25°C in the absence or presence of 318 μM diRL. 50 mg/l phenol (a); 100 mg/l phenol (b); 200 mg/l phenol (c); and 400 mg/l phenol (d). The results are the means of three determinations ± standard deviation
The presence of 318 μM diRL was able to increase the activity of laccase (Fig. 2). However, that increment (about 19%) could not explain how diRL protected laccase and improved the removal of phenol up to 4.3–6.4 folds when the initial concentration of phenol was 400 mg/l (Fig. 3). Previous investigations reported that the adsorption of enzymes on the polymer precipitates of phenol is a reason to cause enzyme inactivation and inhibit phenols removal (Ji et al. 2009; Kulys and Ivanec-Goranina 2009; Modaressi et al. 2005; Sakurai et al. 2003). In this study, the biosurfactants may adsorb onto the hydrophobic polymers of phenol using the orientation of the hydrophobic groups of diRL (Kulys and Ivanec-Goranina 2009; Sakurai et al. 2003), thus suppressing the adsorption of enzyme to the polymer precipitates and helping reserve its activity. In addition, the adsorption of phenol into the aggregate cores of diRL may be another reason for the increased removal of phenol. Enzyme inactivation can also occur by irreversible reactions between the enzyme and phenoxyl radials formed by one electron of the phenolic substrates during the catalytic cycle (Aitken and Heck 1998; Sakurai et al. 2003). Phenol, the low-molecular polarizable compound with an aromatic ring, adsorbs initially at the micelle interface, and then moves deeper into the hydrophobic core of surfactants (Adamczak et al. 1999; Liu et al. 2010; Zeng et al. 2008). These actions may decrease the amount of free phenol, suppress its interaction with laccase and reduce the production of phenoxyl radials (Aitken and Heck 1998). Liu et al. (2010) also reported that the adsorption of phenol into the micelle cores of diRL can decrease the amount of free phenol in aqueous solution and reduce the toxicity of phenol to microorganisms, and finally improve the degradation of phenol.
pH and Temperature
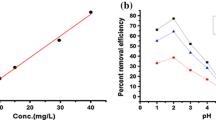
In order to be effective for practical applications, an enzyme must not only be able to exert its catalytic action on a target substrate, but also be stable under various environmental conditions. Figure 4 shows that acid environment was better for phenol removal in the absence of diRL, with the optimal performance at pH 6. The maximum removal efficiency was also obtained at pH 6 in the presence of 318 μM diRL, suggesting that the addition of the biosurfactant did not change the optimal pH for the reaction. The removal efficiency of phenol with diRL was 1.1–2.4 folds that of the controls over a wide range of pH 3–9. Sakurai et al. (2003) also tested the effect of pH on the removal of phenol catalyzed by C. cinereus peroxidase with chemical surfactants. They found that electrostatic interactions of the ionic surfactants with enzyme contribute to the enhancement of phenol removal.
As shown in Fig. 5, the optimal conversion of phenol was obtained at 50°C with or without diRL. At the temperatures below the optimal, the presence of diRL increased the removal efficiency of phenol by 17% approximately. However, when the temperature was higher than 60°C, diRL reduced the removal efficiency of phenol. The results indicate that the removal of phenol catalyzed by laccase became more sensitive to the change of temperature in the presence of diRL, which also demonstrated that diRL changed the characteristic of laccase. Yang et al. (2007) also reported that the presence of chemical surfactants, such as polyethylene glycol hexadecyl ether, sodium di-2-ethylhexylsulfosuccinate and cetyltrimethylammonium bromide, can cause the enzyme tyrosinase to be more sensitive to the change of temperature.
Effects of diRL on phenol removal under various temperature conditions. Conditions: 50 mg/l phenol and 0.05 mg/ml (1.155 U/ml) laccase in Britton-Robinson buffer at pH 6 and 25–70°C after 6 h reaction with or without 318 μM diRL. The results are the means of three determinations ± standard deviation
Conclusion
In this study, the addition of diRL enhanced the removal at various phenol concentrations. Especially when the initial concentration of phenol was 400 mg/l, the removal efficiency with 3.0 CMC diRL was 4.3–6.4 folds that of the controls during the first 24 h. The increased removal rate indicates the less cost of laccase and/or reaction time for the treatment of wastewater polluted by phenols. In addition, the presence of diRL resulted in the complete treatment (over 98%) of phenol. DiRL also enhanced the phenol removal over a wide range of pH and temperature conditions. These results suggest the potential application of diRL in the remediation of phenols catalyzed by laccase.
References
Abuin E, Lissi E, Duarte R (2005) Kinetics of N-glutaryl-l-phenylalanine p-nitroanilide hydrolysis catalyzed by α-chymotrypsin in aqueous solutions of dodecyltrimethylammonium bromide. J Colloid Interface Sci 283:539–543
Adamczak H, Materna K, Urban′ski R, Szymanowski J (1999) Ultrafiltration of micellar solutions containing phenols. J Colloid Interface Sci 218:359–368
Aitken MD, Heck PE (1998) Turnover capacity of Coprinus cinereus peroxidase for phenol and monosubstituted phenols. Biotechnol Progr 14:487–492
Cañas AI, Alcalde M, Plou F, Martínez MJ, Martínez AT, Camarero S (2007) Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol 41:2964–2971
Canfora L, Iamarino G, Rao MA, Gianfreda L (2008) Oxidative transformation of natural and synthetic phenolic mixtures by Trametes versicolor laccase. J Agric Food Chem 56:1398–1407
Coniglio MS, Busto VD, González PS, Medina MI, Milrad S, Agostini E (2008) Application of Brassica napus hairy root cultures for phenol removal from aqueous solutions. Chemosphere 72:1035–1042
Gianfreda L, Sanninoa F, Raoa MA, Bollag J-M (2003) Oxidative transformation of phenols in aqueous mixtures. Water Res 37:3205–3215
Huang X, Wang D, Liu C, Hu M, Qu Y, Gao P (2003) The roles of veratryl alcohol and nonionic surfactant in the oxidation of phenolic compounds by lignin peroxidase. Biochem Biophys Res Commun 311:491–494
Ji G, Zhang H, Huang F, Huang X (2009) Effects of nonionic surfactant Triton X-100 on the laccase-catalyzed conversion of bisphenol A. J Environ Sci 21:1486–1490
Karbassi F, Haghbeen K, Saboury AA, Ranjbar B, Moosavi-Movahedi AA (2003) Activity, structural and stability changes of mushroom tyrosinase by sodium dodecyl sulfate. Colloids Surf B 32:137–143
Kulys J, Ivanec-Goranina R (2009) Peroxidase catalyzed phenolic compounds oxidation in presence of surfactant Dynol 604: A kinetic investigation. Enzyme Microb Technol 44:368–372
Kurniawati S, Nicell JA (2008) Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour Technol 99:7825–7834
Liu Z-F, Zeng G-M, Wang J, Zhong H, Ding Y, Yuan X-Z (2010) Effects of monorhamnolipid and Tween 80 on the degradation of phenol by Candida tropicalis. Process Biochem 45:805–809
Liu X-L, Zeng G-M, Tang L, Zhong H, Wang R-Y, Fu H-Y, Liu Z-F, Huang H-L, Zhang J-C (2008) Effects of dirhamnolipid and SDS on enzyme production from Phanerochaete chrysosporium in submerged fermentation. Process Biochem 43:1300–1303
Modaressi K, Taylor KE, Bewtra JK, Biswas N (2005) Laccase-catalyzed removal of bisphenol-A from water: Protective effect of PEG on enzyme activity. Water Res 39:4309–4316
Noordman WH, Brusseau ML, Janssen DB (2000) Adsorption of a multicomponent rhamnolipid surfactant to soil. Environ Sci Technol 34:832–838
Qiu L, Huang Z (2010) The treatment of chlorophenols with laccase immobilized on sol–gel-derived silica. World J Microbiol Biotechnol 26:775–781
Sakurai A, Masuda M, Sakakibara M (2003) Effect of surfactants on phenol removal by the method of polymerization and precipitation catalysed by Coprinus cinereus peroxidise. J Chem Technol Biotechnol 78:952–958
Tang L, Zeng GM, Shen GL, Li YP, Zhang Y, Huang DL (2008) Rapid detection of picloram in agricultural field samples using a disposable immunomembrane-based electrochemical sensor. Environ Sci Technol 42:1207–1212
Tonegawa M, Dec J, Bollag JM (2003) Use of additives to enhance the removal of phenols from water treated with horseradish and hydrogen peroxide. J Environ Qual 32(4):1222–1227
Varma RJ, Gaikwad BG (2010) Continuous phenol biodegradation in a simple packed bed bioreactor of calcium alginate-immobilized Candida tropicalis (NCIM 3556). World J Microbiol Biotechnol 26:805–809
Xu F (1997) Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem 272:924–928
Yang Z, Deng J, Chen L-F (2007) Effect of ionic and non-ionic surfactants on the activity and stability of mushroom tyrosinase. J Mol Catal B: Enzym 47:79–85
Yuan XZ, Ren FY, Zeng GM, Fu Zhong H, HY Liu J, Xu XM (2007) Adsorption of surfactants on a Pseudomonas aeruginosa strain and the effect on cell surface lypohydrophilic property. Appl Microb Biotechnol 76:1189–1198
Zeng G-M, Xu K, Huang J-H, Li X, Fang Y–Y, Qu Y-H (2008) Micellar enhanced ultrafiltration of phenol in synthetic wastewater using polysulfone spiral membrane. J Membr Sci 310:149–160
Zhong H, Zeng GM, Liu JX, Xu XM, Yuan XZ, Fu HY, Huang GH, Liu ZF, Ding Y (2008) Adsorption of monorhamnolipid and dirhamnolipid on two Pseudomonas aeruginosa strains and the effect on cell surface hydrophobicity. Appl Microb Biotechnol 79:671–677
Zhou MF, Yuan XZ, Zhong H, Liu ZF, Li H, Jiang LL, Zeng GM (2011) Effect of biosurfactants on laccase production and phenol biodegradation in solid-state fermentation. Appl Biochem Biotechnol 164:103–114
Acknowledgments
The work was financially supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT0719), the National Natural Science Foundation of China (50908081, 50978088, 51039001), the Hunan Key Scientific Research Project (2009FJ1010), the Hunan Provincial Natural Science Foundation of China (10JJ7005), the Xiamen Science & Technology Planning Project Fund (3502Z20093040), the Hunan University Graduate Education Innovation Project (531107011019), and the Hunan Provincial Innovation Foundation for Postgraduate (CX2009B078, CX2009B080, CX2010B157).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, ZF., Zeng, GM., Zhong, H. et al. Effect of dirhamnolipid on the removal of phenol catalyzed by laccase in aqueous solution. World J Microbiol Biotechnol 28, 175–181 (2012). https://doi.org/10.1007/s11274-011-0806-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0806-3