Abstract
Heavy metals cannot be biodegraded and they remain in the environment until being removed. Thus, the removal of heavy metals from contaminated water is of special concern for the protection of human and aquatic lives. Studies on polymer inclusion membranes (PIMs) started more than 50 years ago and have shown outstanding separation performance of metal ions. The potential and capabilities of PIMs have made it more favorable than ion exchange and liquid-liquid extraction process. To achieve efficient transport of metal ions, different types of extractant with compatible base polymer have been successfully used along with suitable targeted metal ions. However, selectivity of metal ion is only limited to one type of metal ion based on the extractant used in PIMs. The present review describes the current literature on heavy metal removal using PIMs for the past 3 years. The compatibility of extractant with base polymer and plasticizer is discussed. Most of PIM studies used cellulose triacetate (CTA) and polyvinyl chloride (PVC) as the base polymer, and only a few studies have used other base polymers. These new base polymers have shown better PIMs in terms of stability and separation performance compared to the CTA- and PVC-based PIMs. Moreover, a new invention of dual PIM separation system has allowed simultaneous separation of multiple metal ions. Such improvement in PIM technology can speed up commercialization process and make it viable for large scale and industrial use especially in hydrometallurgy and wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial applications do not only use heavy metals in their manufacturing process but also produce heavy metals in their discharge wastewater and effluents. Without adequate treatment, these heavy metals will be released into water bodies and cause severe damage to the environment and ecosystem. Ion exchange [1], adsorption [2], and liquid-liquid extraction [3] are among methods used to separate heavy metal compounds from wastewater. However, these methods are often inefficient and costly. For example, liquid-liquid extraction has been widely used in separating heavy metals based on their relative solubility in water and organic solvents. Liquid-liquid extraction is highly risky due to toxicity and flammable nature of organic solvents, especially when they are used at industrial scale [4].
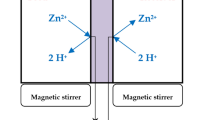
The evolution in membrane development and widely acceptance of membrane technology has developed new technology for treatment of heavy metals in wastewater. Over the last four decades, liquid membranes notably polymer inclusion membranes (PIMs) have been proven to be a better alternative than ion exchange and liquid-liquid extraction methods in extracting and recovering various metal ions [5, 6•]. This is because PIMs can simultaneously conduct extraction and back extraction at the same time unlike liquid-liquid extraction where back extraction only happens after the extraction process takes place. Although PIMs are initially used as sensing component in ion-selective electrodes (ISEs) and optodes, research on PIM-based separation especially in removing heavy metals has become very popular due to their excellent stability and versatility compared to other liquid membrane types especially supported liquid membranes (SLMs) [7••]. To date, research on PIMs for removing various heavy metals such as As(V) [8, 9], Cd(II) [10–11], Cu(II) [12], Cr(IV) [11,12,13], Pb(II) [14•, 15•], and Zn(II) [11,12,13,14,15,16] have been successful and promising.
PIM is made from base polymer, extractant (carrier), and/or plasticizer using a volatile solvent. Cellulose triacetate (CTA) or polyvinyl chloride (PVC) is commonly used as the base polymer to provide mechanical strength to the membrane while extractant is an essential component that is responsible for the ion pair complex formation in order to provide selective membrane permeability for targeted species. However, different extractants demonstrate different transport efficiencies. For example using Aliquat 336 as extractant, less than 30% of Cu(II) is transported to stripping phase using PVC-based PIMs [12]. About 70% of Cu(II) is transported to the stripping phase when D2EHPA is used as extractant in CTA-based PIM with DOP as plasticizer [17•]. Thus, selection of extractant is important in determining the performance of PIMs. The plasticizer is usually used to improve the compatibility of the PIM’s component and increase transport efficiency. A study [18] reported that certain extractants can also act as plasticizer such as D2EHPA, TOPO, Cyanex 272, Cyanex 923, and Aliquat 336, thus simplifying the PIM process.
In this paper, a systematic review is made on the current researches and developments of PIMs focusing on heavy metal removal using different types of extractants, plasticizers, and base polymers. Research challenges and future trend of PIMs are also addressed in this paper.
Extraction and Transport Studies of Heavy Metals Through PIMs
Extractants
Extractant is an important component that provides selectivity of the membrane in removing targeted species. There are many types of extractants namely basic, acidic and chelating, neutral and solvating, and macrocyclic and macromolecular extractants. Many studies have shown that without extractant, the metal ion transport in PIMs cannot occur [19]. Table 1 shows studies on different types of extractants for various target metal ions from recent years. Details of these studies are discussed further in this section.
Basic Extractants
Basic extractants are those with high molecular mass amines such as Aliquat 336, TOA, and TDPNO. Aliquat 336 has been used extensively as extractant in metal ion extraction studies, and most of them are successful either by using batch study or by using transport cell. Annane et al. [20•] studied the CTA-based polymer incorporated Aliquat 336 and NPOE for Cd(II) extraction under flow condition using microdevice system. In this system, the PIM was clamped between two Teflon blocks of donor and acceptor aqueous phase. The donor and acceptor solutions continuously flow to the inlet and outlet respectively using pump and were recycled. The potential of the microchannel was compared with the conventional device by calculating the enrichment factor (EF) of Cd(II) in acceptor phase. After 8 h in the microdevice, the EF reached 100% of Cd(II) at flow rate of 3 mL/min whereas with the conventional cell, the EF was only 80% after 8 h. Moreover, the feasibility and stability of microsystem test showed that Cd(II) can be continuous extracted and recycled up to 16 times without losing extraction efficiency.
A CTA/Aliquat 336 prepared by Yildiz et al. [21••] has shown effective extraction of Co(II) from nickel and cobalt mixture, using 2-NPPE as plasticizer and TBP as modifier. In this study, transport parameters namely plasticizer concentration, Aliquat 336 concentration, pH of acid, stirring speed, and stripping solution type were investigated. Result suggested that optimum condition for separation of Co(II) was found as follows: 27 wt% of plasticizer concentration, 25 wt% of Aliquat 336 concentration, feed solution at pH 4, 900 rpm of feed phase stirring speed, 1 M NH3 + 1 M TEA of stripping solution, 0.5 M of complexing reagent (NH4SCN), and 25 wt% of TBP. By using these optimal conditions, Co(II) was productively extracted with 100% efficiency for the equimolar feed mixtures of 100 mg/L Co(II) + 100 mg/L Ni(II) and 200 mg/L Co(II) + 200 mg/L Ni(II) within 4 to 6 h.
PIM consisting of PVC and Aliquat 336 (3.2% w/v) was used to examine the removal of Cr(VI) from acidic solutions [22••]. Formerly using similar membrane for Cr(VI) extraction, the Aliquat 336 has leached into the solution which probably oxidized by Cr(VI) itself since it exists as strong oxidizing agent especially in acidic solution. They discovered that by adding NaNO3 in the feed solution, the PIM can be stabilized, hence reduced the reduction of Cr(VI) to Cr(III) and increased the extraction of Cr(VI) into PIM. After 6 h, > 92% Cr(VI) was extracted from 10 to 100 mg/L Cr(VI) containing 0.01 mol/L NaNO3 at pH 2.
TIOA has been used by Polat et al. [23••] for the extraction of Cd(II) in synthetic Ni-Cd battery waste using PVC-based with either ONPPE or 2-NPOE as plasticizer. They suggested that ONPPE is more suitable since it results in higher flux (2.72 × 10−7 mol/sm2). Although an increase of TIOA has increased the initial mass flux of Cd(II), a further increase of TIOA (> 30.56% w/w) has decreased the flux due to the formation of extra layer between feed and PIM phase which hinders permeability of the target ions.
Acidic and Chelating Extractants
Acidic and chelating carriers involve exchange of metal ion with hydrogen ion in the extractant. These types of extractants are phosphorus and thiophosphorus acid ester, carboxylic acid, and sulfonic acid compounds. For example, D2EHPA extractant is an organophosphorus acid type. A study using CTA-based PIM incorporated with D2EHPA found that the selectivity of Cr(III) varied with D2EHPA concentrations (5–50% v/v) [24••]. Highest efficiency was observed when D2EHPA content was in the range of 30 to 50% regardless the initial concentration of Cr(III). However, above 50% of D2EHPA, the membrane was tarnished. They also suggested that the dominant transport in the membrane was “jumping” mechanism based on the activation energy obtained which coincides with study by Konczyk et al. [35]. The value indicated that transport of Cr(III) was controlled by chemical reactions occurring at interface of boundary layers.
Wang et al. [25••] conducted a study using D2EHPA and LIX84I as extractant in PVC-based PIM. Unlike the usual, this study proposed dual PIM system to extract Cu(II) and Zn(II) simultaneously from solution containing Zn(II), Cu(II), and Mg(II). The dual system comprises two stripping systems; D2EHPA-based PIM (for Zn(II) transport) and LIX 84I-based PIM (for Cu(II) transport) and one feed compartment. In order to obtain a simultaneous separation, the effects of pH have been studied as the system is highly dependent on pH of feed. The results suggested that feed solution at pH 2 was an excellent selective separation in the dual system. As pH decreased, Zn(II) and Cu(II) can be transported individually in their stripping compartment leaving Mg(II) in the raffinate. The stability test showed that Zn(II) and Cu(II) were still effectively concentrated into the different stripping solutions after four successive cycles using different feeds but the same stripping solutions. In a different study, Wang et al. [26••] also demonstrated that 100% of Cu(II) was transported from feed to receiving phase in 5 h using LIX 84I incorporated with PVDF-HFP PIM. Only a small decreased of extraction rate was observed even after the five successive Cu(II) cycles.
Selective transport of various metal ions across PVC-based PIM using β-diketone derivative (acetylatone, 3-propylacetylacetone, 3-benzylacetylacetone) and ADO as plasticizer was reported by [27••]. The transport studies were carried out in a cell using equimolar quaternary solutions of nitrates of Co(II), Ni(II), Cu(II), and Zn(II) ions, each at a concentration of 0.001 mol/dm3. The transport rate through membrane increases in the order of Zn(II) > Cu(II) > Co(II) > Ni(II) depending on different β-diketone used. It is noted that the transport experiment was conducted at 20 °C and constant feed phase at pH 7.5, using water as receiving phase. Zn(II) was more selective compared to other metal ions when PIM was doped with 3-benzylacetylacetone. But, PIM doped with 3-propylacetylacetone has the highest initial flux of Zn(II) (3.85 μmol/m2/s) and was 93% recovered after 24 h. Differences with transport metal ions are related with the formation of complexation between extractant and metal ions. Zn(II) and Cu(II) were rapidly transported across all studied membranes because they formed the most stable symmetric complexes.
Neutral and Solvating Extractants
Neutral and solvating extractants are phosphorous chemicals that have high selectivity towards actinides and lanthanides. In recent years, many researchers have used phosphonium ionic liquid (IL) for heavy metal removal. Baczynska et al. [28••] proposed Cyphos 101 and 104 for the removal of Fe(II) and Fe(III). Fe(III) removal efficiency is almost 90% with CTA-NPOE PIMs containing 30 and 50 wt% of both ILs. However, the recovery was less effective for the PIM containing Cyphos 101 (< 65%). In contrast, the removal of Fe(II) ions was poor (< 40% removal) with membrane containing Cyphos 101 and 104. According to [36], Fe(II) chloro-complexes are not stable in the aqueous phase and Fe(II) exists mainly as Fe2+ or FeCl+; thus, removal of Fe(II) is not significant. The transportation of Fe(III) across PIM was faster than Fe(II) in all studies, hence confirming the mechanism extraction reaction.
In another study, Baczynska et al. [29••] compared the transport of Zn(II), Fe(II), and Fe(III) ions across CTA-based PIM and PVDF-SLM using Cyphos 101, 104, and 167. The transport of Zn(II) and Fe(III) for both membranes containing Cyphos 101 and 167 was comparable and more efficient with > 80% of ions extracted. In all cases, the extraction of Fe(II) was not significant except with PIM containing Cyphos 104 where the extraction increased to 40%. Transport efficiency is depending on the structure of extractant. Cyphos 104 has larger structure compared to Cyphos 101 and 167, thus showing low mobility in transporting anions. In PIM system, Zn(II) is more selective and the separation increased in the following order: Cyphos 167 > Cyphos 101 > Cyphos 104. However, SLM containing Cyphos 101 has better stability compared to the PIM with the same type of extractant. The extraction and recovery of Zn(II) were maintain the same after three cycles using the same membrane whereas a decrease of 40% was observed in PIM.
Pospiech [30•] studied the transport selectivity between Cd(II) and Cu(II) from chloride solutions using CTA PIM containing ONPOE as plasticizer. Cd(II) and Cu(II) are known to readily form anionic chloride complexes at high chloride concentration; thus, it is difficult to determine the selectivity. Cyphos 101 and 104 was chosen as extractant where the former contained hydrophilic chloride anion and the latter contained phosphinate anion. It was assumed that the difference in anions will affect extraction properties. However, different anion structures in extractant did not change the selectivity of Cd(II) over Cu(II). Their findings indicated that Cd(II) has higher selectivity over Cu(II) in 0.1 mol/dm3 of HCl using Cyphos 104 as extractant with 86% of Cd(II) recovered in 1 mol/dm3 of H2SO4 after 24 h. The initial fluxes of Cd(II) increased from 18.8 to 25.3 μmol m−2 s−1, but the separation coefficient decreased from 28.1 at 0.1 mol/dm3 with the increase of HCl concentration from 0.1 to 2 mol/dm3 in the source phase.
Several studies have demonstrated the separation of actinide ions from acidic solutions using TODGA [31•], C4DGA [32••], and T2EHDGA [33•] as extractant. A CTA-TODGA PIM with NPOE as plasticizer was developed and characterized for the separation of Am3+, Pu4+, UO22+, and Th4+ from 1 M HNO3 feed solution in a batch and transport study [31•]. From the batch study, Am(III) removal increased with an increase of TODGA from 26 to 92% after 2 h when TODGA concentration increased from 15 to 58%. Although both studies show similar trend of removal in the order of Am(III) > Pu(IV) > Th(IV) > U(VI), the transport study has significantly lower extraction than batch study suggesting that large amount of metal ions held up in the membrane. In both cases, the removal of U(VI) was very poor (< 20%).
In case of a C4DGA-based PIM, a similar trend of actinide ion uptake was observed [32••] in batch extraction study whereas in transport study, the order was Pu(IV) > Am(III) > Th(IV). C4DGA was synthesized from a derivative of diglycolamides (DGA) where four DGA units were restructured to the lower rim of the calix[4]arene skeleton as increasing the complexation ability of tri- and tetravalent actinides and also imitating the effectiveness of TODGA. Unlike Pu(IV) and Th(IV), Am(III) was strongly bound to the ligand. Thus, the stripping process was difficult causing lower uptake of Am(III). However, further study is needed to improve the extraction kinetics and recovery of actinide ions as well as expand lifetime of the membrane.
T2EHDGA was used as extractant for the transport of actinide ions in 1 M nitric acid to a receiver compartment containing 1 M AHIBA [33•]. The transport trend was similar with PIM containing C4DGA extractant which was Pu(IV) > Am(III) > Th(IV) > U(IV). The extraction of metal ions in feed phase was much faster than that of the transport ions in the receiving phase, suggesting a higher viscosity and diffusional mass transfer of PIMs containing 14% CTA, 17% NPOE, and 69% T2EHDGA. However, the stability of T2EHDGA-containing PIMs was poor and slowly declines after 2 days and factors responsible are not understood.
Macrocyclic and Macromolecular
Calixarenes, crown ethers, calix crowns, and cyclodextrine are some of the extractants fall in this category. These types of extractant are popular among PIM’s researchers due to their excellent separation selectivity and can form stable complexes with cations, anions, and neutral molecules. A calixarene-based PIM was developed by [34••] for the removal of Cr(VI) from chrome plating bath water. As the calixarene concentration increased from 0.1 to 0.2 M, the transport of Cr(IV) in 0.1 M HCl to acetic acid/ammonium acetate buffer at pH 5 increased from about 20 to 97%. However, a further increase of extractant (0.2 to 0.4 M) has decreased the Cr(IV) transport. Authors also investigate different plasticizers and temperatures on transport removal. Although 2-NPPE showed excellent transport permeability, 2-NPOE was chosen due to comparable kinetic result and cheaper.
Plasticizer
In PIMs, plasticizer is used to improve rigidity of membrane structure by penetrating in between polar group of base polymer and neutralizing them with its own polar group. As a result, the softness and flexibility of the PIMs as well as the metal ion flux will increase. However, the amount of plasticizer needed is depending on the type of plasticizer and the base polymer. This is because a larger concentration of plasticizer can reduce the transport function [21••].
From Table 1, 2-NPOE is the most commonly used plasticizer because of the physicochemical nature which is high dielectric constant and low viscosity. The viscosity and dielectric constant are important factors that governing the stability of metal ion complexes formed in the membrane phase. Ion pairs separate efficiently in high dielectric constant media and influence the balance between the efficiencies of association and dissociation of the metal ion complex from feed to receiving phase [37]. However, a study by Kaya et al. [34••] on the effect of seven plasticizers namely 2-NPPE, 2-NPOE, T2EHP, TBEP, DOA, DOPT, and Bis (2-ethylhexyl) for the removal of Cr(IV) in plating bath water reported that 2-NPPE was the best plasticizer. This was expected because 2-NPPE has the lowest viscosity and high dielectric constant compare to other plasticizer studied. 2-NPPE has achieved the best transport efficiency with recovery of 98%. But, 2-NPOE is more preferred due to lower cost. On top of that, the kinetic result of 2-NPOE was comparable with 2-NPPE. In a different study conducted by Baba et al. [38••], DOP was more suitable plasticizer compared to 2-NPOE in CTA-based PIM using D2EHGA as extractant for Co(II) and Mn(II) separation. This is due to oily surface exhibited by PIM with 2-NPOE. In addition, DOP was less expensive than 2-NPOE.
For many years, Aliquat 336 and a few other extractants described by [26] were assumed to act as plasticizer as it softens the PIMs. However, a study by Abdul Halim et al. [39•] who investigated the characterization of thermal transition in PVC PIMs indicates that Aliquat 336 does not achieve the plasticization by forming a solid solution with PVC and depressing its glass transition (Tg). The mechanism of plasticization may be the formation of a sponge-like structure of PVC containing Aliquat 336 in the sponge pores. Moreover, the Tg of Aliquat did not decrease to below room temperature and is independent of Aliquat 336 content. However, it is undeniable that an increase of Aliquat 336 concentration in PVC-based PIMs has produced transparent membrane and increased the flexibility.
Base Polymer
To date, most of PIM’s researches still use PVC and CTA as a base polymer because of their thermoplastic properties that can easily dissolved in organic solvent due to no cross-linking between polymer strands. Thus, they provide a good mechanical strength to the PIMs. Although PVC-based PIMs are proved to be more stable than CTA, after 20 days, the PIMs swelled [31•]. Previously, various cellulose polymer derivatives including CTA, CAP, CAB, and CTB have been used to increase the durability of PIMs [40]. However, the membrane permeability was found to decrease proportionally and ruined quickly under caustic condition. An attempt of using PVDF and its derivatives as base polymer was also made, but the solubility in common solvent such as THF was low [36, 37].
In the present study, O’Bryan et al. [41••] has employed PVDF-HFP incorporated with Aliquat 336 for the removal of thiocyanate. With 30 wt% Aliquat 336, the membrane exhibited significantly higher extraction and back extraction rates for SCN− and long-term stability compared to the PVC-based PIM with similar Aliquat 336 concentration. A comparison study of CTA, PVC, and PVDF-HFP base polymer was conducted by [26••] for the separation and transport of Cu(II) from ammonium sulfate/ammonia solutions using LIX841. The optimal composition was first studied by varying composition of the based polymers and NPOE using fixed amount of extractant (30 wt%), and the best concentration obtained for CTA, PVC, and PVDF-HFP was 45, 50, and 45% respectively. The results revealed that PVDF-HFP has better extraction and transport efficiency. After 1 h, the membrane has reached equilibrium unlike CTA- and PVC-based PIM which does not reach equilibrium even after 8 h. Even though, PVDF-HFP PIMs appeared slightly opaque after fabrication, it still remained homogenous and stable after used in five successive transport experiments. In fact, 100% of Cu(II) was successfully transported in receiving phase in 5 h with only a small decrease in extraction rate.
A comparison of PVDF-HFP-based PIM and PVC-based PIM was also made by [42••] using Cyphos 101 as extractant for the extraction of vanadium(V). The results revealed that PVDF-HFP PIM rejected vanadium(V) better than PVC PIM. In fact, the PVDF-HFP PIM remained stable even after five extraction and stripping cycles without deterioration in performance. This is due to flexibility chain of PVDF-HFP that leads to less diffusive resistance towards extractant used. Besides, other studies also have successfully produced different types of stable membranes using PVDF-HFP polymer [43•].
Recently, a few studies have conducted on modifying the base polymer and using different polymer materials for PIMs. Kaya et al. [44••] has modified the CTA-based PIM with an addition of 1 wt% of reduced graphene oxide (rGO) and compared their performance with unmodified PIM. In this work, 0.2 M calix[4]arene with chromate anions was used as extractant in both membranes. Results showed that rGO/PIM has successfully improved the kinetic and recovery rate of Cr(IV) in the feed phase containing 2 × 10−4 M K2Cr2O7 in 0.1 M HCl and a buffer solution of acetic acid and ammonium acetate (pH 5) in receiving phase. After ten cycles, the modified PIM was still stable and reproducible due to its hydrophobicity.
Conclusion and Future Trend
Although applications of PIMs have been expanding lately in many other areas including on chemical analysis and analytical applications, research on heavy metal removals using PIMs is still of interest. In this review, current works on PIMs for various metal ions have been highlighted.
Base polymer, extractant, and plasticizer are the three important components in PIMs. Thus, choosing the right component will provide excellent selectivity and permeability of the PIM’s performance. It is noted that PVC and CTA are still popular base polymer used in PIMs. However, in the future, it is expected that the trend will changed as new polymers are discovered such as PVDF-HFP and rGO/CTA. These new base polymers have shown outstanding performance and stability compared to PVC and CTA. As for extractant, it is very crucial as it determines the selectivity of metal ions. Aliquat 336 and D2EHPA are the most popular extractants because of their plasticizing effects, but they are only suitable for certain targeted ions. In order to achieve high selectivity, the concentration of most commercial extractants must reached at least 30 wt%. Thus, finding an extractant that used less amount of concentration but giving similar performance will be a great advantage.
Lastly, the transport system for PIM is important to allow the separation process between targeted ions. Recent years have successfully developed dual PIM system. This system has successfully transported two metal ions into their individual stripping compartment by leaving the third metal in the feed phase. This work demonstrates the versatility and capability of PIMs for industrial applications. Therefore, more researches on enhancing this system are expected in the near future.
Abbreviations
- 2-NPOE:
-
2-Nitrophenyl octyl ether
- 2-NPPE:
-
2-Nitrophenyl pentyl ether
- ADO:
-
Dioctyl adipate
- Aliquat 336:
-
Trioctylmethylammonium chloride
- Am:
-
Americium
- As:
-
Arsenic
- C4DGA:
-
Diglycolamide-functionalized calix[4]arene
- CAB:
-
Cellulose acetate butyrate
- CAP:
-
Cellulose acetate phthalate
- Cd:
-
Cadmium
- Cr:
-
Chromium
- CTA:
-
Cellulose triacetate
- CTB:
-
Cellulose tribenzoate
- Cu:
-
Copper
- Cyphos 101:
-
Trihexyl(tetradecyl)phosphonium chloride
- Cyphos 104:
-
Trihexyl(tetradecyl)phosphonium bromide
- Cyphos 167:
-
Tributyl(tetradecyl)phosphonium chloride
- D2EHPA:
-
Di(2-ethylhexyl) phosphoric acid
- DOP:
-
Dioctyl phthalate
- DOTP:
-
Dioctyl terephthalate
- Fe:
-
Iron
- FeCl:
-
Iron chloride
- HCl:
-
Hydrochloric
- HNO3 :
-
Nitric acid
- ISEs:
-
Ion selective electrodes
- LIX84I:
-
2-Hydroxy-5-nonylacetopnone oxime
- Mg:
-
Magnesium
- Mn:
-
Manganese
- NaNO3 :
-
Sodium nitrate
- Ni:
-
Nickel
- NPOE:
-
Nitrophenyl octyl ether
- NPPE:
-
Nitrophenyl phentyl ether
- ONPOE:
-
1-Nitro-2-octoxybenzene
- ONPPE:
-
o-Nitrophenyl pentyl ether
- Pb:
-
Lead
- PIMs:
-
Polymer inclusion membranes
- Pu:
-
Plutonium
- PVC:
-
Polyvinyl chloride
- PVDF:
-
Polyvinyl difluoride
- PVDF-HFP:
-
Oly(vinylidene) fluoride-co-hexafluoropropene
- rGO:
-
Reduced graphene oxide
- SCN-:
-
Thiocyanate
- SLMs:
-
Supported liquid membranes
- T2EHDGA:
-
N,N,N′,N′-Tetra-2-ethylhexyl diglycolamide
- T2EHP:
-
Tri-(2-ethylhexyl) phosphate
- TBEP:
-
Tris(2-butoxyethyl) phosphate
- TBEP:
-
Tris-(2-butoxyethyl) phosphate
- TDPNO:
-
4-(10-n-tridecyl)pyridine N-oxide
- TEHP:
-
Tris-(ethylhexyl) phosphate
- Th:
-
Hexanuclear
- THF:
-
Tetrahydrofuran
- TIOA:
-
Triioctylamine
- TOA:
-
Trioctylamine
- TODGA:
-
N,N,N′,N′-Tetra-n-octyl diglycolamide
- U:
-
Uranium
- Zn:
-
Zinc
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Da̧browski A, Hubicki Z, Podkościelny P, Robens E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere. 2004;56(2):91–106.
Verbych S, Bryk M, Chornokur G, Fuhr B. Removal of copper (II) from aqueous solutions by chitosan adsorption. Sep Sci Technol. 2005;40(8):1749–59.
Belkhouche NE, Amine Didi M, Villemin D. Separation of nickel and copper by solvent extraction using di-2 ethylhexylphosphoric acid-based synergistic mixture. Solvent Extraction and Ion Exchange. 2005;23(5):677–93.
Benosmane N, Hamdi SM, Hamdi M, Boutemeur B. Selective transport of metal ions across polymer inclusion membranes (PIMs) containing calix [4] resorcinarenes. Sep Purif Technol. 2009;65(2):211–9.
Nghiem LD, Mornane P, Potter ID, Perera JM, Cattrall RW, Kolev SD. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J Membr Sci. 2006;281(1–2):7–41.
• Almeida MI, Cattrall RW, Kolev SD. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J Membr Sci. 2012;415:9–23. This article shows trends in metal ions using PIMs based on specific component in PIMs.
• Almeida MI, Cattrall RW, Kolev SD. Polymer inclusion membranes (PIMs) in chemical analysis—a review. Analytica Chimica Acta. 2017;987:1–4. This article shows the analytical chemistry applications of PIMs by addressing the challenging chemical analysis problem .
Bey S, Criscuoli A, Figoli A, Leopold A, Simone S, Benamor M, et al. Removal of As (V) by PVDF hollow fibers membrane contactors using Aliquat-336 as extractant. Desalination. 2010;264(3):193–200.
Ballinas MD, Rodríguez de San Miguel E, Rodríguez MT, Silva O, Muñoz M, de Gyves J. Arsenic (V) removal with polymer inclusion membranes from sulfuric acid media using DBBP as carrier. Environmental Science & Technology. 2004;38(3):886–91.
Wang L. Extraction study of cadmium (II) and copper (II) using Aliquat 336 Doctoral dissertation. Victoria University of Technology; 1998.
Kozlowski CA, Walkowiak W. Transport of Cr (VI), Zn (II), and Cd (II) ions across polymer inclusion membranes with tridecyl (pyridine) oxide and Tri-n-octylamine. Sep Sci Technol. 2004;39(13):3127–41.
Wang L, Paimin R, Cattrall RW, Shen W, Kolev SD. The extraction of cadmium (II) and copper (II) from hydrochloric acid solutions using an Aliquat 336/PVC membrane. J Membr Sci. 2000;176(1):105–11.
Kozlowski CA, Walkowiak W. Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res. 2002;36(19):4870–6.
• Gherasim CV, Bourceanu G, Olariu RI, Arsene C. Removal of lead (II) from aqueous solutions by a polyvinyl-chloride inclusion membrane without added plasticizer. Journal of Membrane Science. 2011;377(1–2):167–74. This research addresses the usage of PVC and D2EHPA PIMs without using plasticizer in extraction study .
• Gherasim CV, Bourceanu G, Timpu D. Experimental and modeling studies of lead (II) sorption onto a polyvinyl-chloride inclusion membrane. Chemical Engineering Journal. 2011;172(2-3):817–27. This study shows the study of lead (II) sorption through PVC-based PIM model .
• Yilmaz A, Arslan G, Tor A, Akin I. Selectively facilitated transport of Zn (II) through a novel polymer inclusion membrane containing Cyanex 272 as a carrier reagent. Desalination. 2011;277(1–3):301–7. This paper addresses the transport of Zinc(II) through PIMs that used Cyanex 272 as carrier .
• Kavitha N, Palanivelu K. Recovery of copper (II) through polymer inclusion membrane with di (2-ethylhexyl) phosphoric acid as carrier from e-waste. Journal of Membrane Science. 2012;415:663–9. This research showed the permeation of copper ions through PIMs that contain D2EHPA as carrier, CTA as base polymer, and DOP as plasticizer .
Pereira N, St John A, Cattrall RW, Perera JM, Kolev SD. Influence of the composition of polymer inclusion membranes on their homogeneity and flexibility. Desalination. 2009;236(1–3):327–33.
Luo X, He D, Ma M. Simultaneous transport and separation of Cu (II) and Zn (II) in Cu-Zn-Co sulfate solution by double strip dispersion hybrid liquid membrane (SDHLM). Sep Sci Technol. 2010;45(14):2130–40.
• Annane K, Sahmoune A, Montels P, Tingry S. Polymer inclusion membrane extraction of cadmium (II) with Aliquat 336 in micro-channel cell. Chemical Engineering Research and Design. 2015;94:605–10. This paper showed the transport of cadmium through PIMs in microsystem .
•• Yıldız Y, Manzak A, Tutkun O. Selective extraction of cobalt ions through polymer inclusion membrane containing Aliquat 336 as a carrier. Desalination and Water Treatment. 2016;57(10):4616–23. This paper reports to have selective separation of Co(II) using CTA incorporated with Aliquat 336 PIMs .
•• Kagaya S, Maeno T, Ito K, Gemmei-Ide M, Cattrall RW, Kolev SD. Improvement of chromium (VI) extraction from acidic solutions using a poly (vinyl chloride)-based polymer inclusion membrane with Aliquat 336 as the carrier. Analytical Sciences. 2017;33(5):643–6. This research showed the improvement of anionic Cr(VI) of NaNO 3 by using PIM PVC with Aliquat 336 as carrier .
•• Polat C, Eyüpoğlu V, Sara ON. The novel approach to Cd (II) extraction by polymer inclusion membrane using TIOA as carrier. InAIP Conference Proceedings 2016;Vol. 1726, No. 1, p. 020110. AIP Publishing. This paper reported to have extraction of Cd(II) by using PIMs containing TIOA as carrier and PVC as base polymer .
•• Rajewski J, Łobodzin P. Abexperimental analysis of the transport mechanism of chromium (III) ions in the polymer inclusion membrane system stract. Problemy Eksploatacji. 2016. This paper explains well about “jumping mechanism” of chromium(II) in PIMs .
•• Wang D, Hu J, Liu D, Chen Q, Li J. Selective transport and simultaneous separation of Cu (II), Zn (II) and Mg (II) using a dual polymer inclusion membrane system. Journal of Membrane Science. 2017;524:205–13. This research proposed the technique of dual systems in removal of Cu(II), Zn(II), and Mg(II) simultaneously by using PIMs .
•• Wang D, Cattrall RW, Li J, Almeida MI, Stevens GW, Kolev SD. A poly (vinylidene fluoride-co-hexafluoropropylene)(PVDF-HFP)-based polymer inclusion membrane (PIM) containing LIX84I for the extraction and transport of Cu (II) from its ammonium sulfate/ammonia solutions. Journal of Membrane Science. 2017;542:272–9. This research addresses the usage of PIMs containing PVC, CTA, or PVDF-HFP as base polymer, LIX 841 as carrier, and NPOE as plasticizer for extraction of Cu(II) .
•• Witt K, Radzyminska-Lenarcik E, Urbaniak W. Selective transport of zinc ions through novel polymer inclusion membranes (PIMS) containing β-diketone derivatives as carrier reagents. Separation Science and Technology. 2016;51(15–16):2620–7. This paper carried out research about PIMs doped with β-diketone derivatives (acetylacetone (1), 3-propyl-acetylacetone (2), and 3-benzyl-acetylacetone (3)) for the transport of Zn(II), Cu(II), Co(II), and Ni(II) .
•• Baczynska M, Rzelewska M, Regel-Rosocka M, Wisniewski M. Transport of iron ions from chloride solutions using cellulose triacetate matrix inclusion membranes with an ionic liquid carrier. Chemical Papers. 2016;70(2):172–9. This study showed the transport of Fe(II) and Fe(III) through PIM base CTA, Cypos IL 101, and Cypos IL 104 as carrier and NPOE as plasticizer .
•• Baczyńska M, Regel-Rosocka M, Coll MT, Fortuny A, Sastre AM, Wiśniewski M. Transport of Zn (II), Fe (II), Fe (III) across polymer inclusion membranes (PIM) and flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Separation Science and Technology. 2016;51(15–16):2639–48. This work showed the transport of Zn(II), Fe(II), and Fe(III) ions with PIMs containing Cypos IL 104 and Cypos IL 167 as carrier .
• Pospiech B. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Journal of Solution Chemistry. 2015;44(12):2431–47. This research showed the applications of ionic liquids as metal ion carriers and extractant for separation of metal cations from aqueous solution .
• Mahanty BN, Raut DR, Mohapatra PK, Das DK, Behere PG, Afzal M. Comparative evaluation of actinide ion uptake by polymer inclusion membranes containing TODGA as the carrier extractant. Journal of Hazardous Materials. 2014;275:146–53. This research evaluates the separation of actinide ions with PIM base CTA and NPOE as plasticizer .
•• Mahanty BN, Mohapatra PK, Raut DR, Das DK, Behere PG, Afzal M, et al. Polymer inclusion membrane containing a diglycolamide-functionalized calix [4] arene for actinide ion uptake and transport. Journal of Membrane Science. 2016;516:194–201. This work addresses the separation of Am 3+ ,Pu 4+ ,UO 2 2+ , and Th 4+ by using PIM base CTA and C4DGA as carrier and NPOE as plasticizer .
• Mahanty BN, Mohapatra PK, Raut DR, Das DK, Behere PG, Afzal M. Polymer inclusion membranes containing N,N,N',N'-tetra(2-ethylhexyl) diglycolamide: Uptake isotherm and actinide ion transport studies. Ind Eng Chem Res. 2015;54(12):3237–46. This study reported that PIMs containing N , N , N ′, N ′-tetra(2-ethylhexyl) diglycolamide (T2EHDGA), a branched diglycolamide extractant as the carrier, cellulose triacetate (CTA) as the polymer matrix, and 2-nitrophenyloctyl ether (NPOE) as the plasticizer was used for recovery of Am 3+ ,Pu 4+ ,UO 2 2+ , and Th 4+ .
•• Kaya A, Onac C, Alpoguz HK, Yilmaz A, Atar N. Removal of Cr (VI) through calixarene based polymer inclusion membrane from chrome plating bath water. Chemical Engineering Journal. 2016;283:141–9. This research showed the removal of Cr(VI) from chrome plating bath water by using calixarene based polymer .
Konczyk J, Kozlowski C, Walkowiak W. Removal of chromium (III) from acidic aqueous solution by polymer inclusion membranes with D2EHPA and Aliquat 336. Desalination. 2010;263(1–3):211–6.
Regel-Rosocka M, Szymanowski J. Iron (II) transfer to the organic phase during zinc (II) extraction from spent pickling solutions with tributyl phosphate. Solvent extraction and ion exchange. 2005;23(3):411–24.
Fontàs C, Tayeb R, Tingry S, Hidalgo M, Seta P. Transport of platinum (IV) through supported liquid membrane (SLM) and polymeric plasticized membrane (PPM). J Membr Sci. 2005;263(1–2):96–102.
•• Baba Y, Kubota F, Goto M, Cattrall RW, Kolev SD. Separation of cobalt (II) from manganese (II) using a polymer inclusion membrane with N-[N, N-di (2-ethylhexyl) aminocarbonylmethyl] glycine (D2EHAG) as the extractant/carrier. Journal of Chemical Technology and Biotechnology. 2016;91(5):1320–6. This research showed the potential of PIMs containing CTA, D2EHPA, and dioctylphtalate for separation on Co(II) .
• Abdul-Halim NS, Whitten PG, Nghiem LD. Characterising poly (vinyl chloride)/Aliquat 336 polymer inclusion membranes: evidence of phase separation and its role in metal extraction. Separation and Purification Technology. 2013;119:14–8. This study addresses the extraction of Cd(II) and Zn(II) using PVC/Aliquat 36 PIMs .
Gardner JS, Walker JO, Lamb JD. Permeability and durability effects of cellulose polymer variation in polymer inclusion membranes. J Membr Sci. 2004;229(1–2):87–93.
•• O’Bryan Y, Cattrall RW, Truong YB, Kyratzis IL, Kolev SD. The use of poly (vinylidenefluoride-co-hexafluoropropylene) for the preparation of polymer inclusion membranes. Application to the extraction of thiocyanate. J Membr Sci. 2016;510:481–8.
•• Yaftian MR, Almeida MI, Cattrall RW, Kolev SD. Selective extraction of vanadium (V) from sulfate solutions into a polymer inclusion membrane composed of poly (vinylidenefluoride-co-hexafluoropropylene) and Cyphos® IL 101. Journal of Membrane Science. 2018;545:57–65. This work reported the extraction of vanadium by using PVDF-HFP-based PIM containing trihexyltetradecylphosphonium chloride (Cyphos® IL 101) as its carrier .
• Guo L, Zhang J, Zhang D, Liu Y, Deng Y, Chen J. Preparation of poly (vinylidene fluoride-co-tetrafluoroethylene)-based polymer inclusion membrane using bifunctional ionic liquid extractant for Cr (VI) transport. Industrial & Engineering Chemistry Research. 2012;51(6):2714–22. This research showed transport of Cr(VI) using PIMs with bifunctional ionic liquid extractant (Bif-ILE) [trialkylmethylammonium][bis(2,4,4-trimethylpentyl)- phosphinate] ([A336][C272]), [trialkylmethylammonium][di-2-ethylhexylphosphinate] ([A336][P204]), or [trialkylmethylammonium][di-(2-ethylhexyl)orthophosphinate] ([A336][P507]) as the carrier, 1-octyl-3-methylimidazolium hexafluorophosphate or tetrafluoroborate ([C8mim][PF6] or [BF4]) as the ionic liquid plasticizer (ILP), and poly(vinylidene fluoride-co-tetrafluoroethylene) (PVDF) as the polymer .
•• Kaya A, Onac C, Alpoğuz HK, Agarwal S, Gupta VK, Atar N, et al. Reduced graphene oxide based a novel polymer inclusion membrane: transport studies of Cr (VI). Journal of Molecular Liquids. 2016;219:1124–30. This study showed the recovery of chromium using graphene oxide base PIMs .
Acknowledgments
The authors would like to thank the research participants for their time and effort involved with the research.
Funding
This study was funded by the Malaysian Ministry of Higher Education for under the grant FRGS/A08.00/00453A/001/2015/000285.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
This article is part of the Topical Collection on Water Pollution
Rights and permissions
About this article
Cite this article
Zulkefeli, N.S.W., Weng, S.K. & Abdul Halim, N.S. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr Pollution Rep 4, 84–92 (2018). https://doi.org/10.1007/s40726-018-0091-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-018-0091-y