Abstract
Hydrogeochemistry of groundwater was investigated to understand groundwater quality for drinking and irrigation purposes in an unconfined aquifer located in Jangal plain, NE Iran. Groundwater samples were collected and analyzed for determining physicochemical parameters (TDS, EC and pH) and concentration of major ions (Na+, K+, Ca2+, Mg2 +, Cl−, HCO3 −, SO 2 −4 and CO 2 −3 ). Water quality assessment parameters including SAR, Na%, RSC, TH and SI were also calculated. TDS, EC, Na+, K+, Cl− and SO 2 −4 levels in all samples were greater than the maximum permissible limit, while pH, Ca2 +, Mg2 + and HCO −3 levels in most samples were within the standard range. Based on the Piper diagram, most groundwater samples were classified into Na-Cl as dominant water type. The saline water intrusion, evapotranspiration, ion exchange reactions and dissolution of carbonate minerals were found as the main processes controlling the chemistry of groundwater. According to the calculated parameters and the Wilcox and USSL diagrams, high salinity and alkalinity of the groundwater resources pose a major problem for irrigation use. It is obvious that the groundwater unsuitability threatens sustainable crop production and soil quality. Hence, urgent management of groundwater resources is suggested to avoid harmful effects on inhabitants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is the most important source of water for drinking, agricultural and industrial purposes in arid and semiarid regions with shortage of surface water (Wang et al. 2016). Chemical composition and hydrochemistry of groundwater is influenced by natural factors such as geological structures, residence time, weathering of lithological units, soil/rock-water interactions and evapotranspiration within watersheds and aquifers (Tirumalesh et al. 2010; Zhu and Schwartz 2011; Rajesh et al. 2015; Nematollahi et al. 2016). In addition, the continuous increase of population and agricultural and industrial activities, and the consequent lowering of the groundwater table due to over-pumping to meet the increasing water demands, followed by the declining annual natural recharge, have brought groundwater resources under severe constrains worldwide (Fetter 1994; Appelo and Postma 2005; Singh et al. 2014). Since frequency and magnitude of the natural and anthropogenic factors in an area differ in time and space, their influences are reflected in the groundwater hydrochemistry, exhibiting wide spatial and temporal fluctuations (Singh et al. 2007).
Planning and management of groundwater resources guarantee sustainable water supply which is often obtained by identifying water chemistry and quality. Since daily, seasonal and climatic factors affect quality and chemistry of groundwater (Ackah et al. 2011), constant monitoring of water quality is significant to prevent the harmful effects of unsuitable water on humans and the biota. Moreover, irrigation water quality directly influences soil physical and chemical properties (Simsek and Gunduz 2007). Comprehending hydrogeological conditions and hydrochemical processes in aquifers is complicated. Nevertheless, using the raised perception of geochemical evolution of groundwater along with approaches based on geographic information systems (GIS), effective management of water resources could be possible (Elci and Polat 2010; Hamzaoui-Azaza et al. 2011; Sheikhy Narany et al. 2014; Nematollahi et al. 2016).
Increasing agricultural activity, insignificant surface water resources, low annual precipitation, high evaporation and ineffective management of groundwater resources have restricted safe water supply. Preliminarily, Velayati (2000) investigated groundwater hydrochemistry in Jangal plain and concluded that water quality has been degraded by saline water intrusion. In this research, as the major objective, monitoring of groundwater salinity in order to evaluate aquifer vulnerability and identify groundwater suitability for drinking and irrigation purposes under the most severe condition was carried out.
2 Study Area
2.1 General Properties of the Area
The study was undertaken in Jangal plain, which is situated in the south of Roshtkhar (90 % of the total area) and north of Gonabad, Khorasan Razavi province, NE Iran (Fig. 1a, b, c) and lies between east latitudes 34°24′–35°01′ and north longitudes 58°48′–59°33′ (Fig. 1d). Average altitude of the plain is 924 m with respect to average sea level and its total surface area is 2338 km2. Jangal plain is bounded by Roshtkhar and Torbat Heydarieh to the north, Khaf to the east, Gonabad to the south, and Mahvelat to the west. With nearly 6500 inhabitants, the study area indicates fairly good population density of 1 person per hectare of croplands. Due to unsuitable groundwater quality, alternative drinking water resource is provided although the groundwater is the main source of irrigation water. The study area experiences arid to semi-arid climate, with the annual temperature ranging from −14 °C in the winter to 49 °C in the summer.
2.2 Geologic Setting
The study area is structurally and tectonically a part of the dynamic Alpine-Himalayan system, with several major and minor faults influencing the area. According to the tectonosedimentary classification of Iran, Jangal plain belongs to the Central Iran zone (Stöcklin 1974). The main lithological outcrops in the area are marls, shales, sandstones, conglomerates, swamps and marsh deposits, with a high level piedmont fan and valley terrace deposits in the north, clay flats and unconsolidated windblown sand deposits in the west and south, along with low level piedmont fan and valley terrace deposits in the east and northwest of the study area (Fig. 2). Meanwhile, chemical composition of groundwater might be affected by surface water infiltration containing dissolved ions derived from geological units such as ophiolites (colored mélange complex, dunites, harzburgites and serpentinites), conglomerates, sandstones, gypsiferous marls, gypsums, igneous rocks (andesites, diorites and tuffs), alluvial deposits, shales and limestones in the north and outskirt of the study area (Fig. 2).
2.3 Hydrogeology
The study area is hydrologically situated in Jangal catchment area. Average annual rainfall in a 30-year period is 158 mm with an average volume of 369.4 million m3. Rainfall does not occur in the summer while February and March experience most rainfall. The quality of groundwater is influenced by Bajestan playa in the west and southwest (Velayati 2000). The minimum and maximum discharge values of the aquifer are 4.2 and 93.5 L/s, respectively, with an average value of 49.0 L/s. The insignificant surface water resources in the area drive from the low precipitation and high evaporation. The groundwater recharge resources include saline water inflow, irrigation return flow, precipitation, and floodwater and surface water from Kalsalar and Shastdarreh rivers. Aquifer exploitation via 233 wells which mostly have a depth of more than 100 m is also the sole discharge resource. Recently, the severe decrease of precipitation and the overexploitation due to rapid development of agriculture have led to quantitatively critical circumstances in the aquifer. Furthermore, because of recently established Shahid Yaghoubi dam, located in Kalsalar river upstream, floodwater infiltration and consequently aquifer recharge have been restricted. Hence, the groundwater budget which is the sum of inputs (129.6 million m3) minus the sum of outputs (204.5 million m3) is equal to −74.9 million m3 reflecting water table decline. The piezometric map and decline curves of the water table in a 6-year time span (based on 32 piezometric wells) show that the major groundwater flow direction is toward the east of the alluvial plain where an intense decline of the water table is observed (Fig. 3a, b).
3 Materials and Methods
3.1 Sampling and Analyses
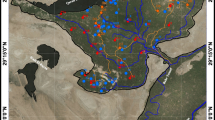
To monitor aquifer vulnerability and groundwater suitability under the most critical conditions, a sampling campaign was conducted during the summer. Thirty two representative groundwater samples from piezometric wells (Fig. 4a, b) were collected in dry and clean polyethylene bottles prewashed with HNO3 (analytical grade, Merck) and rinsed 3 times before collecting the samples. The samples were then sealed and stored at 4 °C in the Torbat Heydarieh regional water organization laboratory until analysis. After sampling, portable instruments were immediately used for in-situ measurement of the electrical conductivity (EC) and pH. The samples then were analyzed for determining concentration of Na+, K+, Ca2+, Mg2+, Cl-, HCO −3 , SO4 2- and CO3 2 − following the standard water quality methods (Table 1). The quality assurance and quality control (QA/QC) of the analytical data were checked using certified procedures, strict examination of standards and reagent blanks, along with replicate analyses. Calculated recovery percent for the analyzed chemical concentrations of standard reference materials was within ±10 %. Using AquaChem software, the data were plotted on the Piper (1944) and Durov (1948) diagrams to understand the chemical characteristics of the groundwater. Total dissolved solids (TDS) were determined using pre-filtered samples evaporated to dryness. The total hardness (TH), sodium percentage (Na %), sodium adsorption ratio (SAR) and residual sodium carbonate (RSC) were calculated by the formulae presented in Table 2. Mineral saturation indices also were computed using PHREEQC computer software version 2 via Eq. (5) in Table 2; where SI is the saturation index, IAP is ion activity product of a particular solid phase, and Ksp is the solubility product of that phase at a given temperature (i.e., the thermodynamic equilibrium constant adjusted to the temperature of the given sample). To reveal relationships between the parameters, correlation coefficients were also determined using IBM SPSS 20.
3.2 Spatial Analysis Based on GIS
GIS was used to ease the overall assessment by analyzing and representing spatial data (Collins et al. 1995). Hydrogeochemical data can be visualized by GIS and provide a reliable mean of monitoring environmental conditions and recognizing hot-spot areas. In the present study, the geographic position of the study area and the sampling wells, the lithological map, the piezometric map, the spatial distribution of the physicochemical parameters and the concentration of the major ions, and the contour map of water table decline were produced using ArcGIS software version 10.1.
4 Results and Discussion
4.1 Groundwater Chemistry
Suitability of groundwater for domestic, industrial, and agricultural purposes depends on its chemical composition. Table 3 indicates descriptive statistics of the major cations and anions, and the physicochemical characteristics. Shapiro-Wilk test was used to check normality of the data. The results show that only HCO −3 and SO4 2- are somewhat normally distributed.
Average pH is 8.1 which reveals that the groundwater is slightly alkaline (Table 3). Since pH varies from 7.3 to 8.6, it is clear that almost all pH values are in the optimum pH limit (6.5 to 8.5) recommended by WHO (2004) for potable water (Fig. 5a).
The value of TDS which is a measure of the degree of quality, ranges from 2608.0 to 8650.0 mg/L, with an average value of 4666.3 mg/L (Table 3). Elevated TDS values influence groundwater suitability for drinking and agricultural use (Fetter 2001). Based on WHO drinking water quality guidelines (2004), 600 and 1000 mg/L are the most desirable and acceptable limits for TDS, respectively. Table 3 reveals that the minimum TDS value in the sampling wells is more than the maximum acceptable limit.
The groundwater EC values vary between 4140.0 and 13730.0 μS/cm, with an average value of 7406.9 μS/cm (Table 3). According to WHO (2004), 1500 μS/cm is the allowable EC threshold in drinking water. Thus, similar to TDS, EC values of the samples exceed the drinking water permissible limit (Fig. 5b, c).
The TH values of the samples were calculated using Eq. (1) in Table 2. The calculated TH values range from 370.0 to 2520.0 mg/L, with an average value of 1004.2 mg/L (Table 3). Regarding TH values, only 9.38 % of the samples fell below the WHO maximum allowable limit (500 mg/L) and the rest had higher values of TH (Fig. 5d). According to the hardness classification of Sawyer et al. (2003), the groundwater samples are very hard (Table 6). Due to the unpleasant taste, there is mainly an aesthetic concern about hard water. It also prevents soap to produce lather, increases water boiling point and may cause precipitation of calcium carbonate in distribution pipes, in water heaters and on plumbing fixtures.
On the average, contents of the major cations and anions are in the order of Na+ > Mg2 + > Ca2 + > K+, and Cl− > SO 2 −4 > HCO −3 > CO 2 −3 , respectively (Fig. 6). Average concentrations of Na+ and K+ in the groundwater samples were 1186.3 and 128.8 mg/L, respectively, which exceed the corresponding desirable limits (Table 3 and Fig. 5e, f). Calcium and magnesium concentrations ranged from 30.1 to 424.8 and 71.7 to 430.2 mg/L, respectively (Table 3). Taste thresholds of Ca2 + and Mg2 +, which depend on associated anions, range from 100 to 300 and 50 to 150 mg/L, respectively (WHO 2004). Ca2 + concentration in 59.38 % of the samples was in the range of WHO standard, whereas 50.00 % of the samples were in Mg2 + permissible limit (Fig. 5g, h). It can be interpreted that in the presence of clay minerals and Fe-Mn oxy-hydroxides and under ion exchange processes, Ca2 + and Mg2 + substitute Na+ and K+ on cation adsorption sites of the adsorbents, leading to Ca2 + and Mg2 + decrease and Na+ and K+ increase in groundwater.
The contents of chloride in the samples varied between 762.2 and 3900.0 mg/L, with an average value of 1787.6 mg/L (Table 3). Chloride contents of the samples were higher than the WHO permissible limit (Fig. 5i). The elevated water chloride content (above 250 mg/L) affects the taste (WHO 2004). It can also develop hypertension and increase the risk for osteoporosis, renal stones and asthma (Maiti 1982).
The average groundwater bicarbonate concentration was 211.8 mg/L (Table 3). Since no health disorder is attributed to the elevated HCO −3 values, the anion is not a health concern but should not exceed 240 mg/L in drinking water (WHO 2004). HCO −3 concentration in 31.25 % of the samples was higher than the permissible limit of WHO, suggesting dissolution of abundant carbonates in the study area (Fig. 5j).
Values of sulfates ranged from 600.4 to 1585.0 mg/L, having a mean value of 1012.3 mg/L (Table 3). The minimum sulfate concentration was two times greater than 250 mg/L (Table 3 and Fig. 5k). Sulfate concentrations above 250 mg/L in drinking water may cause unpleasant taste and corrosion of distribution pipes, while concentrations higher than 500 mg/L may cause risk to human health such as gastrointestinal disorders (WHO 2004).
4.2 Hydrochemical Facies
The Piper diagram demonstrated that Na − Cl water type is the main hydrochemical facies in Jangal plain (Fig. 7). Based on the diagram, it is obvious that the alkaline elements (Na+ and K+) significantly dominated the alkaline earth elements (Ca2 + and Mg2 +), as well as the strong acids (Cl− and SO 2 −4 ) exceeded the weak acids (HCO −3 and CO 2 −3 ).
The Durov diagram (Fig. 8) also reflects simultaneous occurrence of hydrogeochemical processes including dissolution and ion exchange. The alkaline elements abundance could be a result of dissolution of halite and silvite along with ion-exchange processes within the aquifer matrix. Na − Cl water type may also propose the mixture of existing water with high-salinity water taken from intrusion of Bajestan playa saltwater and surface contamination sources including irrigation return flow, septic tank effluents, and domestic wastewater. According to the Piper and Durov diagrams (Figs. 7 and 8, respectively), Na+ and Cl− are the main ions controlling the groundwater chemistry, and thus, the dominant water type.
4.3 Compositional Relations of Dissolved Species
For determining the degree of variables dependency and also measuring the relationship between dependent and independent variables, the coefficient of correlation was used. In this study, spearman rank correlation was used to measure correlation between variables due to non-parametric distribution of variables in the groundwater samples. Table 4 presents the correlation coefficients of water quality characteristics. EC and TDS show maximum correlation coefficient (0.990; p < 0.01), confirming the efficacy of the field measurements.
According to high TDS, EC, Ca2 +, Mg2 +, Na+, K+, Cl− and SO 2 −4 values in the west and south of the aquifer and direction of the groundwater flow (Figs. 3a and 5), intrusion of Bajestan playa saline water from the west and southwest borders into the aquifer could be the most important factor controlling content of the aforementioned ions and parameters. Good positive correlation coefficients between TDS, EC, Ca2 +, Mg2 +, Na+, K+, Cl− and SO 2 −4 also reveal that the ions contribute to the groundwater salinity, supporting the assumption of saline water intrusion (Table 4). Furthermore, ion exchange processes and weathering of Na and K-bearing minerals such as halites, sylvites and clay minerals, and also anthropogenic contamination sources such as local and animal wastes, septic tank seepages and irrigation return flow affect concentration of Na+ and K+.
Since intrusion of Bajestan playa saline water in the aquifer play the main role in high chloride concentration of the groundwater, the Na+ and Cl− relationship is considered to determine the salinity mechanisms in the study area. NaCl dissolution could occur if Na+/ Cl− ratio is equal to 1 (Meybeck 1987). Almost all of the groundwater samples have a Na+/ Cl− ratio below 1 (Fig. 9), which might be considered as salinization process (El Moujabber et al. 2006). Ca2 + also indicates good positive correlation coefficient with Cl− (p < 0.01), showing feasible calcium chloride dissolution (Table 4). In addition, according to Loizidou and Kapetanios (1993), anthropogenic sources including human and animal wastes, septic tank seepages and fertilizers could also influence concentration of chloride in water resources.
Sulfate-bearing minerals dissolution, e.g., gypsum and anhydrite, and weathering of sulfide minerals have been reported as major probable sources of sulfate in groundwater (Fianko et al. 2009). Good positive correlation coefficients between SO 2 −4 and the major cations reflects possible dissolution of sulfate minerals (Table 4). The SO 2 −4 / Ca2 + molar ratio was constructed to evaluate calcium sulfate dissolution in the aquifer (Hounslow 1995). All water samples fell above the equiline, suggesting that sulfate concentration is controlled by more complicated geochemical processes (Fig. 10). Sulfates mainly occur in dissolved state, while Ca2 + may precipitate as calcite minerals and/or experience a Ca2 +/ Na+ base-exchange between water and clay minerals (Desbarats 2009).
The (Ca2 + + Mg2 +) / HCO −3 ratio can be used to identify sources of calcium and magnesium ions. The ratio would be about 0.5 if Mg2 + and Ca2 + were solely derived from dissolution of carbonates (Sami 1992). About 88 % of the samples were above the 0.5:1 line, suggesting that carbonates dissolution is not the only source of Ca2 + and Mg2 + (Fig. 11). Prasanna et al. (2010) reported that Ca2 + might be derived from chemical fertilizers. Ophiolite complex containing abundant Mg-bearing minerals and chemical fertilizers might also be the possible excess Mg2 + sources in groundwater.
The Ca2 + + Mg2 + vs. HCO −3 + SO 2 −4 molar ratio is mostly used to determine mineralization processes in groundwater (Cerling et al. 1989; Fisher and Mullican 1997). The samples fell below the equiline, indicating cation exchange process along with carbonate and sulfate minerals dissolution (Fig. 12). Hence, Na+ and K+ balance the excess negative SO 2 −4 and HCO −3 charges.
A temporal increase in TDS value might occasionally leads to wrong interpretation of saltwater intrusion. Hence, the Revelle coefficient (RC), determined as Cl-/(CO 2 −3 + HCO −3 ), is considered as a criterion to make sure that groundwater salinity is derived from saltwater intrusion. The modified Revelle coefficient classification (Kallergis 2000) was also used to investigate the degree of contamination by saltwater (Table 5).
In accordance with the calculated RC values (2.2 to 33.7), the samples reflected medium to dangerous level of contamination (Fig. 13). The average value of RC (9.5) shows that most of the samples are seriously contaminated. Hence, it seems necessary that the groundwater in the polluted areas is paid special attention.
Groundwater quality to the east of the plain (Kalsalar river entrance) where the alluvial deposits are overlied, due to floodwater infiltration, is more suitable, although clay flat which contains clay-sized minerals covers significant sector of the study area (Fig. 2). The dominance of fine-grained particles leads to an increase of the water residence time, resulting in salinity of groundwater during cation exchange and simple solution processes. The majority of wells and agricultural farms are located in the alluvial deposits. Hence, overexploitation is observed in the alluvial deposits, resulting in water table decline. This also causes a large flow of saline water toward the east, and consequently, low water quality.
4.4 Processes Regulating Groundwater Chemistry
Groundwater quality is significantly affected by hydrochemical reactions among aquifer minerals and groundwater, which is also helpful in comprehending the groundwater genesis (Prasanna et al. 2011). Rock weathering, evaporation and precipitation are important processes regulating chemistry of water. For this reason, to determine the dominant hydrochemical process, the Gibbs diagrams (Gibbs 1970) were applied (Fig. 14). Since the samples lie in the evaporation-crystallization zone of the Gibbs diagrams, the chemistry of groundwater is roughly governed by evaporation process which effectively enriches the dissolved groundwater ions.
As previously mentioned, the Na+/ Cl− ratio is usually applied to recognize the salinity and saline intrusion mechanisms in semi-arid regions. The dominance of evaporation leads to Na+/ Cl− ratio to be constant with increasing EC (Jankowski and Acworth 1997). The Na+/ Cl− ratio against EC diagram displays that the data are mostly plotted along and below the horizontal line with increasing EC (Fig. 15). This means that the evapotranspiration, dissolution of chlorides and anthropogenic chloride contamination are feasible factors affecting Na+/ Cl− ratio.
4.5 Groundwater Irrigation Quality
Investigation of irrigation water suitability due to the excess dissolved ion effect on soil physicochemical characteristics (e.g., reducing permeability) and consequently plant reproduction is considerable. Hence, suitability of irrigation water was investigated by salinity and alkalinity hazard, and also Na%, SAR and RSC. The results are presented in Table 6.
4.5.1 EC and TDS
EC, which also reflects groundwater TDS, is used to monitor salinity hazard to soil and crops (Kelley 1951). Elevated values of groundwater EC cause soil salinity, while high sodium values lead to soil alkalinity (Nagarajan et al. 2010). Classification of Wilcox (1955) based on groundwater EC demonstrates that all samples belong to the unsuitable class (Table 6). The osmotic activity of plant majorly decreases with high EC content, interfering in water and nutrients absorption from the soil. Furthermore, according to the TDS classification of the US Geological Survey (2000), most of the samples (90.6 %) are moderately saline (Table 6).
4.5.2 Sodium Percentage
Sodicity or alkalinity is a serious hazard for soil, thereby sodium leads to damage in soil structure, resulting in compaction and impermeability of the soil (Raju 2007). The sodium percentage of the water samples was calculated using Eq. (2) (Table 2). In accordance with Wilcox (1955) classification, the majority of the samples belong to the water classes of doubtful and unsuitable, respectively (Table 6). In addition to the excess Na+ concentration, Na+ relative activity in exchange reactions may result in high sodium hazard. The irrigation water with a high sodium proportion increases the soil Na+ exchange, as well as decreases permeability of the soil. Therefore, soil ploughing and seedling emergence seems difficult.
4.5.3 Sodium Adsorption Ratio
Alkali/Sodium hazard can also be determined by realizing SAR which is an important index in identifying suitability of irrigation water. Equation (3) in Table 2 was used to compute SAR. Based on SAR classification (Richards 1954), 62.5 % of the samples fell in good class while the remaining belonged to doubtful class (Table 6).
To investigate irrigation water suitability, the Wilcox (1955) diagram which is a plot of sodium percentage vs. salinity, and also the USSL diagram (Richards 1954) which is a plot of SAR vs. EC were also used. The Wilcox diagram exhibited that 53.12 % of the groundwater samples fell in doubtful to unsuitable categories, whereas the rest belonged to unsuitable class (Fig. 16). The USSL diagram illustrates that all of the sampling points lie in the C5S4 and C4S4 fields, indicating extremely high level salinity and alkalinity (Fig. 17). High salinity waters are not suitable and must not be used for irrigation of soils with poor drainage. Thus, it is needed to pay specific attention to salinity management and cultivation of high salt tolerant crops.
4.5.4 Residual Sodium Carbonate
High (HCO −3 + CO 2-3 )/(Ca2 + + Mg2 +) ratio results in Ca2 + and Mg2 + precipitation as carbonates. Hence, RSC was calculated via Eq. (4) in Table 2 to recognize hazardous carbonates and bicarbonates impact on irrigation water quality. RSC values which range from −46.98 to −1.88, having a mean value of −16.50, indicate that the water samples lie in good class (Table 6).
4.6 Saturation Index
To thermodynamically consider the groundwater composition, the SIs were evaluated regarding the major carbonates including calcite, dolomite and aragonite, and evaporites containing gypsum, anhydrite and halite. Values of Saturation indices can be categorized as saturation (−1 < SI < 0), unsaturation (SI < − 1) and oversaturation (SI > 0).
Values of SI varied between 0.89–2.93, 0.07–1.17 and 0.21–1.31 with reference to dolomite, aragonite and calcite, respectively. Moreover, SI values of gypsum, anhydrite and halite ranged from −1.56 to −0.44, −1.77 to −0.65 and −4.89 to −3.89, respectively. On the basis of Fig. 18, carbonates were oversaturated in all groundwater samples, revealing that precipitation occurs. Although saturation state was observed in few samples concerning anhydrite and gypsum, which indicate the equilibrium, unsaturation state dominated evaporites, especially halite, reflecting evaporites dissolution in most of the groundwater samples.
In this regard, dissolution of gypsum and anhydrite is continuously carried out without precipitation along the flow-path across the basin, resulting in an increase in Ca2 + concentration, and consequently, a decrease in the concentrations of HCO −3 and CO 2 −3 by calcium carbonate precipitation. Hence, evaporites do not play a significant role in the groundwater chemistry, whereas carbonates dissolution mostly influences the chemistry of water.
4.7 Groundwater Hydrochemistry in Comparison with Other Similar Studies
Groundwater chemical composition in the selected relevant studies has statistically been compared with the corresponding values in Jangal plain (Table 7). The hydrochemical characteristics of the groundwater in this research were, on average, similar to those measured by Velayati (2000), which reveals acceptable chemical analyses error. However, the maximum values of most major ions and physicochemical properties were higher in the present study, reflecting degradation in the groundwater quality. Comparing with this study, the measured cations, anions and physicochemical parameters in Torbat-Zaveh plain (Nematollahi et al. 2016), situated in the north of the area (Fig. 4a), could be useful to gain a better insight into groundwater salinization. Significant higher contents of the hydrochemical quantities in Jangal plain suggest intrusion of Bajestan playa saline water as a different specific factor controlling the groundwater composition. Except Ca2 + and HCO −3 , average values of the major ions, EC and TDS in the studied aquifer is greater when compared with those in an aquifer in Iran central desert (Baghvand et al. 2010) and Selçuk plain in Turkey (Somay and Gemici 2009). Relative to Jangal plain, Jerba aquifer (Kharroubi et al. 2012), which is affected by salt water intrusion, shows excessive EC and the ions concentration as well.
5 Conclusions
Quality of groundwater for drinking and irrigational purposes has been investigated in an unconfined aquifer. With collecting and analyzing groundwater samples, values of physicochemical parameters including TDS, EC and pH, and major ions content including Na+, K+, Ca2+, Mg2 +, Cl−, HCO3 -, SO 2 −4 and CO 2 −3 have been determined to assess hydrogeochemical characteristics of groundwater. The Piper diagram showed that Na-Cl poses the main water type. The values of water quality parameters and major ions concentrations in comparison with the WHO permissible limits indicate that the groundwater resources are unsuitable for drinking purpose. Moreover, parameters, such as EC, TDS, SAR, Na% and TH, along with the Wilcox and USSL diagrams suggest that the groundwater is mostly not suitable for irrigation purpose. Salinity and alkalinity threaten soil health and thus biota. The study shows that saline water intrusion, evatranspiration, ion exchange and carbonates dissolution are the dominant processes regulating the quality of groundwater. Although alternative drinking water resource is supplied, the authors recommend urgent special management and programming to improve groundwater quality for various aims and avoid harmful effects on inhabitants. For instance, programmed agriculture is suggested to prevent groundwater overexploitation. As well uncontrolled usage of fertilizers could be limited and cropping salt-tolerant plants could be promoted. To overcome the excess irrigation water salinity, soil drainage should be improved as well.
References
Ackah M, Agyemang O, Anim A, Osei J, Bentil N, Kpattah L (2011) Assessment of groundwater quality for drinking and irrigation: the case study of Teiman-Oyarifa Community, Ga East Municipality, Ghana. Proc Int Acad Ecol Environ Sci 1(3–4):186–194
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution. CRC Press
Baghvand A, Nasrabadi T, Nabi Bidhendi G, Vosoogh A, Karbassi A, Mehrdadi N (2010) Groundwater quality degradation of an aquifer in Iran central desert. Desalination 260:264–275
Cerling TE, Pederson BL, Damm KLV (1989) Sodium-calcium ion exchange in the weathering of shales: implication for global weathering budgets. Geology 17:552–554
Collins S, Smallbone K, Briggs D (1995) A GIS approach to modelling small area variations in air pollution within a complex urban environment. Innov GIS 2:245–253
Desbarats AJ (2009) On elevated fluoride and boron concentrations in groundwaters associated with the Lake Saint-Martin impact structure, Manitoba. Appl Geochem 24(5):915–927
Durov SA (1948) Natural waters and graphic representation of their composition. Dokl Akad Nauk SSSR 59:87–90
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
El Moujabber M, Bou Samra B, Darwish T, Atallah T (2006) Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resour Manag 20:161–180. doi:10.1007/s11269-006-7376-4
Elci A, Polat R (2010) Assessment of the statistical significance of seasonal groundwater quality change in a karstic aquifer system near Izmir-Turkey. Environ Monit Assess 172(1):445–462. doi:10.1007/s10661-010-1346-2
Fetter CW (1994) Applied hydrogeology. Macmillan College Publishing Company, New York
Fetter CW (2001) Applied hydrogeology. Prentice Hall, New Jersey, Upper Saddle River
Fianko J, Osae S, Adomako D, Achel D (2009) Relationship between land use and groundwater quality in six districts in the eastern region of Ghana. Environ Monit Assess 153(1–4):139–146
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium sulphate and sodium chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Hamzaoui-Azaza F, Ketata M, Bouhlila R, Gueddari M, Riberio L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss–Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174(1–4):283–298
Hounslow A (1995) Water quality data: analysis and interpretation. CRC Press, New York
Jankowski J, Acworth RI (1997) Impact of debris-flow deposits on hydrogeochemical processes and the developement of dryland salinity in the Yass River Catchment, New South Wales, Australia. Hydrogeol J 5(4):71–88
Kallergis G (2000) Applied environmental hydrogeology, 2nd edn. Technical Chamber of Greece, Athens
Kelley WP (1951) AIkali soils; their formation, properties, and reclamation. Reinhold, New York
Kharroubi A, Tlahigue F, Agoubi B, Azri C, Bouri S (2012) Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones: case of the Jerba coastal aquifer in southeast Tunisia. Environ Earth Sci 67(7):2089–2100
Loizidou M, Kapetanios E (1993) Effect of leachate from landfills on underground water quality. Sci Total Environ 128(1):69–81
Maiti T (1982) The dangerous acid rain. Sci Rep 9(6):360–363
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171(1–4):289–308
Nematollahi MJ, Ebrahimi P, Razmara M, Ghasemi A (2016) Hydrogeochemical investigations and groundwater quality assessment of Torbat-Zaveh plain, Khorasan Razavi, Iran. Environ Monit Assess 188(1):1–21. doi:10.1007/s10661-015-4968-6
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey: Earth Science Information Center, Open-File Reports Section
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–928
Prasanna MV, Chidambaram S, Hameed AS, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168(1–4):63–90
Prasanna MV, Chidambaram S, Kumar GS, Ramanathan A, Nainwal H (2011) Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arab J Geosci 4(1–2):319–330
Rajesh R, Brindha K, Elango L (2015) Groundwater quality and its hydrochemical characteristics in a shallow weathered rock aquifer of southern India. Water Qual Expo Health 7(4):515–524
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52(6):1067–1074
Richards L (1954) Diagnosis and improvement of saline and alkali soils. United States Department of Agriculture, Washington
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139(1):27–48
Sawyer G, McMcarty D, Parkin G (2003) Chemistry for environmental engineering and science, 5th edn. McGraw Hill Education, New York
Sheikhy Narany T, Ramli MF, Aris AZ, Sulaiman WNA, Fakharian K (2014) Spatiotemporal variation of groundwater quality using integrated multivariate statistical and geostatistical approaches in Amol-Babol Plain, Iran. Environ Monit Assess 186(9):5797–5815
Simsek C, Gunduz O (2007) IWQ index: a GIS-integrated technique to assess irrigation water quality. Environ Monit Assess 128(1):277–300. doi:10.1007/s10661-006-9312-8
Singh KP, Malik A, Sinha S, Mohan D, Singh VK (2007) Exploring ground water hydrochemistry of alluvial aquifers using multi-way modeling. Anal Chim Acta 596:171–182
Singh KP, Gupta S, Mohan D (2014) Evaluating influences of seasonal variations and anthropogenic activities on alluvial groundwater hydrochemistry using ensemble learning approaches. J Hydrol 511:254–266
Somay MA, Gemici Ü (2009) Assessment of the salinization process at the coastal area with hydrogeochemical tools and geographical information systems (GIS): Selçuk plain, Izmir, Turkey. Water Air Soil Pollut 201(1–4):55–74
Stöcklin J (1974) Possible ancient continental margins in Iran. In: Bruk CA, Drake CL (eds) The geology of continental margins. Springer, Berlin Heidelberg, pp 873–887
Tirumalesh K, Shivanna K, Sriraman A, Tyagi A (2010) Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. Environ Monit Assess 163(1–4):171–184
Todd DK (1980) Groundwater hydrology, 2nd edn. John Wiley and Sons, New York
US Geological Survey (2000) Classification of natural ponds and lakes. U.S. Department of the Interior, U.S. Geological Survey, Washington, DC
Velayati S (2000) Influence of groundwater overexploitation on quality of water in Jangal plain, Ferdowsi University of Mashhad
Wang J, Wu J, Jia H (2016) Analysis of spatial variation of soil salinization using a hydrochemical and stable isotopic method in a semiarid irrigated basin, Hetao Plain, inner Mongolia, North China. Environ Process 1–11. doi:10.1007/s40710-016-0179-6
WHO (2004) Guidelines for drinking-water quality: recommendations. Vol. 1, 3rd edn. WHO, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Department of Agriculture, Washington, DC
Zhu C, Schwartz FW (2011) Hydrogeochemical processes and controls on water quality and water management. Elements 7(3):169–174
Acknowledgments
The authors would like to thank Mashhad Regional Water experts for making this research possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nematollahi, M.J., Ebrahimi, P. & Ebrahimi, M. Evaluating Hydrogeochemical Processes Regulating Groundwater Quality in an Unconfined Aquifer. Environ. Process. 3, 1021–1043 (2016). https://doi.org/10.1007/s40710-016-0192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0192-9