Abstract
Background
The vast majority of IgE-mediated food allergies in adults are based on sensitization to pollen and subsequent cross-reactions to structurally related allergens in fruit, vegetables, and spices. The effect of allergen immunotherapy (AIT) against pollen on pollen-related food allergy has not been conclusively elucidated.
Methods
A review of studies on AIT in pollen-related food allergy was conducted.
Results
The fact that the published studies show considerable differences in terms of design (e.g., number of subjects, treatment duration, mode of administration, allergen content, oral provocation testing) hampers their evaluation and comparison. Only some of the studies demonstrated an improvement in pollen-related food allergy as a result of AIT with pollen allergens.
Conclusion
Reliable recommendations on the use of AIT with pollen allergens in pollen-related food allergy are not possible as yet. AIT with birch pollen allergens appears to have a positive effect on concomitant food allergy in some patients with birch pollen allergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of IgE-mediated food allergies in adults are based on sensitization to aeroallergens (in particular pollen) with subsequent reactions (cross-reactions) to structurally related, often unstable allergens, particularly in fruit, vegetables, and spices. This type of food allergy is referred to as a secondary food allergy, as distinct from the primary form, which is presumed to involve sensitization via the gastrointestinal tract [1,2,3,4]. Birch pollen-related food allergies are by far the most prevalent in Germany.
Symptoms of pollen-related food allergy typically manifest within a few minutes to up to 2 h following intake of the food. Oropharyngeal contact urticaria (also referred to as oral allergy syndrome, OAS) occurs most commonly. Reactions may also be seen in one or more target organs, including the skin, gastrointestinal tract, respiratory tract, and cardiovascular system [1].
In terms of treatment, and in addition to allergen avoidance, (emergency) anti-allergic drugs are used where required. In contrast to pollen-related respiratory symptoms, there are controversial data on whether allergen immunotherapy (AIT) has a positive effect on pollen-related food allergy [2, 5, 6]. AIT has been attempted with the food allergens themselves, as well as with the associated pollen allergen.
The following review presents the current state of knowledge on the performance of AIT with pollen extracts for pollen-related food allergy, with particular attention to birch-related food allergy.
Triggers of birch-related food allergy
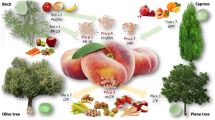
The main plant-based food allergens belong to a handful of protein families: the best known of these are Bet v 1 homologues, lipid transfer proteins, storage proteins, and thaumatin-like proteins [1].
Bet v 1 homologues are widespread in the plant world, and hence also in fruit and vegetables. The types of fruit that are most frequently involved in pollen-related food allergy belong to the Rosaceae (e.g., apple, cherry) and Fagales families (hazelnut). In terms of vegetables, celery and carrot from the Apiaceae family, as well as tomato and bell pepper, are particularly worthy of note ([1]; Table 1). In a Bet v 1-sensitized patient group (n = 221) that was investigated for specific IgE to PR10 proteins using immuno solid-phase allergen chip (ISAC), concomitant sensitization was detected to Cor a 1.04 (98%), Mal d 1 (94%), Aln g 1 (94%), Pru p 1 (86%), Cor a 1.01, Ara h 8 (72%), Gly m 4 (52%), Api g 1 (37%), and Act d 8 (29%) [7].
Allergen immunotherapy and extracts
AIT is a causal immunomodulatory treatment aimed at inducing immune tolerance through the administration of allergen extracts. The effect is triggered by specific blocking antibodies, tolerance-inducing cells, and messengers, which prevent further exacerbation of the allergen-triggered immune response, block the specific immune response, and attenuate the inflammatory response in tissue.
The guidelines [5] specify that the following criteria should be met in order for AIT to be initiated:
-
Detection of IgE-mediated sensitization (preferably using skin tests and/or in vitro diagnostic methods) and an unequivocal link to clinical symptoms (provocation testing where appropriate)
-
Availability of standardized or high-quality allergen extracts
-
Proof of efficacy of the planned AIT for the respective indication and age group
-
Allergen avoidance not possible or inadequate
Subcutaneous or sublingual products are available for immunotherapy. Native foods, allergen extracts, and allergen peptides have been used in studies on patients with food allergy to, e.g., cow’s milk, hen’s egg, and peanut, either subcutaneously, sublingually, orally (selective oral tolerance induction), or epicutaneously [6].
Biomarkers
In vitro and in vivo biomarkers can be used to establish the indication for AIT; some of these are also suited to monitoring disease course or treatment success [5, 6, 8]. It is not possible to say with any certainty at present the extent to which the serological markers used in inhalant pollen allergy, as well as skin prick tests (SPT), are suited to the evaluation of AIT course and success in pollen-related food allergy.
In vitro markers
In order for AIT to be indicated, IgE-mediated sensitization needs to be proven, e.g., by specific serum IgE. Molecular allergy diagnostics have made such significant advances here in recent years that, in addition to Bet v 1 sensitization, IgE antibodies to a number of cross-reactive food allergens can be detected, e.g., to Mal d 1 (apple), Cor a 1 (hazelnut), and Gly m 4 (soy) [9,10,11,12]. It was recently shown that those with concomitant OAS in a birch-allergic patient group had higher levels of specific immunoglobulin E (sIgE) to Bet v 1 compared with those without concomitant pollen-related food allergy [9]. Another working group, on the other hand, found no association between IgE, IgG4, or IgA to Bet v 1 and the clinical onset of OAS [13]. Specific IgG4 determination is generally performed to evaluate the extent to which an immunological response takes place under AIT, although no clinically relevant success can be inferred from this.
Skin testing
SPT can be used to diagnose an IgE-mediated reaction. However, according to the current state of knowledge, there is no evidence to suggest that SPT can be used to evaluate the success of AIT for pollen-related food allergy.
Oral provocation testing
Older studies on AIT for pollen-related food allergy refer exclusively to information from patients in the context of non-controlled consumption in terms of food-related symptoms. Meanwhile, double-blind placebo-controlled food challenge (DBPCFC) is considered the gold standard; however, this test is by no means straightforward. Overall, DBPCFC is still in great need of standardization [14]. This applies to:
-
Timing (e.g., stronger reactions during the pollen season)
-
Provocation meals (e.g., allergen selection/administration, allergen dose, allergen content, blinding, placebo meal)
Due to a lack of commercial availability to date, there has been no standardization in terms of allergen content. When using native foodstuffs, allergen content may differ as a result of, e.g., variety, as well as growing and storage conditions. An attempt was made to counter this situation in soy allergy by using standardized Gly m 4 measurements [15]. There are also preliminary studies in which the major apple allergen, Mal d 1, was used for oral challenge testing [16, 17].
-
Dose escalation schedule (e.g., every 20–30 min) [18]
-
Evaluation criteria and determining the final value (e.g., shifting the threshold value following AIT) [18].
Threshold values are set using the lowest observable adverse event level (LOAEL), defined as the lowest dose of the food at which symptoms or clinical signs manifest. Studies only rarely provide detailed information on the DBPCFC evaluation criteria used. Unfortunately, there is no consensus-based standard as yet on to how objective signs and subjective symptoms can be measured and evaluated. The evaluation of placebo reactions, which are not uncommon, also presents a challenge [14, 18].
Measuring quality of life
The Food Allergy Quality of Life Questionnaire-Adult Form (FAQLQ-AF) is the first freely available questionnaire designed to measure health-related quality of life in adults with food allergy. It was recently shown in an adult patient collective with exclusively pollen-related food allergy that the questionnaire can also be used for this entity. It was reported that OAS impairs quality of life to the same extent as food allergy symptoms that go beyond OAS [19].
Data on allergen-specific immunotherapy for pollen-related food allergy
AIT with birch pollen extracts causes the induction of Bet v 1-specific (s)IgG4 antibodies. There has been discussion that these sIgG4 antibodies cross-react with related food allergens, e.g., Mal d 1 and Cor a 1, thereby inhibiting the IgE binding of these allergens. This inhibitory effect could be measured as early on as 1 year following AIT and further increased after 3 years (cumulative allergen dose 500 µg). A reduction in sIgE antibodies to food allergens was also demonstrated, albeit only after 12 months of AIT. Interestingly, IgG4 antibodies in this investigation failed to recognize more than 35% of epitopes of Bet v 1, Mal d 1, and Cor a 1, meaning that the sIgG4 induced by AIT apparently does not cover or prevent all IgE activities [20].
However, based on the available in vitro data, one can indeed expect AIT with birch pollen extracts to have a clinical effect on birch-related food allergy [21].
When evaluating the existing studies (Table 1), one must bear in mind that there are significant differences in study design [14, 22], particularly in terms of:
-
Extracts with varying allergen contents used
-
Mode of administration (sublingual, subcutaneous)
-
Group size (mostly extremely small groups, probably statistically underpowered)
-
Food allergens investigated (hazelnut, apple, soy)
-
Performance and evaluation of provocation testing
This makes a comparison virtually impossible. Only a handful of studies included a placebo-controlled intervention, while others chose a treatment-free control group to determine treatment effect (Table 2; [22,23,24,25,26,27,28,29,30]). The double-blind food challenge with threshold value determination, defined as the gold standard [2], was fulfilled in only some of the studies.
Overall, a number of studies did indeed yield evidence that AIT with pollen allergens has a clinical effect on birch-related food allergy. It is possible that even better effects can be achieved by using molecular food allergens (e.g., Mal d 1) in allergen extracts, as recently shown in a study on birch-related apple allergy [31].
The lack of AIT’s clinical effect in other studies has been linked, first, to an overly low number of cases and, second, to the different pollen allergen extracts used. Thus, it was speculated that in order to achieve a positive effect on pollen-related food allergy the allergen dose used would need to be higher than the dose usually used in pollen-induced respiratory symptoms (in particular allergic rhinoconjunctivitis) [32]. For the most part, allergen extracts with 12.5–25 µg of major allergen were used to investigate the effect of AIT on birch-related food allergy (Table 2). Whether a high-dose extract with 80 µg of folded recombinant Bet v 1 is more successful in birch-related food allergy was investigated in a randomized placebo-controlled multicenter study using birch-related soy allergy as an example (birch-related soy allergy and immunotherapy/BASALIT study) [20, 30, 33, 34].
In general, the multicenter BASALIT study [30] is characterized by:
-
Randomized placebo-controlled study both in terms of diagnosis using oral food challenge [14] and treatment
-
The use of high-dose (80 µg) Bet v 1 extract for subcutaneous AIT
-
The use of a standardized challenge meal with controlled Gly m 4 allergen content [15]
-
Standardized criteria to evaluate subjective symptoms and objective signs [14]
-
A high number of subjects compared with previous studies: 82 patients tested positive in oral provocation testing (OPT) to soy, 56 of which were randomized to interventional placebo-controlled AIT (verum–placebo ratio: 2:1)
The BASALIT study showed that 1‑year AIT has a clear immunological effect on sIgG4 antibody production to Bet v 1. A significant increase in sIgG4 antibodies to Gly m 4 and Cor a 1 was also documented.
In terms of clinical effect, the verum group showed an improvement in the threshold dose for objective signs (LOAEL), with post-interventional reactions to the challenge meal only occurring at higher doses compared with the baseline investigation. However, what was remarkable was that similar improvements in LOAEL were also observed in some of the subjects in the placebo group. It is unfortunate, therefore, that statistical significance was not achieved for this effect (p = 0.08), which—despite the comparatively high number of subjects included—was attributed to the fact that only 56% of the sample size calculated as necessary for a clearly demonstrable effect could be recruited [30]. The BASALIT study demonstrates the difficulty associated with conducting studies to investigate the effect of AIT in pollen-related food allergy.
Outlook
A recent study showed, both in vitro and in a mouse model, that vaccination with a hybrid molecule directed against the three relevant T‑cell epitopes, Bet v 1, as well as cross-reactive apple and hazelnut epitopes, is capable of inducing protective antibodies in pollen-related food allergy [35]. It is possible that synthetically produced immuno-regulatory peptides could be used in the context of AIT in the future [36]. Another potential treatment option for food allergy may be the use of the anti-IgE antibody omalizumab, for which the first cases of treatment success have been shown, either alone or in combination with oral immunotherapy [37, 38].
Summary: what is the evidence for efficacy?
It is important to note that, on the whole, knowledge regarding the use of AIT in pollen-related food allergy has been limited to date. This is due to the fact that there are only scant randomized placebo-controlled treatment studies that conducted their investigations using defined AIT extracts. To complicate matters further, there are no standardized target parameters for the evaluation of food allergy severity that are suited to measuring the extent of symptoms before and after AIT. Finally, there are no readily available, standardized challenge meals for which a defined allergen content can be assumed. Therefore, all things considered, one must currently assume an evidence level of 1b (at least one sufficiently large, methodologically high-quality randomized study) to 2a (at least one high-quality study without randomization), since even recent high-quality studies were unable to recruit a sufficient number of patients [29]. As such, the grade of recommendation that can currently be given is essentially only level B (findings based on reliable level-2 or level-3 studies or on extrapolations from level-1 studies) and does not reach level A (findings based on reliable level-1 studies).
In summary, one would have to say that AIT with Bet v 1 that is successful in terms of respiratory symptoms does not necessarily improve pollen-related food allergy. However, it certainly appears to have positive effects on concomitant food allergies in some patients. One can assume that precise criteria are still lacking on which subgroup of Bet v 1-sensitized individuals would in actual fact also benefit in terms of pollen-related food allergy. The evidence to date does not permit pollen-related food allergy alone to be considered an indication for AIT. Therefore, in accordance with the guidelines, AIT with pollen allergens should only be performed if the indication is made on the basis of concomitant respiratory symptoms [2].
Abbreviations
- AIT:
-
Allergen immunotherapy
- BASALIT:
-
Birch-related soy allergy and immunotherapy
- DBPCFC:
-
Double-blind placebo-controlled food challenge
- FA:
-
Food allergy
- FAQLQ-AF:
-
Food Allergy Quality of Life Questionnaire-Adult Form
- IgA:
-
Immunoglobulin A
- IgE:
-
Immunoglobulin E
- IgG:
-
Immunoglobulin G
- ISAC:
-
Immuno solid-phase allergen chip
- LOAEL:
-
Lowest observed adverse effect level
- OAS:
-
Oral allergy syndrome
- OPT:
-
Oral provocation testing
- sIgE:
-
Specific immunoglobulin E
- SPT:
-
Skin prick test
References
Treudler R, Simon JC. Pollen-related food allergy: An update. Allergo J Int. 2017;26:41–53.
Worm M, Reese I, Ballmer-Weber B, Beyer K, Bischoff SC, Classen M, et al. Guidelines on the management of IgE-mediated food allergies. S2k-Guidelines of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Medical Association of Allergologists (AeDA), the German Professional Association of Pediatricians (BVKJ), the German Allergy and Asthma Association (DAAB), German Dermatological Society (DDG), the German Society for Nutrition (DGE), the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS), the German Society for Oto-Rhino-Laryngology, Head and Neck Surgery, the German Society for Pediatric and Adolescent Medicine (DGKJ), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Society for Pneumology (DGP), the German Society for Pediatric Gastroenterology and Nutrition (GPGE), German Contact Allergy Group (DKG), the Austrian Society for Allergology and Immunology (ÖGAI), German Professional Association of Nutritional Sciences (VDOE) and the Association of the Scientific Medical Societies Germany (AWMF). Allergo J Int. 2015;24:256–93.
Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, et al. Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70:1079–90.
Worm M, Jappe U, Kleine-Tebbe J, Schäfer C, Reese I, Saloga J, et al. Food allergies resulting from immunological cross-reactivity with inhalant allergens. Guidelines from the German Society for Allergology and Clinical Immunology (DGAKI), the German Dermatology Society (DDG), the Association of German Allergologists (AeDA) and the Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2014;23:1–16.
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282–319
Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI Allergen Immunotherapy Guidelines Group. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815.
Blankestijn MA, Knulst AC, Knol EF, Le TM, Rockmann H, Otten HG, et al. Sensitization to PR-10 proteins is indicative of distinctive sensitization patterns in adults with a suspected food allergy. Clin Transl Allergy. 2017;23(7):42.
Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485–98.
Ciprandi G, Comite P, Ferrero F, Bignardi D, Minale P, Voltolini S, et al. Birch allergy and oral allergy syndrome: The practical relevance of serum immunoglobulin E to Bet v 1. Allergy Asthma Proc. 2016;37:43–9.
Tolkki L, Alanko K, Petman L, Skydtsgaard MB, Milvang PG, Seppälä U, et al. Clinical characterization and IgE profiling of birch (Betula verrucosa)—allergic individuals suffering from allergic reactions to raw fruits and vegetables. J Allergy Clin Immunol Pract. 2013;1:623–31.
Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013;13:110–7.
Treudler R. Update on in vitro allergy diagnostics. J Dtsch Dermatol Ges. 2012;10:89–97. quiz 98–99.
Guhsl EE, Hofstetter G, Lengger N, Hemmer W, Ebner C, Fröschl R, et al. IgE, IgG4 and IgA specific to Bet v 1‑related food allergens do not predict oral allergy syndrome. Allergy. 2015;70:59–66.
Treudler R, Franke A, Schmiedeknecht A, Ballmer-Weber BK, Worm M, Werfel T, et al. Standardization of double blind placebo controlled food challenge with soy within a multicentre trial. Clin Transl Allergy. 2016;6:39.
Holzhauser T, Franke A, Treudler R, Schmiedeknecht A, Randow S, Becker WM, et al. The BASALIT multicentre trial: Gly m 4 quantification for consistency control of challenge meal batches and towards Gly m 4 threshold data. Mol Nutr Food Res. 2017;61 https://doi.org/10.1002/mnfr.201600527.
Kinaciyan T, Nagl B, Faustmann S, Kopp S, Wolkersdorfer M, Bohle B. Recombinant Mal d 1 facilitates sublingual challenge tests of birch pollen-allergic patients with apple allergy. Allergy. 2016;71:272–4.
Kollmann D, Geroldinger-Simic M, Kinaciyan T, Huber H, Ebner C, Lidholm J, et al. Recombinant Mal d 1 is a reliable diagnostic tool for birch pollen allergen-associated apple allergy. J Allergy Clin Immunol. 2013;132:1008–10.
Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–74.
Beyer S, Franke A, Simon JC, Treudler R. Measurement of health-related quality of life in adult patients with birch pollen-associated food allergy. J Dtsch Dermatol Ges. 2016;14:397–404.
Subbarayal B, Schiller D, Möbs C, de Jong NW, Ebner C, Reider N, et al. Kinetics, cross-reactivity, and specificity of Bet v 1‑specific IgG4 antibodies induced by immunotherapy with birch pollen. Allergy. 2013;68:1377–86.
Treudler R, Simon JC. Schwere Sojaallergie im Erwachsenenalter: Hilft eine spezifische Immuntherapie? Hautarzt. 2012;63:307–12.
Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28:1368–73.
Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–9.
Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–6.
Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004;48:441–8.
Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–43.
Mauro M, Russello M, Incorvaia C, Gazzola G, Frati F, Moingeon P, et al. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156:416–22.
van Hoffen E, Peters KA, van Neerven RJ, van der Tas CW, Zuidmeer L, van leperen-van Dijk AG, et al. Effect of birch pollen-specific immunotherapy on birch pollen-related hazelnut allergy. J Allergy Clin Immunol. 2011;127:100–1.
Geroldinger-Simic M, Kinaciyan T, Nagl B, Baumgartner-Durchschlag U, Huber H, Ebner C, et al. Oral exposure to Mal d 1 affects the immune response in patients with birch pollen allergy. J Allergy Clin Immunol. 2013;131:94–102.
Treudler R, Franke A, Schmiedeknecht A, Ballmer-Weber B, Worm M, Werfel T, et al. BASALIT-trial: Double-blind placebo-controlled allergen immunotherapy with rBet v 1‑FV in birch-related soy allergy. Allergy. 2017;72:1243–53.
Kinaciyan T, Nagl B, Faustmann S, Frommlet F, Kopp S, Wolkersdorfer M, et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen-related apple allergy. J Allergy Clin Immunol. 2018;141:1002–8.
Asero R. Effects of birch pollen SIT on apple allergy: A matter of dosage? Allergy. 2004;59:1269–71.
Treudler R, Kramer S, Kleine-Tebbe J, Simon JC. Steigende Popularität von Soja: Wie werden Birkenpollenallergiker richtig beraten? Allergo J. 2010;19:243–50.
Treudler R, Werner M, Thiery J, Kramer S, Gebhardt C, Averbeck M, et al. High risk of immediate-type reactions to soy drinks in 50 patients with birch pollinosis. J Investig Allergol Clin Immunol. 2008;18:483–4.
Hofer H, Asam C, Hauser M, Nagl B, Laimer J, Himly M, et al. Tackling Bet v 1 and associated food allergies with a single hybrid protein. J Allergy Clin Immunol. 2017;140(10):525–33.
Klimek L, Pfaar O, Worm M. New opportunities for allergen immunotherapy using synthetic peptide immuno-regulatory epitopes (SPIREs). Expert Rev Clin Immunol. 2016;12:1123–35.
Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: A double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018;3:85–94.
Asero R. Disappearance of severe oral allergy syndrome following omalizumab treatment. Eur Ann Allergy Clin Immunol. 2017;49:143–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Treudler has received fees for consultancy and lectures, as well as travel expenses or study grants, from the following companies: Allergopharma J. Ganzer KG, ALK-Abello, Novartis, Shire. L. Klimek has received study funds, fees for consultancy activities and/or expert appraisals, as well as for lecturing and/or training activities, and/or paid participation on a scientific advisory board from ALK-Abelló, Allergopharma, Bencard, Bionorica, Biomay, Boehringer Ingelheim, Cytos, Circassia, Euroimmun, Dr. Fooke, HAL, GSK, Leti, Lofarma, MEDA/Mylan, Novartis, Stallergenes, Thermofisher, and Roxall.
Rights and permissions
About this article
Cite this article
Treudler, R., Klimek, L. Allergen immunotherapy for oral allergy syndrome: what is the evidence for efficacy?. Allergo J Int 28, 50–56 (2019). https://doi.org/10.1007/s40629-018-0081-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-018-0081-z