Abstract
Antioxidant enzymes are important components in the defense against arsenic (As) stress in plants. Here, we tested the hypothesis that Salvinia molesta, an aquatic fern, counteracts the harmful arsenite (AsIII) effects by activating scavenging reactive oxygen species (ROS) enzymes. Thus, our objective was to investigate the role of the superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and ascorbate peroxidase (APX) in S. molesta tolerance to AsIII and indicate the use of this plant in remediation of contaminated water. Plants were grown in nutrient solution at pH 6.5 and exposed to 0, 5, 10, or 20 µM AsIII for 96 h (analyses of As absorption, mineral nutrient content, and relative growth rate) and for 24 h (analyses of oxidative stress indicators and enzymatic antioxidant defenses). In the floating leaves, there was a greater basal activity of the antioxidant enzymes and less accumulation of As than in submerged leaves. The submerged leaves, which function as roots in S. molesta, accumulated more As than floating leaves, and SOD and CAT activities were inhibited. Thus, there was a greater production of ROS and oxidative stress. Our results show that S. molesta presents enzymatic antioxidant defenses to alleviate AsIII toxicity and are more effectives in the floating leaves. These results are important to elucidate the AsIII tolerance mechanisms in S. molesta and the possibility of their use in contamined water phytoremediation. Additional studies exposing plants to more prolonged stress and using AsIII concentrations closer to those found in contaminated environments will confirm this claim.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) is an environmental pollutant and its concentrations have increased in freshwater sources around the world, through both geological processes and anthropogenic activities such as mining, burning fossil fuels, and agricultural use of fertilizers, pesticides, and herbicides containing As. The most common inorganic As forms in the aquatic environment are arsenate (AsV) and arsenite (AsIII) (Fazi et al. 2016). The latter is considered more toxic and also more present in groundwater sources. In many regions of the world, water intake with high As concentrations represents a serious threat to the health of human populations because of the pollutant toxicity and carcinogenic potential (Hettick et al. 2015; Kumar et al. 2016). This scenario makes it urgent to solve or mitigate this social and environmental problem.
There are many conventional technologies based on physical and chemical processes that are available to remove As from the aquatic environment, but most of them combine costly and complex processes of implantation and maintenance (Fazi et al. 2016; Nicomel et al. 2016). The phytoremediation appears in this scenario as an alternative technique for the removal of pollutants from air, soil and water, with the benefits of having low cost and ease of implantation, as well as being environmentally sustainable. Phytoremediation takes advantage of the ability of some plants to absorb and accumulate toxic elements in their tissues and includes several processes namely, phytoextraction, phytostabilization and rhizofiltration (Jasrotia et al. 2017; Sarwar et al. 2017). Usually aquatic plants perform rhizofiltration, where contaminants are removed by absorption and adsorption being accumulated in the roots (Rahman and Hasegawa 2011; Newete and Byrne 2016).
The effective results using phytoremediation depends on plant ability to absorb, accumulate and tolerate the deleterious effects of the pollutant. The primary AsIII toxic effect its to promote overproduction of reactive oxygen species (ROS) within plant cells (Sharma 2012). ROS are highly reactive molecules capable of oxidizing membrane lipids, nucleic acids and proteins (Das and Roychoudhury 2014). Oxidative stress is the main deleterious AsIII effect in plants (Talukdar 2013; Singh et al. 2015a; Farooq et al. 2015), although it may also inhibit the catalytic function of enzymes by binding to their sulfhydryl groups, leading to metabolic damage (Sharma 2012; Farooq et al. 2016a).
To avoid and mitigate oxidative damage promoted by toxic metals, plants have many defense mechanisms such as antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT) and peroxidases, which eliminate ROS in different subcellular compartments. These enzymes play recognizable role on macrophyte As tolerance (Farnese et al. 2014; Andrade et al. 2016; Leão et al. 2017; da-Silva et al. 2018).
Salvinia molesta is a floating and free-living pteridophyte. Each plant is composed of two floating oblong-shaped green leaves and a set of submerged long and filiform brown leaves, covered with hairs that absorb water and nutrients, and act as a root (Miranda and Schwartsburd 2016). S. molesta is native to Brazil, although it is currently found in many other countries. It is considered one of the worst 100 invasive species in the world (Luque et al. 2014), and presents rapid biomass production and reproduction rates (Barros and Henares 2015). These latter S. molesta features are important for the phytoremediation and support the relevance of researches to find a use for the species.
S. molesta has the ability to absorb toxic elements from water, such as lead, mercury (Kumari et al. 2017), and As (Hariyady et al. 2013). In this work we evaluated the S. molesta potential to absorb, accumulate and tolerate AsIII. We tested the hypothesis that S. molesta can counteract the harmful AsIII effects by antioxidant enzymes and ROS removal. Our objective was to evaluate the role of enzymatic antioxidant defenses in S. molesta tolerance to AsIII in a short-term exposure and indicate the use of this plant in remediation of contaminated water.
2 Material and methods
2.1 Plant material and treatment conditions
Specimens of Salvinia molesta D. S. Mitchell (Salviniaceae) were collected in an artificial lake in the Botanical Garden of Universidade Federal de Viçosa, Viçosa, Minas Gerais State, Brazil (20°45′24″S 42°52′23″W). Plants were surface sterilized with 1% sodium hypochlorite for 1 min and then extensively rinsed with running tap water and maintained in demineralized water for 24 h. Then, they were transferred to polyethylene flasks with 10 L of Clark’s nutrient solution pH 6.5 (Clark 1975), and maintained in a growth room for an acclimation period of 7 days with controlled temperature (25 ± 2 °C), irradiance (250 μmol m−2 s−1) and a 16-h light photoperiod.
Plants with similar sizes were then subjected to the following treatments: control (Clark nutrient solution 1/4 strength pH 6.5) and three As concentrations (5, 10 and 20 µM), provided as sodium arsenite (NaAsO2) in a nutrient solution (Clark nutrient solution 1/4 strength pH 6.5). Experiments were set up with three replicates, consisting of 5 g of fresh weight plants in glass pots with 0.5 L of solution.
The exposure time to treatments was 96 h to analyzes of arsenic absorption, mineral nutrients and relative growth rate (RGR), and 24 h for the other evaluations, under the same conditions described above. At the end of the exposure, plants were harvested and floating and submerged leaves were divided, washed in deionized water and immediately analyzed or stored at − 80 °C.
2.2 Determination of arsenic and nutrients concentration
The floating and submerged leaves of S. molesta were washed in deionized water, and placed in a conventional oven at 80 °C, until constant dry mass was obtained. The dry plant material was crushed and digested in a nitric-perchloric acid mixture (2:1 v/v) (Marin et al. 1993) and the concentrations of arsenic (As), calcium (Ca), magnesium (Mg), phosphorus (P) and sulfur (S) were determined through inductively coupled plasma emission spectroscopy (ICP-AES) (Optima 3300 DV, Perkin-Elmer, Norwalk, CT, USA). The accuracy of the method was verified by analysis of certified reference materials (Lemna minor (BCR-670)), from the National Institute of Standards and Technology (Gaithersburg, MD, USA).
2.3 Arsenic translocation factor (TF)
The As translocation factor was calculated by the equation; TF = [M]shoots/[M]roots where [M]shoots is the As concentration in floating leaves (µg g−1 dry weight (DW)) and [M] roots is the As concentration in submerged leaves in (µg g−1 DW).
2.4 Determination of relative growth rate (RGR)
The relative growth rate (RGR) of plants was calculated using the equation proposed by Hunt (1978): Rw = (ln w1 − ln w0) × 1000/(t1 − t0), where Rw represents relative growth rate; ln w1 and ln w0 represents neperian logarithm of the mass at the end and beginning of the experiment, respectively; and t1 − t0 represents duration of the experiment (days).
2.5 Concentration of reactive oxygen species
To measure the concentration of the superoxide anion radical (O .−2 ), 50 mg of floating leaves and submerged leaves were incubated in an extraction medium consisting of 100 μM ethylenediaminetetraacetic acid (EDTA) disodium salt, 20 μM NADH and 20 mM sodium phosphate buffer, pH 7.8 (Mohammadi and Karr 2001). The reaction was initiated by adding 100 μL of 25.2 mM epinephrine in 0.1 N HCl. The samples were incubated at 28 °C under stirring for 5 min. The absorbance was read at 480 nm for 5 min. Superoxide anion radical production was assessed by determining the accumulated adenochrome, using the molar absorption coefficient of 4.0 × 103 M−1 (Boveris et al. 2002).
The hydrogen peroxide (H2O2) concentration was determined using 200 mg of floating leaves and submerged leaves that were homogenized in an extraction medium consisting of 50 mM potassium phosphate buffer, pH 6.5, containing 1 mM hydroxylamine and centrifuged at 10,000×g for 15 min at 4 °C. Subsequently, 50 μL aliquots of the supernatant were added to a reaction medium containing 100 μM ammonium iron (II) sulfate (Fe(II)NH4SO4), 25 mM sulfuric acid, 250 μM xylenol orange, and 100 mM sorbitol (Gay and Gebicki 2000). The samples were kept in the dark for 30 min, and the absorbance was read at 560 nm. The H2O2 concentrations were estimated based on a calibration curve prepared with H2O2 standards.
2.6 Rate of electrolyte leakage (EL)
The cell damage was evaluated through an assessment of membrane integrity, quantifying electrolyte leakage according to Faria et al. (2013). Floating leaves discs and submerged leaves apices were obtained after the treatments, rinsed thoroughly in distilled water and maintained in 10 mL of distilled water in sealed vials for 6 h at room temperature. The electrolyte leakage was estimated from the electrical conductivity in the solution containing the plant tissues samples using an electrical conductivity meter (DM31, Digimed, Brazil). The conductivity was expressed as a percentage of the total conductivity measured after incubating the vials at 90 °C for 2 h.
2.7 Assessment of the antioxidant enzymes activity: superoxide dismutase (SOD), catalase (CAT), peroxidase (POX) and ascorbate peroxidase (APX)
To assess enzyme activity, 0.3 g fresh weight (FW) of floating leaves and submerged leaves were homogenized in extraction medium containing 0.1 M potassium phosphate buffer, pH 6.8; 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1% (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 12,000×g for 15 min. at 4 °C and the supernatant was used to assess superoxide dismutase (SOD), catalase (CAT), peroxidase (POX) and ascorbate peroxidase (APX) activities.
The SOD activity (SOD, EC 1.15.1.1) was measured according to the inhibition of p-nitroblue tetrazolium (NBT) photoreduction, according to the method of Giannopolitis and Ries (1977). The enzymatic activity was expressed in SOD units corresponding to the amount of enzyme required to inhibit 50% of of NBT photoreduction per minute (Beauchamp and Fridovich 1971).
The CAT activity (CAT, EC 1.11.1.6) was estimated as the rate of decomposition of H2O2 during the first minute of the reaction at 240 nm (Havir and McHale 1987) and calculated using a molar extinction coefficient of 36 mol−1 L cm−1. The enzymatic activity was expressed in micromoles H2O2 min−1 g−1 FW.
The POX activity (POX, EC 1.11.1.7) was estimated as the rate of production of purpurogallin at 420 nm according to the proposed by Nakano and Asada (1981) with a molar extinction coefficient of 2.47 mmol−1 L cm−1 (Chance and Maehley 1955). The enzymatic activity was expressed in micromoles purpurogallin min−1 g−1 (FW).
The APX activity (APX, EC 1.11.1.11) was assessed as the rate of ascorbate oxidation at 290 nm (Nakano and Asada 1981) using a molar extinction coefficient of 2.8 mmol−1 L cm−1. The enzymatic activity was expressed in micromoles ascorbate min−1 g−1 FW.
2.8 Data analysis
The experiment was performed using a completely randomized design. Analysis of variance was performed and the means of the arsenite concentrations and types of plant leaves (floating and submerged) were compared using Tukey’s test at 5% significance. The statistical analyses were performed with the statistical software SAS 9.1 (SAS Institute Inc. 2004).
3 Results
3.1 Concentration of arsenic, translocation factor of arsenic, and relative growth rate
The As concentration in floating leaves increased according to the increment of the pollutant in solution and reached 103 μg g−1 DW following treatment with 20 μM AsIII. The highest As concentration in submerged leaves was observed for 10 μM AsIII, but it decreased for 20 μM. The As concentration was higher in the submerged leaves compared to the floating leaves at all concentrations (Table 1). The As translocation factor (TF) in S. molesta ranged from 0.28 to 0.68 and it was higher in plants exposed to 20 μM AsIII compared to 5 and 10 μM AsIII (Table 1).
The relative growth rate (RGR) of floating leaves did not vary at 5 μM AsIII compared to the control, but it was 34% and 60% lower than the control at 10 and 20 μM AsIII, respectively. The growth of submerged leaves was affected in all AsIII treatments. There was an intense deterioration of the submerged S. molesta leaves and tissue residues were accumulated in the culture solution at the end of the exposure period, resulting in negative values at the two highest AsIII concentrations. The RGR was higher in floating leaves for all AsIII treatments (Table 1).
3.2 Mineral nutrient concentration
The calcium (Ca) concentration did not vary in the floating leaves, while it increased in the submerged leaves of the plants exposed to 10 and 20 μM AsIII. A higher concentration of Ca was recorded in the submerged leaves than in the floating leaves for all treatments (Table 2).
There was a reduction in the magnesium (Mg) concentration in floating leaves exposed to 20 μM AsIII compared to the control. In the submerged leaves, the Mg concentration decreased after plants were exposed to AsIII, but did not vary in the different concentrations. In all AsIII treatments, the Mg concentration was higher in the floating leaves than in the submerged leaves (Table 2).
The phosphorus (P) concentration did not vary in the floating leaves of plants exposed to arsenite compared to the control, but there was a reduction in 20 µM, compared to 5 and 10 µM AsIII. In submerged leaves, the P concentration decreased after exposure to 10 and 20 µM AsIII. A higher P concentration was observed in the floating leaves than in the submerged leaves for all treatments (Table 2).
The sulfur (S) concentration did not differ from control in the floating leaves. In the submerged leaves, S was reduced in all AsIII treatments, but did not differ among them. A higher S concentration was observed in the submerged leaves than in the floating leaves of the control and 20 μM AsIII treatments (Table 2).
3.3 Reactive oxygen species concentration and cell membrane damage
The superoxide anion radical (O .−2 ) concentration increased in S. molesta floating leaves exposed to AsIII, but did not differ between 10 and 20 μM treatments. In submerged leaves, the increase in O .−2 concentration occurred according to the increase in the AsIII concentration (Table 3).
In floating leaves, the hydrogen peroxide (H2O2) concentrations increased only at 20 μM AsIII. In the submerged leaves, the H2O2 concentration decreased at 20 μM compared to 10 μM AsIII. The O .−2 and H2O2 concentrations were higher in the floating leaves in all treatments compared to submerged leaves (Table 3).
The rate of electrolyte leakage (EL) did not vary in floating leaves. In submerged leaves, there was an increase of 142% and 198% at 10 and 20 μM AsIII, respectively, compared to control. The EL was higher in submerged leaves compared to floating leaves in the two largest AsIII concentrations (Table 3).
3.4 Antioxidant enzyme activity
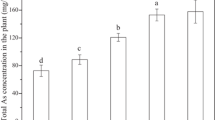
All AsIII treatments promoted an increase in SOD activity in S. molesta floating leaves. In the submerged leaves, there was no difference between control and AsIII concentrations. SOD activity was reduced at 20 μM AsIII compared to 5 and 10 µM treatments. Higher SOD activity was recorded in the floating leaves for all AsIII treatments compared to submerged leaves (Fig. 1a).
Activity of antioxidant enzymes: a superoxide dismutase (SOD), b catalase (CAT), c peroxidase (POX), d ascorbate peroxidase (APX) in S. molesta floating leaves (black bar) and submerged leaves (grey bar) after 24 h of AsIII exposure. Values are the mean of three replicates (n = 3) ± SD. Means followed by different capital letters for the same plant part and lowercase letters between plant parts, show significant difference at P < 0.05, according to Tukey test. FW fresh weight
CAT activity increased in the floating leaves only at 5 μM and 20 μM AsIII compared to contol. In the submerged leaves, there was no difference among treatments. There was higher CAT activity in the floating leaves for all treatments compared to submerged leaves (Fig. 1b).
The POX activity in floating leaves increased only in plants exposed to 20 μM AsIII. Increase of 61% were observed in the submerged leaves at 20 μM AsIII compared to control. The POX activity was always higher in floating leaves compared to submerged leaves (Fig. 1c).
APX activity was increased at 5 μM and 10 μM AsIII in the floating leaves. In submerged leaves it increased with the pollutant dose. Higher APX activity was recorded in floating leaves than in submerged leaves (Fig. 1d).
4 Discussion
Except for hyperaccumulator species, As translocation is restricted in most plants, including macrophytes. This supports As accumulation in submerged leaves rather than in floating leaves of S. molesta (Zhao et al. 2009; Rahman and Hasegawa 2011; Freitas-Silva et al. 2016). This response is mainly due to the formation of complexes among AsIII, phytochelatins (PCs), and glutathione (GSH) in root cells. The complexes are then retained in vacuoles, which decreases As reactivity and cell damage (Liu et al. 2010; LeBlanc et al. 2013; Farooq et al. 2016a). This defense mechanism may explain the low TF and the highest As accumulation in S. molesta submerged leaves.
Although S. molesta did not present characteristics to tell its As phytoremediation capacity, such as As accumulation higher than 1000 μg g−1 dry weight and a TF > 1, its use should not be excluded. These characteristics are found in some terrestrial and hyperaccumulating plants (Kumar et al. 2015; Fahyiga and Saha 2016). Therefore, it is not appropriate to use them to define the phytoremediation potential of macrophytes. In floating aquatic species absorption occurs on roots surface and in the aerial part that is in contact with the environment (Rezania et al. 2016). Thus, the whole plant contributes to pollutant stabilization, absorption and its removal from the water.
It is not possible to distinguish between As absorbed and adsorbed by S. molesta. The adsorption of As on the root surface in some plant species requires the formation of an iron plaque in the presence of oxygen (Yamaguchi et al. 2014; Fresno et al. 2016). This iron plaque has a much higher affinity for AsV ion sequestration than AsIII and acts as a barrier to the metalloid transport into the plant (Chen et al. 2005; Rahman and Hasegawa 2011; Hu et al. 2015). S.molesta plants was exposed to AsIII in a non-oxygenated nutrient solution. Thus, we can believe that the adsorption was minimal compared to the As absorption by the submerged leaves.
The AsIII can be transported from the inside of the plant to the environment via efflux (Li et al. 2016; Han et al. 2016). The aquaporins are intrinsic membrane proteins able to bidirectional transport in some plant species and can perform AsIII symplast efflux, as a defense mechanism (Pommerrenig et al. 2015; Xu et al. 2015; Afzal et al. 2016). Efflux and translocation to floating leaves may be the reasons why pollutant accumulates in S. molesta submerged leaves and does not follow the increases in AsIII concentration in solution. Damage caused by AsIII in the cell membranes can inhibit the function of aquaporins and affect the polutant’s absorption, as well as the acquisition of water and nutrients by plants (Hoffman and Schenk 2011). Reductions of Mg, P, and S concentrations in S. molesta support this hypothesis of stress-induced cell membrane damage.
Nutritional deficiency during stress can impair the photosynthetic process and plant energetic metabolism, as well as compromise antioxidant system defenses (Finnegan and Chen 2012; Dixit et al. 2015; Shen et al. 2016). Moreover, a reduction in root growth and size in plants exposed to As decreases the availability of essential nutrients in leaves and roots (Reed et al. 2015). However, increases in Ca may be a defense mechanism, because the nutrient acts as a secondary messenger in response to stress factors and improves the plant antioxidant defenses (Ahmad et al. 2015; Rahman et al. 2015; Edel et al. 2017). Calcium also participates in the cell wall and membrane stabilization and regulates the metabolic process related to other nutrients uptake (White and Broadley 2003; Hepler 2005). Additionally, Ca and P ions are able to form complexes with toxic elements and immobilize them in the cell wall (Parrota et al. 2015), reducing their potential damage.
The severe reduction in biomass observed in submerged leaves prove the high AsIII toxicity. The ROS increase can cause intense oxidative damage and promote plant tissue necrosis, affecting plant biomass (Talukdar and Talukdar 2013; Upadhyaya et al. 2014). The same happened with macrophytes Ceratophyllum demersum (Khang et al. 2012) and Nasturtium officinale (Ozturk et al. 2010) in which AsIII caused biomass loss. Thus, it is possible that AsIII damage to submerged leaves induced a decrease in water absorption and contributes to inhibiting plant growth. In addition, the reduction of photosynthesis promoted by As also impairs the biomass gain (Sharma 2013, Gusman et al. 2013, Farooq et al. 2016b).
It should be considered that the AsIII concentrations in this study are higher than most of those naturally found in in countries with remarkable levels of water contamination (Chakraborti et al. 2016). Therefore, a high amount of pollutant increased the toxic effects on plant growth.
Corroborating the study hypothesis, the AsIII induced oxidative stress in S. molesta submerged leaves as evidenced by the increases in O .−2 and electrolyte leakage. Membranes are the primary ROS targets in plant cells and when its structure is disturbed, electrolyte leakage occurs. Thus, membrane damage can function as a biomarker of As toxicity (Finnegan et al. 2012; Anjum et al. 2015). The membrane integrity of the floating leaves denote lesser AsIII effects in the plant shoots. This may be the result of lower As accumulation coupled with a greater activity of antioxidant enzymes.
The ROS generation in plants exposed to toxic metals can trigger the expression of antioxidant enzyme genes, as well as increase the activity of these enzymes for stress acclimatization (Ahammed et al. 2013; Rout and Sahoo 2013; Ahmad et al. 2015; Farooq et al. 2015). SOD is the first line of antioxidant enzymatic defense in plants because it controls the O .−2 removal in cell (Gill et al. 2015). Increases in SOD activity and greater As tolerance have been reported in different plants (Mishra et al. 2011; Gupta et al. 2013; Kanwar et al. 2015; Dixit et al. 2015). In S. molesta floating leaves, SOD activity may have helped to protect cells from oxidative damage.
SOD activity can be inhibited by As (Du et al. 2017). Because of its affinity for the sulfhydryl (-SH) proteins groups, AsIII disturbs the catalytic functions of enzymes (Shen et al. 2013). Inhibition of antioxidant enzymes allows hydroxyl radical (OH•) generation, a highly reactive molecule with extreme cellular oxidizing capacity (Halliwell and Gutteridge 2015). If this happens, oxidative stress is intensified. A similar situation may have occurred in S. molesta submerged leaves, in which the SOD activity was negligible and there was significant increases in O .−2 concentrations.
The SOD activity produces H2O2 (Gill et al. 2015). This helps to explain the insignificant variation in H2O2 in the submerged leaves. However, this direct relationship between increase in SOD activity and H2O2 concentration was not verified in the floating leaves. The performance of H2O2-eliminating enzymes support these results. Thereby, CAT was an important enzyme for H2O2 control in floating leaves. CAT is responsive to As mainly in plant leaves (Mishra et al. 2011; Gusman et al. 2013). Thus, CAT activity was lower in S. molesta submerged leaves which act as roots, and was not changed in response to AsIII. Catalase has a lower affinity for H2O2 than peroxidases enzymes (Mhamdi et al. 2010; Das and Roychoudhury 2014). Therefore, peroxidases is important in ROS control.
Increases in POX and APX activities suggests an important role for these enzymes in AsIII tolerance in S. molesta. POX and APX are recognized members of the antioxidant defense system against As in macrophytes such as Pistia stratiotes (Farnese et al. 2014), Azolla caroliniana (Leão et al. 2017), and Spirodela intermedia (da-Silva et al. 2017). The peroxidases’ activity indicates defense mechanisms mediated by antioxidants, which protect plant cell structures and reduce As toxic effects (Dave et al. 2013; Dixit et al. 2015). In addition, APX is an enzyme component of the ascorbate–glutathione cycle, as one of the most efficient ROS elimination system in As stressed plants (Singh et al. 2015b).
In the S. molesta floating leaves, while the APX activity increased in the two lowest AsIII concentrations, POX activity was stimulated only under 20 µM AsIII. Similarly, APX activity increased in submerged leaves at all AsIII concentrations, while POX showed a relevant increase only in the most stressed plants.This result suggests the coordinated action of these two enzymes in the peroxide elimination, depending on the As stress intensity. Differences in the enzymes affinity for their substrate may be the cause of this responses. Indeed, it is known that APX has a high affinity for H2O2 and acts on its elimination even when it is in low concentrations in the cell (Sofo et al. 2015; Anjum et al. 2016).
The antioxidant enzymes activity seems to act in a coordinated way in order to alleviate AsIII toxic effects in S. molesta and avoided cell damage in the floating leaves. There is also a remarkable difference in the activity level of the enzymes between the plant parts. The CAT, POX and APX activities in the floating leaves are much higher than in submerged leaves, even in the control condition. The higher ROS production in photosynthetic tissues, further increased by photorespiration (Demidchik 2015; Del Río and López-Huertas 2016; Mitller 2017), can explain these results. Even under optimal environmental conditions, the intense ROS production by photosynthesis requires a higher concentration of antioxidant enzymes in leaves (Hajiboland 2014).
It is known that the differences in As compartmentalization in cells can also influence plant defense responses (Shaibur and Kawai 2009). Thus, while sequestration mechanisms using metal chelating molecules such as glutathione and phytochelatin can be more effective in root cells, antioxidant enzymes may be more important in leaf cells (Begum et al. 2016, Silva et al. 2017). Additionally, a higher accumulation of toxic metals in the roots is suggested to be a protective mechanism for the plant’s photosynthetic apparatus (Liu et al. 2010; Gomes et al. 2012; Gusman et al. 2013).
The results obtained in this study show the response of enzymatic antioxidant system in S. molesta to AsIII exposure. This defense mechanism has been shown to be important for As tolerance in plants and this trend was also observed in this study. However, further studies assessing the S. molesta antioxidant defenses in a longer stress, and the use of equivalent concentrations to those found in contaminated environments are still necessary to establish the plant AsIII tolerance and its potential for use in contamined water phytoremediation.
References
Afzal Z, Howton TC, Sun Y, Mukhtar MS (2016) The roles of aquaporins in plant stress responses. J Dev Biol. https://doi.org/10.3390/jdb4010009
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, LSP Tran (2015) Alleviation of cadmium toxicity in Brassica juncea L.(Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE. https://doi.org/10.1371/journal.pone.0114571
Andrade HM, Oliveira JA, Farnese FS, Ribeiro C, Silva AA, Campos FV, Neto JL (2016) Arsenic toxicity: cell signalling and the attenuating effect of nitric oxide in Eichhornia crassipes. Biol Plant 60:173–180
Anjum NA, Sofo A, Scopa A et al (2015) Lipids and proteins–major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121
Anjum NA, Sharma P, Gill SS et al (2016) Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Barros JPA, Henares MNP (2015) Biomass reduction of Salvinia molesta exposed to copper sulfate pentahydrate (CuSO4.5H2O). Rev Ambient Água. https://doi.org/10.4136/ambi-agua.1633
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay aplicable to acrylamide gels. Anal Biochem 44:276–287
Begum MC, Islam MS, Islam M, Amin R, Parvez MS, Kabir AH (2016) Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem 104:266–277
Boveris A, Alvarez S, Bustamante J, Valdez L (2002) Measurement of superoxide radical and hydrogen peroxide production in isolated cells and subcellular organelles. Methods Enzymol 349:280–287
Chakraborti D, Rahmana MM, Chatterjeea A et al (2016) Fate of over 480 million inhabitants living in arsenic and fluoride endemic Indian districts: magnitude, health, socio-economic effects and mitigation approaches. J Trace Elem Med Biol 38:33–45
Chance B, Maehley AC (1955) Assay of catalase and peroxidase. Methods Enzymol 2:755–764
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Clark RB (1975) Characterization of phosphatase of intact maize roots. J Agric Food Chem 23:458–460
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. https://doi.org/10.3389/fenvs.2014.00053
da-Silva CJ, Canatto RA, Cardoso AA, Ribeiro C, de Oliveira JA (2018) Oxidative stress triggered by arsenic in a tropical macrophyte is alleviated by endogenous and exogenous nitric oxide. Braz J Bot 41:21–28
Dave R, Singh PK, Tripathi P et al (2013) Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch Environ Contam Toxicol 64:235–242
Del Río LA, López-Huertas E (2016) ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol 57:1364–1376
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Dixit G, Singh AP, Kumar A et al (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Du L, Xia X, Lan X, Liu M, Zhao L, Zhang P, Wu Y (2017) Influence of arsenic stress on physiological, biochemical, and morphological characteristics in seedlings of two cultivars of maize (Zea mays L.). Water Air Soil Pollut 228:255
Edel KH, Marchadier E, Brownlee C, Kudla J, Hetherington AM (2017) The evolution of calcium-based signalling in plants. Curr Biol 27:667–679
Faria AP, Lemos-Filho JP, Modolo LV, França MGC (2013) Electrolyte leakage and chlorophyll a fluorescence among castor bean cultivars under induced water deficit. Acta Physiol Plant 35:119–128
Farnese FS, Oliveira JA, Lima F, Leão GA, Gusman GS, Silva LC (2014) Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz J Biol 74:108–112
Farooq MA, Li L, Ali B et al (2015) Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ Sci Pollut Res 22:10699–10712
Farooq AM, Islam F, Ali B (2016a) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2016.08.004
Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, Zhou W (2016b) Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 25:350–366
Fayiga AO, Saha KU (2016) Arsenic hyperaccumulating fern: implications for remediation of arsenic contaminated soils. Geoderma 284:132–143
Fazi S, Amalfitano S, Casentini B et al (2016) Arsenic removal from naturally contaminated waters: a review of methods combining chemical and biological treatments. Rend Fis Acc Lincei 27:51–58
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol. https://doi.org/10.3389/fphys.2012.00182
Freitas-Silva LD, de Araújo TO, da Silva LC, Oliveira JA, Araújo JM (2016) Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol Environ Saf 124:1–9
Fresno T, Peñalosa JM, Santner J, Puschenreiter M, Prohaska T, Moreno-Jiménez E (2016) Iron plaque formed under aerobic conditions efficiently immobilizes arsenic in Lupinus albus L roots. Environ Pollut 216:215–222
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem 284:217–220
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Anjum NA, Gill R (2015) Superoxide dismutase–mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res Int 22:10375–10394
Gomes MP, Carvalho M, Marques TCLLSM, Duarte DM, Nogueira COG, Soares AM, Garcia QS (2012) Arsenic-sensitivity in Anadenanthera peregrina due to arsenic-induced lipid peroxidation. Int J Appl Sci Technol 2:55–63
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puertas MC, Sandalio LM (2013) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gusman GS, Oliveira JA, Farnese FS, Cambraia J (2013) Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol Plant 35:1201–1209
Hajiboland R (2014) Reactive oxygen species and photosynthesis. In: Ahmad P (ed) Oxidative damage to plants, 3rd edn. Academic Press, India, pp 1–63
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Han YH, Fu JW, Chen Y, Rathinasabapathi B, Ma LQ (2016) Arsenic uptake, arsenite efflux and plant growth in hyperaccumulator Pteris vittata: role of arsenic-resistant bacteria. Chemosphere 144:1937–1942
Hariyadi, Yanuwiadi B, Polii B, Soemarno (2013) Phytoremediation of arsenic from geothermal power plant waste water using Monochoria vaginalis, Salvinia molesta and Colocasia esculenta. Int J Biosci 3:104–111
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hettick BE, Cañas-Carrell JE, French AD, Klein DM (2015) Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J Agric Food Chem 19:7097–7107
Hofffman H, Schenk M (2011) Arsenite toxicity and uptake rate of rice (Oryza sativa L.) in vivo. Environ Pollut 159:2398–2404
Hu M, Li F, Liu C, Wu W (2015) The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci Rep 5:13611. https://doi.org/10.1038/srep13611
Hunt R (1978) Plant growth analysis (Studies in Biology). Edward Arnold Ltd., London
Jasrotia S, Kansal A, Mehra A (2017) Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl Water Sci. 7:889–896
Kanwar MK, Poonam, Bhardwaj R (2015) Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol Environ Saf 115:119–125
Khang VH, Hatayama M, Inoue C (2012) Arsenic accumulation by aquatic macrophyte coontail (Ceratophyllum demersum L.) exposed to arsenite, and the effect of iron on the uptakeof arsenite and arsenate. Environ Exp Bot 83:47–52
Kumar S, Dubey RS, Tripathi RD, Chakrabarty D, Trivedi PK (2015) Omics and biotechnology of arsenic stress and detoxification in plants: current updates and prospective. Environ Int 74:221–230
Kumar M, Rahman MM, Ramanathan AL, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Kumari S, Kumar B, Sheel R (2017) Biological control of heavy metal pollutants in water by Salvinia molesta. Int J Curr Microbiol App Sci 6:2838–2843
Leão GA, Oliveira JA, Felipe RTA, Farnese FS (2017) Phytoremediation of arsenic-contaminated water: the role of antioxidant metabolism of Azolla caroliniana Willd. (Salviniales). Acta Bot Bras 31:161–168
LeBlanc MS, McKinney EC, Meagher RB, Smith AP (2013) Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol 163:1–9
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis thaliana. Plant Physiol 152:2211–2221
Luque GM, Bellard C, Bertelsmeier C (2014) The 100th of the world’s worst invasive alien species. Biol Invasions 16:981–985
Marin AR, Pezeshki SR, Masscheleyn PH, Choi HS (1993) Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic and photosynthesis in rice plants. J Plant Nut 16:865–880
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Miranda CV, Schwartsburd PB (2016) Aquatic ferns from Viçosa (MG, Brazil): Salviniales (Filicopsida; Tracheophyta). Braz J Bot 39:935–942
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Mohammadi M, Karr AL (2001) Superoxide anion generation in effective and ineffective soybean root nodules. J Plant Physiol 158:1023–1029
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-especific peroxidase en spinach chloroplasts. Plant Cell Physiol 22:867–880
Newete SW, Byrne MJ (2016) The capacity of aquatic macrophytes for phytoremediation and their disposal with specific reference to water hyacinth. Environ Sci Pollut Res Int 23:10630–10643
Nicomel NR, Leus K, Folens K, Voort PV, Du Laing GD (2016) Technologies for arsenic removal from water: current status and future perspectives. Int J Environ Res Public Health 13:62. https://doi.org/10.3390/ijerph13010062
Ozturk F, Duman F, Leblebici Z, Temizgul R (2010) Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ Exp Bot 69:167–174
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00133
Pommerrenig B, Diehn TA, Bienert GP (2015) Metalloido-porins: essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci 238:212–227
Rahman MA, Hasegawa H (2011) Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83:633–646
Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidante defense and glyoxalase systems and stress markers. BioMed Res Int. https://doi.org/10.1155/2015/340812
Reed ST, Ayala-Silva T, Dunn CB, Gordon GG (2015) Effects of arsenic on nutrient accumulation and distribution in selected ornamental plants. Agric Sci 6:1513–1531
Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Rout JR, Sahoo SL (2013) Antioxidant enzyme gene expression in response to copper stress in Withania somnifera L. Plant Growth Regul 71:95–99
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shaibur MR, Kawai S (2009) Effect of arsenic on visible symptom and arsenic concentration in hydroponic Japanese mustard spinach. Environ Exp Bot 67:65–70
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Sharma I (2013) Arsenic-induced oxidative stress and antioxidant defense system of Pisum sativum and Pennisetum typhoides: a comparative study. Res J Biotech 8:48–56
Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic binding to proteins. Chem Rev 113:7769–7792
Shen J, Song L, Müller K et al (2016) Magnesium alleviates adverse effects of lead on growth, photosynthesis, and ultrastructural alterations of Torreya grandis seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01819
Silva AA, Oliveira JA, Campos FV, Ribeiro C, Farnese FS (2017) Role of glutathione in tolerance to arsenite in Salvinia molesta, an aquatic fern. Acta Bot Bras. https://doi.org/10.1590/0102-33062017abb0087
Singh AP, Dixit G, Kumar A (2015a) Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci. https://doi.org/10.3389/fpls.2015.01272
Singh VP, Singh S, Kumar J, Prasad SM (2015b) Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 252:1217–1229
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Talukdar D (2013) Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19:69–79
Talukdar D, Talukdar T (2013) Superoxide-dismutase deficient mutants in common beans (Phaseolus vulgaris L.): genetic control, differential expressions of isozymes, and sensitivity to arsenic. BioMed Res Int. https://doi.org/10.1155/2013/782450
Upadhyaya H, Shome S, Roy D, Bhattacharya MK (2014) Arsenic induced changes in growth and physiological responses in Vigna radiata seedling: effect of curcumin interaction. Am J Plant Sci 5:3609–3618
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Xu W, Dai W, Yan H et al (2015) Arabidopsis NIP3;1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol Plant 8:722–733
Yamaguchi N, Ohkura T, Takahashi Y, Maejima Y, Arao T (2014) Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ Sci Technol 48:1549–1556
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgements
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Federal University of Viçosa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, A.A., de Oliveira, J.A., de Campos, F.V. et al. Phytoremediation potential of Salvinia molesta for arsenite contaminated water: role of antioxidant enzymes. Theor. Exp. Plant Physiol. 30, 275–286 (2018). https://doi.org/10.1007/s40626-018-0121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-018-0121-6