Abstract
Influences of sulfur (S) on the accumulation and detoxification of arsenic (As) in Vallisneria natans (Lour.) Hara, an arsenic hyperaccumulating submerged aquatic plant, were investigated. At low sulfur levels (<20 mg/L), the thiols and As concentrations in the plant increased significantly with increasing sulfate nutrient supply. If sulfur levels were above 20 mg/L, the thiols and As concentrations in the plant did not increase further. There was a significant positive correlation between thiols and As in the plant. As(III) is the main form (>75%) present in the plant after exposure to As(V). Sulfur plays an important role in the arsenic translocation and detoxification, possibly through stimulating the synthesis of thiols and complexation of arsenite-phytochelatins. This suggests that addition of sulfur to the arsenic-contaminated water may provide a way to promote arsenic bioaccumulation in plants for phytoremediation of arsenic pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Arsenic (As), a well-known carcinogen, is ubiquitous in the environment and considered to be a major environmental pollutant (Smith et al. 2002; Mondal et al. 2006). Millions of people all over the world, especially in South-East Asia, have suffered As poisoning from consumption of As-contaminated water or foods (Nordstrom 2002; Xia and Liu 2004; Yu et al. 2017).
Submerged plants are those that grow fully immersed in the water. They get their nutrients directly from the water through their roots, stems and leaves and play a vital role in aquatic systems. Some species of submerged plants could be used for As phytoremediation because of their high As hyperaccumulating ability, such as Vallisneria neotropicalis (Lafabrie et al. 2011), Hydrilla verticillata (L.f) Royle (Xue and Yan 2011) and Callitriche lusitanica (Favas et al. 2012). Therefore, it is urgent to understand the mechanism of arsenic accumulation and detoxification in submerged plants for As phytoremediation.
Sulfur (S) is one of the essential elements for all living organisms because it forms part of important life sustaining molecules such as cysteine, thioredoxin, metallothionein, enzymes and vitamins (Hell 1997; Saito 2000). These compounds can improve plant tolerance to heavy metals through complexation and/or sequestration into vacuoles (Oliveira et al. 2014). For example, Zhang et al. (2011) reported that low sulfate-pretreated rice accumulated less arsenite than high sulfate pretreated plants, but the arsenite concentrations in shoots of low sulfate pretreated rice were higher than those of high sulfate pretreated. Sulfur deprivation in rice increased the translocation of arsenic from roots to shoots; Oliveira et al. (2014) examined the effects of S on arsenic uptake by Pteris vittata (L.) and found that addition of sulfate enhanced As uptake and translocation by 26%–28%.
Vallisneria natans (Lour.) Hara, a widely distributed submerged aquatic plant, is a promising plant species for phytoremediation of arsenic contaminated water (Chen et al. 2015). The objectives of this study were to determine the influences and mechanism of sulfur on the arsenic accumulation by V. natans and provide a way to improve arsenic removal from contaminated water.
Materials and Methods
Vallisneria natans were obtained from the Xiangjiang River, China. Then the plants were planted in a greenhouse pond until new shoots and roots had developed. After pre-culture, three replicates of four equally-sized plants (30 g total weight) were transferred to 10 L pots and exposed to 0.2 mg/L As and different levels of S (0, 5, 10, 20, 40 mg/L). As and S were added as Na2HAsO4 and MgSO4. Plants were harvested at 7 d and rinsed repeatedly with de-ionized water. Half of the plant samples were oven dried to constant weight at 60°C for 72 h, the remaining half were frozen in liquid nitrogen and stored at −80°C for analysis of thiol compounds and malondialdehyde (MDA).
The oven-dried plant samples were ground with a stainless steel plant mill. Then, 0.5 g plant materials were digested with HNO3–H2O2 on a heating block at 150°C until the digest had cleared. Finally, the concentrations of total As and As(III) were measured by an atomic fluorescence atomic absorption spectrometry coupled with a hydride generation system (Agilent 240FS, USA) following the method of Chen et al. (2015). The detection limit of this method was 0.5 μg/L. At 1.0 μg/L the relative standard deviation of this method was 2.4%–6.8%. The recovery of the added standards was 92%–106%. Analytical reagents and deionized water were used throughout.
The thiols and malondialdehyde (MDA) content of the plants were estimated according to Rama Devi and Prasad (1998). About 0.5 g fresh frond of plants from each treatment was homogenized in 4 mL ice-cold 0.5% thiobarbituric acid (TBA) in 10% trichloroacetic acid (TCA) and centrifuged at 14,000×g for 15 min at 4°C. Thiols and malondialdehyde (MDA) were measured at 560 and 600 nm with a fluorescence spectrophotometer using the method of Rama Devi and Prasad (1998).
Data are presented as mean(s) ± standard deviation (SD). One-way or two-way analysis of variance (ANOVA) was performed to test the significant differences between treatments using windows-based SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The level for statistical significance was set at p < 0.05. Pearson’s correlation coefficients were determined to reveal potential correlations between As concentrations and thiol levels in the plant after exposure to different sulfur treatments.
Results and Discussion
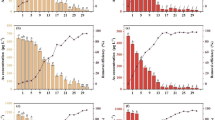
To understand the influences of sulfur on As uptake by V. natans, the interactions between the uptake of As(V) and S were investigated. The effects of sulfur on As uptake by plants are summarized in Fig. 1, different letters indicate significant differences between treatments. After exposure to 0.2 mg/L As without sulfur addition (Control) for 7 d, the total arsenic concentration in V. natans was 70.7 mg/kg (DW). Increasing S nutrient supply increased the As concentration in the plant significantly at low S levels (<20 mg/L). If sulfur levels were above 20 mg/L, the As concentration in the plant did not increase further. Compared with the control, addition of 20 or 40 mg/L S enhanced As uptake by 197% and 203%, respectively. Results indicated that As concentrations in V. natans were influenced by sulfur addition. Increasing S nutrient supply enhanced As accumulation in the plant significantly at low S levels (<20 mg/L).
As(III) is the dominant species of arsenic in the plant, regardless of whether As is supplied as As(V) or As(III) (Wang et al. 2002). This is in consistent with our results that more than 75% of As in V. natans was As(III) under different treatments (Fig. 2). Figure 2 showed that the percentages of As(III) were also influenced by sulfur addition and had the same trend as the total As concentrations in the plant.
As(III) may be detoxified by chelation with thiol (SH)-containing compounds (Raab et al. 2005). Thiols, including phytochelatins (PCs) and glutathione (GSH), can function as non-enzymatic antioxidants. Reduction of As(V) to As(III) and complexation of As(III) with glutathione and phytochelatins, followed by sequestration of these complexes in vacuoles, is a major strategy for As detoxification in plants (Bleeker et al. 2006; Liu et al. 2010). Phytochelatins are synthesized from reduced glutathione by the enzyme phytochelatin synthase under the stress of arsenic or heavy metals (Clemens et al. 1999). Reduced glutathione is not only a substrate for PCs synthesis but also a reductant for enzymatic or nonenzymatic reduction of As(V) to As(III) (Bleeker et al. 2006; Dhankher et al. 2006). Increased synthesis of GSH under As stress has been observed in hypertolerant (Hartley-Whitaker et al. 2001), hyperaccumulator (Zhao et al. 2003; Cai et al. 2004) and non-hyperaccumulator plants (Srivastava et al. 2007). Nuclear magnetic resonance analysis showed that As(V) can be reduced to As(III) by glutathione (Delnomdedieu et al. 1994). Pickering et al. (2000) found that nearly 100% of arsenic was bound to thiols in Brassica juncea roots and shoots by X-ray absorption spectroscopy. The results of Raab et al. (2005) showed that up to 40% of As was arsenic-phytochelatin compound present in sunflower (Helianthus annuus) after 3 h. High S treatment resulted in the increase of As accumulation, likely due to As complexation through enhanced synthesis of thiolic ligands, such as non-protein thiols and phytochelatins (Dixit et al. 2015).
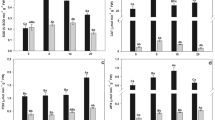
However, synthesis of thiol compounds lead to a growing demand for sulfur (McMahon and Anderson 1998). In our study, at low S levels (<20 mg/L), the concentrations of thiols in the plant increased with increasing S nutrient supply (Fig. 3). Treatments indicated by different letters are significantly different. At the same time, the total As concentrations and percentage of As(III) increased in the plant (Fig. 2), It is likely that the accumulated arsenic reduced to As(III) and combined with thiols which improve As accumulation capacity of V. natans. However, increasing sulfur concentration in the nutrient solution (from 20 to 40 mg/L), did not increase the synthesis of thiols further. This may be because the ability for synthesis was already maximal. Similarly, the concentration of arsenic and percentage of As(III) in the plant didn’t increase any more. There was a significant positive correlation between thiols and arsenic uptake by plant (r = 0.89). Therefore, increasing the use of sulfur fertilizer stimulate the synthesis of thiols that could be considered as an important mechanism for As bioaccumulation and detoxification in submerged plants.
The accumulation of metals in various parts of plants is often accompanied by oxidative stress and cell damage, which is directly connected with the metal tolerance capacity of the plants (Rama Devi and Prasad 1998). Usually, cell membrane is the primary site of injury by heavy metals (Kärenlampi et al. 2000). MDA can be used as an indicator for evaluating the degree of injury (Rama Devi and Prasad 1998). The variation of MDA in V. natans showed that increasing S nutrient supply reduced oxidative stress significantly. Different letters indicate significant differences between treatments (Fig. 4). It may be because that higher sulfur concentration in solution stimulated the synthesis of thiols which can combine with As(III). Thus, subsequent sequestration of As in vacuoles can reduce As stress. Of course, if the ability for synthesis was already maximal, the function of alleviating As stress didn’t increase any more.
In conclusion, increasing sulfate nutrient supply (<20 mg/L) significantly increased the arsenic bioaccumulation and detoxification in Vallisneria natans (Lour.) Hara, an arsenic hyperaccumulating plant, possibly by stimulating the synthesis of thiols and complexation of arsenite-phytochelatins. Sulfur plays an important role in the arsenic translocation and detoxification. Elucidation of the detailed mechanism needs further study, but it indeed provides a potentially useful way to improve the removal of arsenic from contaminated water.
References
Bleeker PM, Hakvoort HW, Bliek M, Souer E, Schat H (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45:917–929
Cai Y, Su J, Ma LQ (2004) Low molecular weight thiols in arsenic hyperaccumulator Pteris vittata upon exposure to arsenic and other trace elements. Environ Pollut 129:69–78
Chen G, Liu X, Brookes PC, Xu J (2015) Opportunities for phytoremediation and bioindication of arsenic contaminated water using a submerged aquatic plant: Vallisneria natans (Lour.) Hara. Int J Phytoremediation 17:249–255
Clemens S, Kim EJ, Neumann D, Schroeder JI (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. Embo J 18:3325–3333
Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ (1994) Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact 90:139–155
Dhankher OP, Rosen BP, Mckinney EC, Meagher RB (2006) Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc Natl Acad Sci USA 103:5413–5418
Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwivedi S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Favas PJC, Pratas J, Prasad MNV (2012) Accumulation of arsenic by aquatic plants in large-scale field conditions: opportunities for phytoremediation and bioindication. Sci Total Environ 433:390–397
Hartley-Whitaker J, Ainsworth G, Vooijs RBW, Schat H (2001) Phytochelatins are involved in differential arsenate tolerance in Holcuslanatus. Plant Physiol 126:299–306
Hell R (1997) Molecular physiology of plant sulfur metabolism. Planta 202:138–148
Kärenlampi S, Schat H, Vangronsveld J, Verkleij JAC, Lelie DVD, Mergeay M, Tervahauta AI, Romantschuk M (2000) Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ Pollut 107:225–231
Lafabrie C, Major KM, Major CS, Cebrian J (2011) Arsenic and mercury bioaccumulation in the aquatic plant, Vallisneria neotropicalis. Chemosphere 82:1393–1400
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152:2211–2221
McMahon PJ, Anderson JW (1998) Preferential allocation of sulphur into y-glutamylcysteinyl peptides in wheat plants grown at low sulphur nutrition in the presence of cadmium. Physiol Plant 104:440–448
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479
Nordstrom DK (2002) Public health. Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Oliveira LMD, Ma LQ, Santos JAG, Guilherme LRG, Lessl JT (2014) Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata (L.). Environ Pollut 184:187–192
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122:1171–1177
Raab A, Schat H, Meharg AA, Feldmann J (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168:551–558
Rama Devi S, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3:188–195
Smith AH, Lopipero PA, Bates MN, Steinmaus CM (2002) Public health. Arsenic epidemiology and drinking water standards. Science 296:2145–2146
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and Antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, Mcgrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Xia Y, Liu J (2004) An overview on chronic arsenism via drinking water in PR China. Toxicology 198:25–29
Xue P, Yan C (2011) Arsenic accumulation and translocation in the submerged macrophyte Hydrilla verticillata (L.f.) Royle. Chemosphere 85:1176–1181
Yu Y, Guo Y, Zhang J, Xie J, Zhu Y, Yan J, Wang B, Li Z (2017) A perspective of chronic low exposure of arsenic on non-working women: risk of hypertension. Sci Total Environ 580:69–73
Zhang J, Zhao Q, Duan G, Huang Y (2011) Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ Exp Bot 72:34–40
Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, Mcgrath SP (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159:403–410
Acknowledgements
This work was supported by the Natural Science Foundation of China (Nos. 41501343, 31671635, 31400374 and 51408214), Scientific Research Foundation of Hunan Provincial Education Department (Nos. 14B066 and 15C0534).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Feng, T., Li, Z. et al. Influence of Sulfur on the Arsenic Phytoremediation Using Vallisneria natans (Lour.) Hara. Bull Environ Contam Toxicol 99, 411–414 (2017). https://doi.org/10.1007/s00128-017-2135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2135-1