Abstract
In savanna environments, plants have specific leaf traits to deal with high irradiance. These traits allow plants to show high carbon assimilation capacity. However, under encroachment, reduced light availability may act as a filter on traits of plants established under typical savanna conditions. Here we studied morpho-physiological traits of species exclusively found in typical and forested savanna conditions to evaluate how encroachment selects for specific leaf traits in such environments. We also evaluated if species occurring in distinct encroached situations would show plasticity to deal with light variations. We studied two species exclusively found in typical savanna (TS, open condition), two species exclusively found in forested savanna (FS, encroached condition) and two species growing along a gradient of tree encroachment (typical, dense and forested savanna). We measured specific leaf area (SLA), maximum photosynthetic rate in an area basis (Amax), stomatal conductance (gs), water use efficiency (WUE), leaf carbon (C) and nitrogen (N) concentrations. We found that herbaceous species exclusively found in TS possess higher Amax, gs, WUE and C in comparison with plants from forested savanna. Such strategies are necessary to thrive under environments with elevated irradiances. In turn, species from FS showed elevated SLA and foliar N concentration, strategies linked to capture diffuse light in forested environments. Species capable of thriving in sites with distinct degrees of encroachment changed their leaf traits according with light availability. We conclude that differences in leaf traits between typical and forested savanna species may explain the non-occurrence of typical savanna species when their environment become encroached. Only those species capable of showing a certain degree of plasticity may survive under such distinct encroached states.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The assessment of leaf traits values is of fundamental importance to understand plant establishment, growth and persistence in the environment (Poorter and Bongers 2006; Violle et al. 2007). This is particularly the case for traits associated with carbon gain, considering morphological (specific leaf area) and physiological aspects (maximum photosynthesis and stomatal conductance) (Pérez-Harguindeguy et al. 2013). Many studies reported how these traits enable plant persistence in savanna environments (Lemos Filho 2000; Prado et al. 2004; Franco et al. 2005; Habermann et al. 2011; Souza et al. 2015; Rossatto and Franco 2017); which are characterized by a series of selective pressures such as fire, rainfall seasonality, lower content of soil nutrients and elevated irradiance (Franco 2002). Such studies, however, focused only on tree species, a diverse, but not unique, component of the savanna ecosystem.
The savannas of Brazil (“Cerrado”) are characterized by a great richness of species, showing diversified types of growth forms (Mendonça et al. 2008). The arboreal component is very diverse, but it is outnumbered by the herbaceous component in a proportion of 7:1 (Mendonça et al. 2008). The greater richness and diversity of such component is found at the typical savanna vegetation (regionally called cerrado sensu stricto) and some authors argue that these aspects are linked with the great availability of light niches in this environment, in contrast with more closed ones (Ludwig et al. 2004; Pinheiro et al. 2016). In fact, richness and diversity of exclusive species from typical savanna decrease when this vegetation starts a process of tree encroachment: encroached environments show higher canopy cover and lower irradiation reaching plants in the understory, which are the main drivers of species richness and diversity (Pinheiro et al. 2016).
Light availability is one of the most important factors affecting plant survival (Givnish 1988; Bond and Midgley 2001), mainly because it is a fundamental resource for plants to perform photosynthesis and produce necessary carbohydrates for its survival and growth (Cunningham 1997). Light availability is very variable considering vegetation types: in forests, this resource is abundant in the canopy, but scarce in the understory given the stratification and elevated density of trees (Niinemets 2007). In contrast, light is highly available in savanna environments, given the low stature and low density of its arboreal component (Franco and Lüttge 2002; Finch et al. 2004).
The high irradiance in typical savanna sites is a significant factor that selects for specific leaf traits (Eamus et al. 1999; Franco 2002; Ludwig et al. 2004). Savanna tree species possess traits reported to deal with this high irradiance: in terms of morphology, many of the savanna plants possess thicker leaves with low specific leaf area, prominent cuticle and a dense and compact leaf structure (Bieras and Sajo 2009; Rossatto et al. 2015). Physiological aspects include elevated assimilation rates on an area basis, high water use efficiency and low nitrogen content (Franco et al. 2005). In contrast, species from forest environments show high specific leaf area and nutrient concentration, while showing low maximum assimilation rates (Rossatto et al. 2013). Such aspects are well reported for trees; however, physiological aspects of the herbaceous component have been occasionally reported for savannas (Rossatto and Franco 2017).
The understanding of how light availability selects leaf strategies in savanna environments is of utmost importance because savannas can be transformed into forests under fire absence (Murphy and Bownman 2012; Durigan and Ratter 2016). This transformation by means of encroachment may imply the creation of a strong environmental filter, which can select specific traits, especially those for carbon acquisition. This is especially the case for herbaceous plants, which grow in the understory and can be drastically affected by the encroachment caused by the advance of forests in direction to typical savanna sites under fire absence (Durigan and Ratter 2016). In fact, studies have shown that few species can appear in vegetations differing in the degree of tree encroachment: the majority of species are exclusively found in typical or forested savanna (encroached situation), and only few can appear in both states (Pinheiro et al. 2016). The presence of exclusive and common species at different degrees of tree encroachment provides an interesting opportunity to test whether savanna and forest herbaceous plants differ in their leaf traits; additionally, these data can provide information about possible occurrence of species in different environments.
Here we analyzed leaf morpho-physiological traits to understand the functional responses of herbaceous savanna plants in response to a tree encroachment gradient. Given the high light availability in the understory, created by the lower canopy cover in typical savanna environment, we propose that herbaceous savanna species possess leaf traits related to persistence under high irradiances, and in this way, their leaf strategies would drastically differ from herbaceous plants growing under encroached situation. We hypothesized that plants exclusively found in typical savanna will possess leaves with low specific leaf area and elevated gas exchange rates; while plants in forested savanna will show leaves with higher specific leaf area and lower rates of gas exchange. In contrast, we expected that species capable of surviving along the entire tree encroachment gradient will show some degree of plasticity, and will adequate their leaf traits to each light condition found along the tree encroachment gradient.
2 Materials and methods
2.1 Study site and experimental design
We conducted our study at the Assis Ecological Station (AES), located in the municipality of Assis, São Paulo state (SP) (22º33′20″W and 50º21′27″S). The average annual precipitation at AES is approximately 1.400 mm, with a rainy period between September and May and the dry season spanning between June and August. Average temperatures are 22 °C. AES is one of the fewest ecological stations with a considerable variety of Cerrado plant physiognomies, ranging from typical savannas (cerrado sensu stricto) to forested physiognomies (cerradão, gallery and semi-decidous forest) (Durigan et al. 1999). Within the AES area, the typical savanna vegetation underwent encroachment in the past 50 years due to fire prevention and suppression techniques and, as consequence, many typical savanna sites became denser and even forested (Durigan and Ratter 2006; Pinheiro and Durigan 2012).
The study site consisted of regions where it was possible to find a gradient of tree encroachment spanning from typical savanna to forested savanna. In such regions we assembled five transects, in a way that each transect possessed 165 m in length and 4 m wide, representing three different stages of tree encroachment (see details on Pinheiro et al. 2016). These stages were: typical savanna (TS; a vegetation type possessing discontinuous tree layer and a herbaceous-grass stratum); dense savanna (DS; a TS site at intermediate stage of encroachment possessing a dense tree layer) and forested savanna (FS; previously a TS vegetation that now possesses a continuous tree layer). We measured leaf area index (LAI) and photosynthetic active radiation (PAR) reaching the understory to characterize the light environment of each condition.
Based on the study by Pinheiro et al. (2016), which provided data on floristics and structure of herbaceous communities along a gradient of tree encroachment, we selected exclusive (found exclusively in one situation: TS or FS) and common species (found in all situations: TS, DS and FS) (Table 1). To recognize the leaf physiological strategies of exclusive species we selected a pair of monocots (Axonopus and Merostachys) and a pair of eudicots (Miconia falax and Miconia paucidens) (Table 1). To understand if species found in all studied encroachment situations possess leaf plasticity, we selected two species (one monocot, Rhynchospora and one eudicot, Psychotria) (Table 1). For both cases we used one monocot and one eudicot to control the phylogenetical influence. Leaf gas exchange, specific leaf area and carbon and nitrogen concentrations in the leaves were assessed.
2.2 Variables measured
To collect LAI and PAR data, measurements were performed in five plots per encroachment condition (TS, DS and FS) using a CI-110-24P-ID plant canopy imager (CID Bioscience, Camas, WA, USA). Hemispherical photographs were taken during early morning in October 2014 (07:00 am, rainy season) to measure the LAI. PAR measurements were taken with a CI-110-24P-ID (that has an integrated ceptometer with 24 photodiodes) and data were collected every 30 min in a period of 4 h between 08:00 a.m. and 12:00 p.m in sunny days. Each studied situation possessed differences in canopy cover and light irradiance reaching the understory (Fig. 1).
Values of canopy cover (LAI—leaf area index) and irradiance (PAR) reaching the herbaceous plants in the understory along a gradient of tree encroachment. Data retrieved from Pinheiro et al. (2016)
We measured six leaf traits reported as key traits to deal with light environment variations (Lüttge 2008): one morphological (specific leaf area—SLA) and five related to physiological processes: maximum assimilation rate on an area basis (Amax), stomatal conductance (gs), water use efficiency (WUE), leaf carbon and nitrogen content in the dry matter (in %). For each case, traits were measured on one healthy and completely expanded leaf, from ten individuals of each species in each encroachment condition. Gas exchange traits were measured with a portable open photosynthesis system (LCpro-SD, Analytical Development Co., Hoddesdon, U.K.) during the wet season (October 2014). We determined the maximum CO2 assimilation rate (Amax) and stomatal conductance (gs) on a leaf area basis under ambient CO2 (399-410 ppm), a light intensity of 1400 µmol m−2 s−1 and with temperatures maintained at 26.5 ± 1.25 °C. WUE was calculated as the ratio between Amax and gs. After gas exchange measurements, each leaf was collected and scanned on a flatbed scanner (150 dpi resolution) and its area (in cm2) determined using the free software Image J. After leaf area determination, these leaves were weighted on a scale (±0.0001 g), the SLA was then determined as the ratio between leaf area (cm2) and leaf dry weight (g). Leaves used for SLA determination were grinded and a subsample (100 mg) was used for total C and N determinations. Leaf C and N content were determined using an Elemental Analyzer CN628 (LECO Corporation) in the Plant Physiology Laboratory (University of Brasilia).
2.3 Data analysis
To verify differences in the studied traits between encroachment conditions, we performed a MANOVA analysis, since trait values were determined at the same leaf in each sampled individual and these showed high degree of correlation. All data were checked against the premises of normality (Kolmogorov–Smirnov test) and variances homogeneity (Levene’s test). When the assumptions were not met, data was transformed using log10. For all analyses, we considered alpha as 0.05. We used the principal components analysis (PCA) to verify whether species occurring along the encroachment gradient shows similar syndrome of functional leaf traits to that found for typical or forested savanna situations. Specifically, we expected that R. albiceps and P. hoffmannseggiana individuals growing in typical savanna would be grouped with species exclusively found in the savanna site (M. fallax and A. pressus). In contrast, when their specimens were growing in dense conditions, they would group with species exclusively found in the forested savanna (M. skvortzovii and M. paucidens). All data were standardized (z-transformation) before applying the analysis. For the PCA, we used the variance–covariance matrix method (Gotelli and Ellison 2004). Only the two most significant axes were presented in this analysis. PCA was performed with PAST 2.17b free software (Hammer et al. 2001).
3 Results
3.1 Functional strategies of exclusive and common species
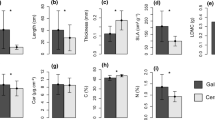
We found significant differences between leaf traits of the monocot and eudicot species (MANOVA Wilks = 0.00005, df = 18, F = 36.33 and P < 0.01). Independently of phylogeny, when compared with forested species, typical savanna species showed higher values of Amax, gs, WUE and Leaf C (Fig. 2a, b, c, e), but lower values for SLA and leaf N concentration (Fig. 2d, f). These patterns were very similar for the common species occurring along the gradient of tree encroachment (Fig. 3). Both, Psychotria (MANOVA Wilks = 0.012, df = 12, F = 3.83 and P = 0.045) and Rhynchospora (MANOVA Wilks = 0.002, df = 12, F = 8.83 and P < 0.01), increased their values of Amax, gs and leaf C concentration from the forested to the typical savanna (Fig. 3a, b, e). Leaf N and SLA decreased from forested savanna to typical savanna (Fig. 3d, f). Different from the exclusive species, common species showed higher WUE in forested environment when compared to typical savanna (Fig. 3c).
Average values for studied leaf traits in the exclusive species found at forested and typical savanna. a maximum leaf assimilation rate (Amax); b stomatal conductance (gs); c water use efficiency (WUE); d specific leaf area (SLA); e leaf carbon concentration and f leaf nitrogen concentration. Vertical bars indicate standard error of means (n = 10 per species). Letters indicate differences between forest and typical savanna and asterisks denotes differences between monocot and eudicot species (α = 0.05)
Average values for studied leaf traits in the common species found along a gradient of tree encroachment. a maximum leaf assimilation rate (Amax); b stomatal conductance (gs); c water use efficiency (WUE); d specific leaf area (SLA); e leaf carbon concentration and f leaf nitrogen concentration. Vertical bars indicate standard error of means (n = 10 per species). Letters indicate differences between species and conditions (α = 0.05)
3.2 Principal component analysis
The first and second axes of the PCA explained, respectively, 54.85 and 26.25% of the variation in the analyzed traits. Using PCA it was possible to visualize a clear separation between the species exclusively found in typical and forested savanna (Fig. 4). Typical savanna species were placed on the left of the first axis, which was characterized by elevated rates of Amax and gs. In contrast, typical forest species were placed on the right of the first axis, where SLA and leaf nitrogen were higher (Fig. 4). The species which are capable of growing along the entire gradient of tree encroachment were placed at different positions: while Psychotria tended to be placed at the same side of the typical savanna species, Rhynchospora appeared side by side with typical forest species. The individuals growing in typical savanna conditions were placed closer to typical savanna species than those growing in forested environment.
Principal component analysis using all studied traits. Species: Mfal S = Miconia fallax (savanna); Axo S = Axonopus pressus (savanna); Mer F = Merostachys skvortzovii (forested savanna); Mpau F = Miconia paucidens (forest); Rhyn S = Rhynchospora albiceps (savanna); Rhyn D = R. albiceps (dense savanna); Rhyn F = R. albiceps (forested savanna); Psy S = Psychotria hoffmannseggiana (savanna); Psy D = P. hoffmannseggiana (dense savanna) and Psy F = P. hoffmannseggiana (forested savanna). Traits: Amax = maximum photosynthetic rate; gs = stomatal conductance; WUE water use efficiency, SLA specific leaf area, Leaf C leaf carbon and Leaf N leaf nitrogen
4 Discussion
As expected, differences in canopy cover and light irradiance were found, as well as clear differences between exclusively typical and forested savanna herbaceous species. Typical savanna species showed leaf traits linked with persistence under elevated irradiances, while species from forested environment showed leaf traits to persist under low irradiances. Species that occurred along the gradient of encroachment changed their leaf traits to deal with environmental conditions, thriving in each situation.
Under the typical savanna conditions, where canopy cover is low and irradiance is high, plants may adjust their leaf strategies in a manner that they can capture the necessary energy to perform photosynthesis, but would also have to deal with excessive light (Franco et al. 2007; Lawlor and Tezara 2009). The species exclusively found in the savanna environment showed lower values of specific leaf area, which are commonly reported to be related with investment in leaf and cuticle thickness, mechanisms that can filter excessive light (Niinemets 1999). This type of leaf structure are related to high leaf carbon but lower leaf nitrogen content (Evans and Poorter 2001), a response reported worldwide by the leaf economic spectrum (Shipley et al. 2006). The investment in leaf thickness in such species are normally followed by larger layers of palisade parenchyma (Rossatto et al. 2015), which in turn can provide elevated carbon assimilation rates on an area basis (Evans and Poorter 2001). These elevated rates, coupled with the higher gs result in savanna plants showing elevated WUE. This fact was mainly reported for tree species (Habermann et al. 2011; Da Veiga and Habermann 2013), and herbaceous species seems to follow a similar pattern.
In contrast to the typical savanna species, forested savanna species showed leaves with lower SLA, but with higher leaf nitrogen content. These aspects are an important way to increase light capture, since it involves thinner leaves with higher chlorophyll content, as a necessary strategy to capture the diffuse light in the forest environment (Givnish 1988). These strategies, however, were adequate to the forested site only; if these plants were exposed to the higher irradiances of savanna sites, their leaf structure and physiological parameters would probably not respond adequately to the excess of photon flux, leading to severe damage in the photosynthetic apparatus, followed by leaf death (Dai et al. 2009). This mechanism may explain why the majority of exclusively herbaceous forest species does not appear at savanna sites that are more open (Pinheiro et al. 2016).
Species growing along the gradient of encroachment showed similar responses to the patterns described previously. When growing in savanna environment, leaf traits were related to conditions where elevated irradiance is present, and when growing in forested environment they showed leaf traits related to conditions where low irradiance is present. Under savanna conditions, both, Psychotria and Rhynchospora, showed leaves with lower SLA and leaf N, which were coupled with elevated carbon assimilation rates, contrasting with the forested savanna conditions, where they showed leaves with high SLA, high N and low Amax. This demonstrates that some species can grow under different irradiances, pointing out that phenotypic plasticity may be an important event that can explain possible survival of typical savanna plants under encroached conditions. Adjustments of leaf traits to distinct light environments have been reported for many savanna tree species, (Rossatto and Kolb 2010; Bedetti et al. 2011; Habermann et al. 2011), and this may be the first report of such responses in herbaceous species from this vegetation.
When analyzed in a multivariate space, the results demonstrated clear differences between exclusive species of typical and forested savanna, independently of their phylogenetic relationship. Both the monocot and the eudicot grouped by their environment of origin suggest that environment has a strong effect on the leaf traits. In fact, many physiological traits (such as Amax and gs) are more affected by environment rather than by phylogeny (Rossatto et al. 2009, 2013). Concerning the species that appeared along the entire gradient, these were grouped more by their affinity as species rather than encroached situation; in this way, independently of where they were growing, Psychotria and Rhynchospora tended to form two separate groups. Even forming these groups, those ones thriving under savanna conditions were more closely placed to the typical savanna species than those growing under dense or forested savanna conditions.
Our results point out important insights concerning the occurrence of savanna herbaceous species under a scenario of forest advance into typical savanna. Structural changes in the vegetation cover can affect light availability necessary for plants (seedlings, saplings and even adults leaves) to achieve their compensation and saturation points, which in turn can limit the carbon gain and survival under these circumstances (Lemos-Filho et al. 2010). Several studies reported the disappearance of typical savanna species when savanna sites are under encroachment (Durigan and Ratter 2006; Pinheiro and Durigan 2012; Pinheiro et al. 2016). In fact, Pinheiro et al. (2016) suggested that species are disappearing in encroached sites because light availability may be filtering certain morpho-physiological strategies. Our data gives support to this idea, since at encroached sites, plants with low specific leaf area and high leaf nitrogen can thrive, and then have the capacity to capture the diffuse light. Strategies reported for typical savanna species are not adequate to capture diffuse light since the majority of carbon and nutrients are invested in non-photosynthetic tissues (Rossatto et al. 2015). Detailed studies involving light response curves are necessary to determine the light compensation and saturation points of typical savanna species under lower irradiances.
In summary, we demonstrated differences in leaf traits between exclusively typical and forested savanna species and that these differences may in part explain the non-occurrence of typical savanna species when their environment becomes encroached. We also show that species that appear along different degrees of canopy cover may have a certain degree of leaf plasticity, since they are capable of modifying their leaf traits according with the degree of light availability.
References
Bedetti CS, Aguiar DB, Jannuzzi MC, Moura MZD, Silveira FAO (2011) Abiotic factors modulate phenotypic plasticity in an apomictic shrub [Miconia albicans (SW.) Triana] along a soil fertility gradient in a Neotropical savanna. Aust J Bot 59:274–282
Bieras AC, Sajo MG (2009) Leaf structure of the cerrado (Brazilian savanna) woody plants. Trees 23:451–471
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol Evol 16(1):45–51
Cunningham SA (1997) The effect of light environment, leaf area, and stored carbohydrates on inflorescence production by a rain forest understory palm. Oecologia 111(1):36–44
Da Veiga EB, Habermann G (2013) Instantaneously measured traits may detect non-plastic ecophysiological performances in response to drought, explaining distributions of Styrax species in the Cerrado. Trees 27(6):1737–1745
Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65(2):177–182
Durigan G, Ratter JA (2006) Successional changes in cerrado and cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962-2000. Edinburgh J Bot 63:119–130
Durigan G, Ratter JA (2016) The need for a consistent fire policy for Cerrado conservation. J Appl Ecol 53:11–15
Durigan G, Bacic MC, Franco GADC, Siqueira MF (1999) Inventário florístico do cerrado na Estação Ecológica de Assis, SP. Hoehnea 26:149–172
Eamus D, Myers B, Duff G, Williams D (1999) Seasonal changes in photosynthesis of eight savanna tree species. Tree Phys 19(10):665–671
Evans J, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24(8):755–767
Finch DA, Bailey WG, McArthur LJB, Nasitwitwi M (2004) Photosynthetically active radiation regimes in a southern African savanna environment. Agric For Meterol 122(3):229–238
Franco AC (2002) Ecophysiology of woody plants. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil. Columbia University Press, New York, pp 179–197
Franco A, Lüttge U (2002) Midday depression in savanna trees: coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 131(3):356–365
Franco AC, Bustamante MMC, Caldas LS, Goldstein G, Meinzer FC, Kozovits AR, Rundel P, Coradin VTR (2005) Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit. Trees 19:326–335
Franco AC, Matsubara S, Orthen B (2007) Photoinhibition, carotenoid composition and the co-regulation of photochemical and non-photochemical quenching in neotropical savanna trees. Tree Phys 27(5):717–725
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Funct Plant Biol 15(2):63–92
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Sunderland
Habermann G, Ellsworth PF, Cazoto JL, Simão E, Bieras AC (2011) Comparative gas exchange performance during the wet season of three Brazilian Styrax species under habitat conditions of cerrado vegetation types differing in soil water availability and crown density. Flora 206(4):351–359
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot 103(4):561–579
Lemos Filho JP (2000) Photoinhibition in three” cerrado” species (Annona crassifolia, Eugenia dysenterica e Campomanesia adamantium), in the dry and rainy seasons. Braz J Bot 23(1):45–50
Lemos Filho JP, Barros CFA, Dantas GPM, Dias LG, Mendes RS (2010) Spatial and temporal variability of canopy cover and understory light in a Cerrado of Southern Brasil. Braz J Biol 70:19–24
Ludwig F, de Kroon H, Berendse F, Prins HH (2004) The influence of savanna trees on nutrient, water and light availability and the understorey vegetation. Plant Ecol 170(1):93–105
Lüttge U (2008) Physiological ecology of tropical plants, 2nd edn. Springer, Berlin
Mendonça RC, Felfili JM, Walter BMT, Silva-Júnior MC, Rezende AB, Filgueiras TS, Nogueira PE, Fagg CW (2008) Flora vascular do bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora, vol 2. Planaltina, Embrapa Cerrados, pp 213–228
Murphy BP, Bowman DM (2012) What controls the distribution of tropical forest and savanna? Ecol Lett 15(7):748–758
Niinemets Ü (1999) Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144(1):35–47
Niinemets U (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Env 30(9):1052–1071
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61(3):167–234
Pinheiro ES, Durigan G (2012) Diferenças florísticas e estruturais entre fitofisionomias do Cerrado em Assis, SP, Brasil. Revista Árvore 36:181–193
Pinheiro LFS, Kolb RM, Rossatto DR (2016) Changes in irradiance and soil properties explain why typical non-arboreal savanna species disappear under tree encroachment. Aust J Bot 64(4):333–341
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecol 87(7):1733–1743
Prado CHBA, Wenhui Z, Cardoza Rojas MH, Souza GM (2004) Seasonal leaf gas exchange and water potential in a woody cerrado species community. Braz J Plant Phys 16(1):7–16
Rossatto DR, Franco AC (2017) Expanding our understanding of leaf functional syndromes in savanna systems: the role of plant growth form. Oecologia 183(4):953–962
Rossatto DR, Kolb RM (2010) Gochnatia polymorpha (Less.) Cabrera (Asteraceae) changes in leaf structure due to differences in light and edaphic conditions. Acta Bot Bras 24:605–612
Rossatto DR, Hoffmann WA, Franco AC (2009) Características estomáticas de pares congenéricos de cerrado e mata de galeria crescendo numa região transicional no Brasil Central. Acta Bot Bras 23:689–698
Rossatto DR, Hoffmann WA, Silva LDCR, Haridasan M, Sternberg LS, Franco AC (2013) Seasonal variation in leaf traits between congeneric savanna and forest trees in Central Brazil: implications for forest expansion into savanna. Trees 27(4):1139–1150
Rossatto DR, Kolb RM, Franco AC (2015) Leaf anatomy is associated with the type of growth form in Neotropical savanna plants. Bot 93(8):507–518
Shipley B, Lechowicz MJ, Wright I, Reich PB (2006) Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87(3):535–541
Souza MCD, Bueno PC, Morellato LP, Habermann G (2015) Ecological strategies of Al-accumulating and non-accumulating functional groups from the cerrado sensu stricto. An Acad Bras Ciênc 87(2):813–823
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116(5):882–892
Acknowledgements
We acknowledge the financial support of BIOTA FAPESP (Grants 2013/18049-6 and 2014/15304-8) and CNPq (Grant 301589/2015-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlos, N.A., Rossatto, D.R. Leaf traits combinations may explain the occurrence of savanna herbaceous species along a gradient of tree encroachment. Theor. Exp. Plant Physiol. 29, 155–163 (2017). https://doi.org/10.1007/s40626-017-0091-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-017-0091-0