Abstract
Objectives
Old age was identified as a strong risk factor for acute kidney injury (AKI). Our objectives were to provide estimates of AKI, risk factors and outcomes in patients ≥ 75 years for whom data are scarce.
Methods
Observational studies and randomized controlled trials between 2005 and 2021 with patients of mean or median age ≥ 75 years, reporting AKI according to current definitions. Data on AKI incidence, risk factors and mortality were analyzed separately in unselected (UC) and acute heart failure (AHF) cohorts.
Results
Twenty-six observational studies and 4 randomized controlled trials with 51,111 UC and 25,414 AHF patients were included. Ages averaged 79.4 and 79.8 years, respectively. Pooled risk ratios (RRs) of AKI rates were 26.29% (95% confidence intervals (CI) 13.20–41.97) (UC) and 24.21% (95% CI 20.03–28.65) (AHF). In both cohorts, AKI was associated with decreased estimated glomerular filtration rate at baseline, chronic kidney disease (UC: RR 1.80 (95% CI 1.15–2.80), AHF: RR 1.51 (95% CI 1.26–1.95) and hypertension (UC: RR 1.30 (95% CI 1.09–1.56), AHF: RR 1.07 (95% CI 1.05–1.09). RRs of AKI in patients on renin-angiotensin-inhibitors were 0.87 (95% CI 0.78–0.97) and 0.88 (95% CI 0.78–0.98) in UC and AHF, respectively. AKI was consistently associated with increased risk of in-hospital mortality (UC: RR 3.15 (95% CI 2.28–4.35), AHF: RR 4.28 (95% CI 2.53–7.24).
Conclusion
AKI is frequent in patients ≥ 75 years. While reduced renal function at baseline, CKD and hypertension were associated with AKI development, renin-angiotensin-inhibitors may be protective. Older AKI patients showed higher short-term mortality rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a heterogeneous group of serious conditions characterized by a rapid decrease in glomerular filtration rate (GFR) and/or renal output [1, 2]. In general, AKI occurs in the setting of acute illness. It is frequent and affects approximately 10–15% of adults admitted to hospital [1, 2]. Diagnostic and staging criteria for AKI were first standardized in 2004 and the understanding of the epidemiology of AKI has improved since then [2,3,4]. Despite this progress, AKI continues to be associated with high morbidity and mortality independently of other severe conditions. As there is currently no therapy for AKI, it is important to identify its exposures and susceptibility factors [1, 2]. When identified, prevention or modification of the respective risk factors may, as recently demonstrated, reduce AKI rate and severity [5, 6].
Older age has been associated with particularly high AKI rates and identified as an independent risk factor for AKI [2, 7,8,9,10,11]. Decreased ability to compensate renal insults and increased reduction of GFR may cause excessive vulnerability to develop AKI in older persons [11,12,13]. Furthermore, AKI increases the rates of chronic kidney disease (CKD) and mortality especially in this age group [9,10,11,12,13]. However, current data are limited as participants were usually categorized as older persons at an age of only 65 years. Worldwide, increased life expectancy has resulted in a continuous, disproportionate and rapid growth in the population of individuals 75 years and over [14, 15]. At present, people of 75 years and over are considered older persons and termed as such in the following text. In addition, most studies on AKI involving older persons were small, few applied current definitions of AKI and reported risk factors and outcomes. In view of this, the epidemiology of AKI in the population of 75 years and older is of particular interest as interventions in this age group may be most rewarding for the prevention of AKI.
Recently, numerous studies have been published which focused on AKI in older patients with acute heart failure (AHF), also termed cardiorenal syndrome type 1. Cardiorenal syndrome type 1 has a distinct pathophysiology, is highly frequent and increases morbidity and mortality substantially, especially in older persons [16, 17]. Data are scarce as to whether older patients with cardiorenal syndrome type 1 differ considerably from unselected cohorts (UC) with regard to AKI rates, demographics and outcomes. Therefore, we studied this selected population of older patients with AHF separately and compared them to UC to verify our analyses. Our systematic review and meta-analysis aimed to provide pooled estimations of AKI incidence, severity, susceptibility factors, exposures, and outcomes in both older UC and older AHF patients.
Methods
This systematic review and meta-analysis was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology [18].
Search strategy, study selection and inclusion criteria
Searches were conducted in Embase, Google Scholar, PubMed, and Scopus. The search strategy used the combinations of the search terms (“acute kidney injury” OR “acute renal failure” OR “cardiorenal syndrome”) AND (“old” OR “elderly”) and was limited to studies in English, French, and German published from January 1, 2005 through April 30, 2021. We chose this starting date for the search on the grounds of a landmark publication at the end of 2004, which introduced the first uniformly accepted and applied, standardized diagnostic and staging criteria for AKI, ‘Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease’ (RIFLE) [3]. The more recent definitions, Acute Kidney Injury Network (AKIN), Kidney Disease: Improving Global Outcomes (KDIGO) and its derivative worsening renal function (WRF) are based upon RIFLE with minor modifications [2, 4]. Before 2004, numerous AKI definitions existed which preclude the comparison of studies. In addition, we manually screened the reference lists of all identified publications and prior reviews in order to identify additional publications.
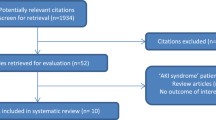
Publications were identified by one reviewer (SHR) and titles, journal and authors checked by another (KS) to identify duplicates (Fig. 1). After removing duplicate publications, records were screened by both reviewers using the abstracts. Publications which were considered eligible and thus included in this meta-analysis (1) were randomized controlled trials (RCTs) or observational studies published in peer-reviewed journals, (2) had a mean or median age of patients ≥ 75 years, (3) reported the data on AKI according to RIFLE, AKIN, KDIGO or WRF criteria, and (4) comprised a control group without AKI. In the AHF cohort, only patients with cardiorenal syndrome type 1, defined as any rapid deterioration of cardiac function by myocardial ischemia, arrhythmia, valvular heart disease, cardiomyopathies or other cardiac conditions, whether as new onset or as acutely decompensated heart failure causing acute renal impairment, were considered as having AKI [16, 19]. We intentionally excluded older patients receiving transcatheter aortic valve and hip replacement from the UC since (1) these cohorts undergo highly selected procedures, (2) they demonstrate very low risk of developing AKI (hip replacement), and (3) the data presently available would over-represent this population (transcatheter aortic valve). In addition, there are several current comprehensive systematic reviews and meta-analyses on AKI in these cohorts [20,21,22,23,24,25].
All articles identified as eligible were assessed as full-text by one reviewer (SHR) and checked by another (KS), again for inclusion criteria, adequacy of data with regard to AKI incidence, severity, susceptibility factors, exposures, outcomes, and multiple publications. That way we identified the studies included in our quantitative and qualitative analysis (Fig. 1). When multiple publications from the same study were found, data from the most inclusive report was used and the remaining articles were not considered.
Data extraction and definitions of variables
Two reviewers (KS and SHR) independently extracted data from full publications of the studies included in the analyses. Discrepancies were resolved by consensus or, if required, by discussion with the third researcher (AK). The following data were extracted from each study, where available: year and country of publication, study design, number of total and AKI patients, age, gender, AKI definition and criteria, stages and types of AKI (community or hospital acquired), renal replacement therapy (RRT) due to AKI, hypertension, diabetes mellitus, ischemic heart disease, chronic heart failure, CKD, malignancy, cardiac and major non-cardiac surgery, rhabdomyolysis, sepsis, shock, trauma, further major comorbidities (cerebral and peripheral artery disease, chronic obstructive lung disease, cirrhosis, et cetera), administration of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, combined as renin–angiotensin-system (RAS) inhibitors, non steroidal anti-inflammatory drugs (NSAIDs), and/or intraarterial iodinated radiographic contrast, all preceding the development of AKI, baseline serum creatinine and estimated GFR (eGFR), all-cause, in-hospital, and 1 year mortality rates. AKI and its stages were recorded according to RIFLE, AKIN, KDIGO or WRF criteria [2,3,4, 16]. Subsequently KDIGO and WRF were subsumed as KDIGO. Stage R from RIFLE and stages 1 from AKIN or KDIGO criteria, stages I and 2, and stages F and 3 were combined as stages 1–3, respectively. We primarily analyzed the outcomes of (1) incidence and severity of AKI and (2) susceptibility factors, exposures, and all cause mortality rates of AKI, separately in the populations of UC and AHF patients. In addition, sensitivity analyses were performed in the 2 distinct cohorts for hospitalized and non-hospitalized patients to provide more robust results.
Quality assessment
We assessed the quality of the included cohort studies using the Newcastle–Ottawa Scale (NOS) [26]. Total NOS scores were categorized into groups with low (7–9), high (4–6), and very high risk of bias (0–3 points) [27]. We applied the Cochrane Collaboration's tool to assess the risk of bias in RCTs [28], which were rated in each category as low risk (2 points), some concerns (1 point) or high risk of bias (0 points), and points were summed. The quality of RCTs was rated as excellent (12–14 points), good (9–11 points), moderate (6–8 points) or poor (≤ 5 points).
Statistical analysis
Variables were analyzed when at least 3 studies reported these data. Continuous variables were expressed as means ± SD or median (25th; 75th percentile). Categorical variables were expressed as percentages and total counts. The effect size was estimated by calculating standardized mean differences (SMD) with 95% confidence interval (CI) in continuous, and rate or risk ratios (RR) with 95% CI in categorical variables [28, 29]. We interpreted SMD values of 0–0.19 as insignificant, 0.20–0.49 as marginal, 0.50–0.79 as moderate, and 0.80–1.00 as large. Significant results were displayed using forest plots. We used the Cochran Q test to examine heterogeneity among studies [30]. P < 0.05 was considered as heterogeneous. I2 statistics were used to quantify the magnitude of heterogeneity [29]. An I2 value of ≤ 25% rendered insignificant, of 26–50% low, of 51–75% moderate and of > 75% high heterogeneity [29]. Random effect models were used in the case of I2 values ≥ 50%, whereas fixed effect models were used otherwise. Potential reporting biases were assessed by visual inspection of funnel plots. Funnel plot asymmetry was checked by Egger's test [31]. For all analyses two-sided P < 0.05 were considered as statistically significant.
Patient and public involvement
Patients and public were not involved in this study. As all patient data was anonymous, approval by the local ethics committee was not required. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
Literature search
The initial search yielded 4751 publications (Fig. 1). After removing duplicates, 2785 titles and abstracts were evaluated for eligibility. Hereafter, another 2484 records were excluded. The full texts of the remaining 301 articles were analyzed. Finally, 30 publications met the inclusion and exclusion criteria, 15 each from UC and with AHF patients. All were included in the following qualitative and quantitative analyses (Fig. 1) [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
Study description
There were 26 observational studies and 4 RCTs (Table 1). Most studies were small with widely varying numbers of participants (N = 291 (168; 836), range 50–25,521). This analysis included 51,111 patients in the UC and 25,414 with AHF. Most of the studies were carried out in Europe (n = 13) and Asia (n = 11), few in North America (n = 5) and Australia (n = 1).
Assessment of study quality, risk of bias and heterogeneity
The quality of the included studies was generally good. The median NOS score of the observational studies was 8 (8; 9). Twenty-five observational studies (96%) featured a low risk of bias according to the NOS (Suppl. Table 1). One study had a NOS score of 6 indicating a high risk of bias. The mean score of RCTs was 10 ± 1 points. Three RCTs were rated as good, and one as moderate (Suppl. Table 2). As demonstrated in Fig. 3A–J and Tables 2 and 3, most pooled data of AKI incidence, demographic characteristics, susceptibility factors and exposures were heterogeneous, both in the UC and AHF patients. There was no obvious asymmetry of the funnel plot suggesting no marked reporting bias for AKI incidence (Fig. 2). Egger’s tests confirmed the funnel plot symmetry (P = 0.12).
Demographic characteristics, AKI, susceptibility factors, exposure and mortality
Baseline characteristics of patients with and without AKI from both cohorts in the primary analyses are presented in Tables 2 and 3. Age did not substantially differ in the pooled data of patients from the UC and those with AHF.
In the studies of the primary analysis, AKI was predominantly defined according to KDIGO (n = 18) and by serum creatinine criteria (n = 27) (Table 1). The pooled AKI rates were 26.29% (95% CI 13.20–41.97) in the UC and 24.21% (95% CI 20.03–28.65) in AHF patients (Fig. 3A and B). AKI was moderately more frequent in the UC cohort [rate ratio 1.09 (95% CI 1.06–1.11); P < 0.001]. The analysis of AKI severity was based on 11 studies with 24,849 patients in the UC (Q 1144.3; P < 0.001; I2 99.13%) and on 4 studies with 2730 patients in the AHF cohort (Q 61.4; P < 0.001; I2 95.11%). While more than 60% of AKI patients in the UC featured more severe stages 2 and 3, almost 80% of AHF patients with AKI developed moderate stage 1 (P < 0.001) (Fig. 4). In contrast, a significantly higher proportion of AHF patients with AKI received RRT compared to those in the UC [14.94% (95% CI 10.93–19.92) vs. 5.59% (95% CI 4.09–7.46); rate ratio 3.18 (95% CI 2.29–4.32); P < 0.001]. The latter analysis was based on 3 studies with 823 AHF patients (Q 12.7; P = 0.002; I2 84.29%) and 9 studies with 19,282 patients in the UC (Q 233.1; P < 0.001; I2 96.57%).
A, B Pooled incidence of acute kidney injury in the unselected cohort (A) and in patients with acute heart failure (B). C, D Pooled risk ratio (RR) of acute kidney injury in patients with and without chronic kidney disease in the unselected cohort (C) and with acute heart failure (D). E, F Pooled risk ratio (RR) of acute kidney injury in patients with and without hypertension in the unselected cohort (E) and with acute heart failure (F). G, H Pooled risk ratio (RR) of acute kidney injury in patients with and without renin-angiotensin-inhibitors (RAS-I) in the unselected cohort (G) and with acute heart failure (H). I, J Pooled risk ratio (RR) of acute kidney injury in patients with and without chronic heart failure in the unselected cohort (I) and with acute heart failure (J). Incidences or RR with 95% confidence intervals (95% CI) are provided for fixed or random effect models according to the results of heterogeneity testing
No differences in age were found between patients with and without AKI in both cohorts (Tables 2, 3). Both cohorts were predominantly female. While in the UC there were significantly more male patients with AKI, no gender difference was detected in AHF patients with and without AKI. Pooled data from the AHF cohort demonstrated moderately higher baseline values of serum creatinine and lower eGFR values compared to the UC (Tables 2, 3). AKI patients in the UC had moderately reduced baseline eGFR.
In the UC and AHF patients, preexisting CKD was the strongest consistent susceptibility factor associated with AKI (Fig. 3C, D). Pooled data showed that hypertension was associated with significantly higher rates of AKI in both UC and AHF patients (Fig. 3E, F). In both cohorts, the administration of RAS inhibitors was associated with substantially lower rates of subsequent AKI (Fig. 3G, H). Chronic heart failure, diabetes mellitus and male gender were significantly more frequent in AKI patients, however only in the UC (Fig. 3I, J, Tables 2, 3). The use of NSAIDs was the only exposure associated with an increased risk of AKI, which could be demonstrated only in the UC (Tables 2, 3). There were no differences in the rates of other exposures such as ischemic heart disease, malignancy, sepsis, administration of intraarterial iodinated radiographic contrast or other nephrotoxins between UC patients with and without AKI (Table 2). Data on these variables were insufficient or unavailable in AHF patients. Studies from neither cohort provided sufficient data on other susceptibility factors and exposures such as ethnicity, other major comorbidities, cardiac and major, non-cardiac surgery, rhabdomyolysis, shock, trauma, or hypovolemia.
There was a significantly higher all-cause in-hospital mortality rate in AKI patients compared to those without AKI. This applied to both patients in the UC and those with AHF (Fig. 5A, B). The pooled data of 1‐year mortality did not demonstrate a significant difference between patients with and without AKI in the AHF cohort (Table 3). Data on this outcome in the UC were insufficient to perform an analysis.
Hospitalized and non-hospitalized patients
The majority of studies covered hospitalized patients (N = 17), a smaller proportion of non-hospitalized patients (N = 6) or both (N = 4), while 3 studies did not specify. The rate of AKI in non-hospitalized patients was significantly higher compared to hospitalized patients [33.98% (95% CI 21.11–48.22) vs. 22.66% (95% CI 18.25–27.39); rate ratio 1.50 (95% CI 1.39–1.63); P < 0.001]. While non-hospitalized patients had a higher proportion of AKI stage 1 [73.94% (95% CI 56.87–87.92), AKI stage 3 and AKI requiring RRT were more frequent in hospitalized patients (15.09% (95% CI 6.91–25.73) and 12.66% (95% CI 3.56–30.95), respectively]. The sensitivity analyses generally yielded results with regard to susceptibility factors, exposures, and increased in-hospital and one-year mortality which were consistent with the primary analyses (Tables 4, 5). In addition to the primary analysis, intraarterial iodinated radiographic contrast was associated with the development of AKI in hospitalized patients.
Discussion
Our systematic review and meta-analysis indicate that AKI is highly frequent in patients aged 75 years and older, which is the age group with the most rapid growth worldwide and who are currently considered older persons [14, 15]. The AKI rate was of a similar magnitude in the 2 independently studied populations, an unselected cohort and a selected one of AHF patients. Higher AKI stages indicated a large proportion of severe AKI in the UC. However, the higher rate of AHF patients with AKI who received RRT may also suggest frequent, severe AKI in that cohort. The pooled data suggest that reduced renal function at baseline and CKD were consistently and strongly associated with the development of AKI in both cohorts, followed by hypertension as AKI risk factors in older persons. Chronic heart failure was identified as the strongest potential risk factors for AKI, but exclusively in the UC. Of note, RAS inhibitors may protect older patients against AKI, as shown in both cohorts. The use of NSAIDs was the only exposure associated with the development of AKI, an exclusive finding in the UC. In addition, short-term mortality was substantially higher in AKI patients in both cohorts.
This analysis is the first to present a large dataset of AKI and its epidemiology in unselected patients as well as in those with AHF, all of whom were 75 years or older. Our finding that approximately one quarter of older patients developed AKI, both in an unselected cohort and in one with acute heart failure, extends our current knowledge. This is a particularly high incidence rate of AKI compared to the variance of mostly between 10 to 15% with a maximum of 18.5% in younger or mixed age cohorts, using current AKI definitions [1, 2, 62]. Similarly, high rates of AKI were reported in some but not all smaller and selected cohorts of older patients receiving transcatheter aortic valve replacement [20,21,22,23,24]. In contrast, analysis of older patients receiving hip replacement demonstrated considerably lower incidence rates, consistent with their overall low risk for postoperative complications [25]. It is a novel finding that AHF patients differed substantially from the UC cohort. In AHF patients, the rate of AKI was moderately, and that of severe AKI substantially, lower. However, AHF patients received RRT more frequently compared to those in the UC cohort. This may be explained by more frequent hypervolemia and decreased response to diuretics in AHF patients leading to dilution of serum creatinine but higher RRT requirement. The former may arbitrarily cause lower AKI stages as AKI was predominantly defined by serum creatinine in the available studies. Compared to younger or mixed age cohorts, AKI in the UC was more severe although not in AHF patients [1, 2, 62]. Wide variations of RRT therapy in previous data on AKI in older patients ranging from 2.8 to 19% make comparison with our results difficult [20, 21, 23, 25]. The lack of uniform indications of when to perform RRT may primarily explain these variations [2].
Our findings that age-related and pathologically decreased renal function as well as hypertension may be the most important risk factors for AKI development corresponds to previous data and current concepts of susceptibility to AKI in older persons [11,12,13, 20, 22, 25]. As a strong and consistent finding, our data suggest that RAS inhibitors could potentially protect against AKI in the population of individuals 75 years and over. While experimental data indicate an activation of RAS during ischemic AKI and a mitigation of AKI by RAS inhibitors, there is also concern about hypotension or vasoplegia caused by RAS inhibitors, possibly aggravating AKI [63,64,65]. While most clinical studies and meta-analyses are consistent with our data that the use of RAS inhibitors is associated with less frequent AKI, other data demonstrated no association between these parameters or even an adverse effect on AKI [65,66,67,68,69,70]. Therefore, the issue of AKI prevention by RAS inhibitors remains controversial.
One of our cohorts consisted entirely of AHF patients, and chronic heart failure showed the strongest association with AKI in the other cohort. This may underscore the importance of heart failure as a risk factor for AKI development as previously described [2, 16, 17, 19]. These findings may be of clinical relevance as adequate treatment of chronic heart failure, chronic kidney disease and hypertension, and treatment with RAS inhibitors—when indicated for other diseases—could prevent AKI in older persons. Besides the detrimental impact of NSAIDs, data on exposures or AKI risk factors in the available studies in older patients is very limited. As a result, no statements on other typical AKI risk factors can be made based on the present data. Future studies are therefore urgently needed to identify additional exposures which should be avoided or modified to decrease the high rate of AKI in this age group. Furthermore, medium or long-term outcome, such as renal recovery, major adverse kidney events or one-year mortality after AKI remain to be determined in older patients.
This analysis has several strengths and limitations. First, our comprehensive study with its large sample size may provide representative results for AKI in older persons as defined by current definitions. Highly consistent results from 2 separate cohorts—one of unselected patients and another in a selected population—and also from the sensitivity analyses—substantially strengthen our findings and conclusions, although the division of the two cohorts is somewhat arbitrary. Additionally, all but very few studies included in this meta-analysis were of high quality and showed low risk of bias. Secondly, as predominantly observational studies were available our results describe association and not necessarily causalities and there may be potential confounding, information and selection bias. Thirdly, we observed substantial heterogeneity across studies in AKI frequency, susceptibility factors, exposures and mortality. Despite appropriate statistical analyses such as sensitivity analysis and meta-regression, the effects of bias and heterogeneity cannot be completely eliminated. Fourthly, 90% of all studies applied only serum creatinine but not urine output criteria to define AKI. This may have resulted in underestimation of AKI [71]. Finally, our review did not include studies in all languages.
In summary, AKI is highly frequent and severe in the population of individuals 75 years or over. While reduced renal function at baseline, CKD and hypertension were associated with AKI development, RAS inhibitors may be protective. Older patients with AKI showed substantially higher short-term mortality rates. Further research is required to collect more meaningful clinical outcomes and to identify more modifiable risk factors so as to design future strategies of AKI prevention in this particularly vulnerable population.
Registration and availability of data
The study was registered on Systematic Review Data Repository Plus (ID #2786). The complete dataset of this study is available as supplemental material of this publication.
References
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet 394:1949–1964
Kellum JA, Lameire N, Aspelin P et al (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:S1-138
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative Group. Crit Care 8:R204-212
Mehta RL, Kellum JA, Shah SV et al (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Meersch M, Schmidt C, Hoffmeier A et al (2017) Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 43:1551–1561
Gocze I, Jauch D, Gotz M et al (2018) Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg 267:1013–1020
Pascual J, Liaño F (1998) Causes and prognosis of acute renal failure in the very old. Madrid acute renal failure study group. J Am Geriatr Soc 46:721–725
Xue JL, Daniels F, Star RA et al (2006) Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17:1135–1142
Ishani A, Xue JL, Himmelfarb J et al (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20:223–228
Kayatas K, Sahin G, Tepe M et al (2014) Acute kidney injury in the elderly hospitalized patients. Ren Fail 36:1273–1277
Anderson S, Eldadah B, Halter JB et al (2011) Acute kidney injury in older adults. J Am Soc Nephrol 22:28–38
Schmitt R, Melk A (2017) Molecular mechanisms of renal aging. Kidney Int 92:569–579
Rosner MH, La Manna G, Ronco C (2018) Acute kidney injury in the geriatric population. Contrib Nephrol 193:149–160
World Population Ageing-the United Nations (2017) https://www.un.org/en/evelopment/desa/population/publications/pdf/ageing/WPA2017Highlights.pdf. Accessed 01 Nov 21
Ageing Europe-European Commission (2019) https://ec.europa.eu/eurostat/documents/3217494/10166544/KS-02-19-681-EN-N.pdf. Accessed 01 Nov 21
Ronco C, Bellasi A, Di Lullo L (2019) Implication of acute kidney injury in heart failure. Heart Fail Clin 15:463–476
Holgado JL, Lopez C, Fernandez A et al (2020) Acute kidney injury in heart failure: a population study. ESC Heart Fail 7:415–422
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
McDonagh TA, Metra M, Adamo M et al (2021) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726
Elhmidi Y, Bleiziffer S, Deutsch MA et al (2014) Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis 107:133–139
Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB (2016) The risk of acute kidney injury following transapical versus transfemoral transcatheter aortic valve replacement: a systematic review and meta-analysis. Clin Kidney J 9:560–566
Liao YB, Deng XX, Meng Y et al (2017) Predictors and outcome of acute kidney injury after transcatheter aortic valve implantation: a systematic review and meta-analysis. EuroIntervention 12:2067–2074
Shah K, Chaker Z, Busu T et al (2019) Meta-analysis comparing renal outcomes after transcatheter versus surgical aortic valve replacement. J Interv Cardiol 2019:3537256
Ma M, Gao WD, Gu YF, Wang YS, Zhu Y, He Y (2019) Clinical effects of acute kidney injury after transcatheter aortic valve implantation: a systematic review and meta-analysis. Intern Emerg Med 14:161–175
Thongprayoon C, Kaewput W, Thamcharoen N et al (2019) Acute kidney injury in patients undergoing total hip arthroplasty: a systematic review and meta-analysis. J Clin Med 8:66
Wells GA, Shea B, O’Connell D et al (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Lo CK, Mertz D, Loeb M (2014) Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14:45
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Cochran WG (1950) The comparison of percentages in matched samples. Biometrika 37:256–266
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Ali T, Khan I, Simpson W et al (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18:1292–1298
Chao CT, Lin YF, Tsai HB, Wu VC, Ko WJ (2003) Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg 217:240–250
Chao CT, Lin YF, Tsai HB, Hsu NC, Tseng CL, Ko WJ (2013) In nonagenarians, acute kidney injury predicts in-hospital mortality, while heart failure predicts hospital length of stay. PLoS ONE 8:e77929
Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA (2015) Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis 65:860–869
Karakose F, Akkoyunlu ME, Erkoc R et al (2015) Geriatric patients with known acute kidney injury and normal renal function at the time of admittance to the intensive care unit/assessment of RRT requirement and mortality: retrospective case–control study. Wien Klin Wochenschr 127:290–296
Chao CT, Tsai HB, Wu CY et al (2015) The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep 5:13925
Baek SH, Lee SW, Kim SW et al (2016) Frailty as a predictor of acute kidney injury in hospitalized elderly patients: a single center, retrospective cohort study. PLoS ONE 11:e0156444
Chaumont M, Pourcelet A, van Nuffelen M, Racapé J, Leeman M, Hougardy JM (2016) Acute kidney injury in elderly patients with chronic kidney disease: do angiotensin-converting enzyme inhibitors carry a risk? J Clin Hypertens 18:514–521
Kate RJ, Perez RM, Mazumdar D, Pasupathy KS, Nilakantan V (2016) Prediction and detection models for acute kidney injury in hospitalized older adult. BMC Med Inform Decis Mak 16:39
Porter CJ, Moppett IK, Juurlink I et al (2017) Acute and chronic kidney disease in elderly patients with hip fracture: prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol 18:20
Turgutalp K, Bardak S, Horoz M et al (2017) Clinical outcomes of acute kidney injury developing outside the hospital in elderly. Int Urol Nephrol 49:113–121
Alshelleh SA, Oweis AO, Alzoubi KH (2018) Acute kidney injury among nonagenarians in Jordan: a retrospective case–control study. Int J Nephrol Renovasc Dis 11:337–342
Morton S, Isted A, Avery P, Wang J (2018) Is frailty a predictor of outcomes in elderly inpatients with acute kidney injury? A prospective cohort study. Am J Med 131:1251–1256
Oweis AO, Alshelleh SA (2018) Incidence and outcomes of acute kidney injury in octogenarians in Jordan. BMC Res Notes 11:279
Harbrecht BG, Broughton-Miller K, Frisbie M et al (2019) Risk factors and outcome of acute kidney injury in elderly trauma patients. Am J Surg 218:480–483
Kociol RD, Greiner MA, Hammill BG et al (2010) Long-term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol 105:1786–1789
Lassus JP, Nieminen MS, Peuhkurinen K et al (2010) Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J 31:2791–2798
Verdiani V, Lastrucci V, Nozzoli C (2010) Worsening renal function in patients hospitalized with acute heart failure: risk factors and prognostic significances. Int J Nephrol 2011:785974
Breidthardt T, Socrates T, Noveanu M et al (2011) Effect and clinical prediction of worsening renal function in acute decompensated heart failure. Am J Cardiol 107:730–735
Eren Z, Ozveren O, Buvukoner E, Kaspar E, Degertekin M, Kantarci G (2012) A single-centre study of acute cardiorenal syndrome: incidence, risk factors and consequences. Cardiorenal Med 2:168–176
Murphy JC, Kozor RA, Figtree G et al (2012) Procedural and in-patient outcomes in patients aged 80 years or older undergoing contemporary primary percutaneous coronary intervention. EuroIntervention 8:912–919
Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H (2014) Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med 25:67–72
Palazzuoli A, Pellegrini M, Ruocco G et al (2014) Continuous versus bolus intermittent loop diuretic infusion in acutely decompensated heart failure: a prospective randomized trial. Crit Care 18:R134
Shirakabe A, Hata N, Yamamoto M et al (2014) Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ J 78:911–921
Shirakabe A, Hata N, Kobayashi N et al (2015) Serum heart-type fatty acid-binding protein level can be used to detect acute kidney injury on admission and predict an adverse outcome in patients with acute heart failure. Circ J 79:119–128
Hu W, He W, Liu W, Fang X et al (2016) Risk factors and prognosis of cardiorenal syndrome type 1 in elderly chinese patients: a retrospective observational cohort study. Kidney Blood Press Res 41:672–679
Kimura K, Momose T, Hasegawa T et al (2016) Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. J Cardiol 67:399–405
Tamaki S, Sato Y, Yamada T et al (2017) Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure and preserved left ventricular ejection fraction—prospective randomized controlled study. Circ J 81:740–747
Leistner DM, Munch C, Steiner J et al (2018) Impact of acute kidney injury in elderly (≥80 years) patients undergoing percutaneous coronary intervention. J Interv Cardiol 31:792–798
Dodson JA, Hajduk A, Curtis J et al (2019) Acute kidney injury among older patients undergoing coronary angiography for acute myocardial infarction: the SILVER-AMI study. Am J Med 132:e817–e826
Sparrow HG, Swan JT, Moore LW, Gaber AO, Suki WN (2019) Disparate outcomes observed within kidney disease: improving global outcomes acute kidney injury stage 1. Kidney Int 95:905–913
Kontogiannis J, Burns KD (1998) Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol 274(1 Pt 2):F79–F90
Cheng SY, Chou YH, Liao FL et al (2016) Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep 6:34265
Yacoub R, Patel N, Lohr JW, Rajagopalan S, Nader N, Arora P (2013) Acute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: a meta-analysis of observational studies. Am J Kidney Dis 62:1077–1086
Cheungpasitporn W, Thongprayoon C, Srivali N et al (2015) Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: a systematic review and meta-analysis. Nephrol Dial Transplant 30:978–988
Chou YH, Huang TM, Wu VC et al (2019) Associations between preoperative continuation of renin-angiotensin system inhibitor and cardiac surgery-associated acute kidney injury: a propensity score-matching analysis. J Nephrol 32:957–966
Whiting P, Morden A, Tomlinson LA et al (2017) What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open 7:e012674
Hollmann C, Fernandes NL, Biccard BM (2018) A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Anal 127:678–687
Bell S, Dekker FW, Vadiveloo T et al (2015) Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery–development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 351:h5639
Quan S, Pannu N, Wilson T et al (2016) Prognostic implications of adding urine output to serum creatinine measurements for staging of acute kidney injury after major surgery: a cohort study. Nephrol Dial Transplant 31:2049–2056
Acknowledgements
We kindly thank Mrs. Naomi Mader and Judith Mader for their expert revision of our manuscript.
Funding
The study was funded by the Dr. Werner Jackstädt Foundation. The Dr. Werner Jackstädt Foundation was informed about the design and conduct of the study but had no role in the development of the research questions, study design, analysis, interpretation, conclusions, writing or submission of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither potential conflicts of interest nor competing interests were reported by the authors.
Ethical approval
For this type of study, ethical approval is not required.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stille, K., Kribben, A. & Herget-Rosenthal, S. Incidence, severity, risk factors and outcomes of acute kidney injury in older adults: systematic review and meta-analysis. J Nephrol 35, 2237–2250 (2022). https://doi.org/10.1007/s40620-022-01381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01381-2