Abstract
Acute kidney injury (AKI) is a frequent complication of multiple myeloma and is associated with increased short-term mortality. Additionally, even a single episode of AKI can eventually lead to end-stage renal disease (ESRD), significantly reducing quality of life and long-term survival. In the setting of multiple myeloma, severe AKI (requiring dialysis) is typically secondary to cast nephropathy (CN). Renal injury in CN is due to intratubular obstruction from precipitation of monoclonal serum free light chains (sFLC) as well as direct tubular toxicity of sFLC via stimulation of nuclear factor (NF)κB inflammatory pathways. Current mainstays of CN treatment are early removal of precipitating factors such as nephrotoxic drugs, acidosis and dehydration, together with rapid reduction of sFLC levels. Introduction of the proteasome inhibitor bortezomib has significantly improved the response rates in multiple myeloma due to its ability to rapidly reduce sFLC levels and has been referred to as “renoprotective” therapy. As an adjunct to chemotherapy, several new extracorporeal techniques have raised interest as a further means to reduce sFLC concentrations in the treatment of CN. Whether addition of extracorporeal therapies to renoprotective therapy can result in better renal recovery is still a matter of debate and there are currently no guidelines in this field. In this positon paper, we offer an overview of the available data and the authors’ perspectives on extracorporeal treatments in CN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is characterized by a malignant proliferation of plasma cells associated with production and release of intact immunoglobulins and/or serum free light chains (sFLC). The disease has heterogeneous clinical manifestations, and both acute and chronic kidney disease (CKD) represent one of the most frequent complications. The incidence of renal disease is not precisely determined, but up to 50 % of patients may develop renal involvement during the course of the disease [1, 2]. The most serious complication is dialysis-requiring acute kidney injury (AKI) (1–13 %) [3], caused in >90 % of cases by cast nephropathy (CN). In MM patients, massive production of sFLC overwhelms the absorptive capacity of the proximal tubule leading to both intratubular obstruction of the distal tubules as well as direct proximal tubular injury through the nuclear factor (NF)κB pathway [4]. Among patients with AKI, the rate of renal function recovery traditionally has been poor, correlating to inferior survival [5, 6]. On the other hand, patients obtaining recovery of renal function and independence from renal replacement therapy (RRT) have a better outcome, similar to those with normal renal function [7–9]. Knowledge of the molecular mechanisms that favor sFLC precipitation in renal tubules is increasing, leading to different therapeutic scenarios [4]. Nevertheless, so far, an aggressive reduction of sFLC is still the major goal in preventing and treating CN.
Therefore, severe AKI in MM must be considered a medical emergency in which all efforts should focus on early renal recovery to improve patients’ survival. Recently, new therapeutic approaches have been proposed using novel drugs possibly combined with extracorporeal removal of sFLC. A recent analysis [9] showed that adjusted incident rates of end-stage renal disease (ESRD) from MM declined by nearly 20 % between 2001 and 2002 and 2009–2010 in the US, a trend that was largely independent of major demographic characteristics. Mortality rates for MM were much higher than for other causes of ESRD, especially in the first year of dialysis, but the likelihood of death declined by 28 % from 2001–2002 to 2009–2010. Together, these data support the conclusion that the addition of aggressive treatment of CN with bortezomib may have contributed to these outcomes, but the combined efficacy of bortezomib treatment and sFLC removal compared to bortezomib alone needs to be further investigated.
In this Position paper, we offer an overview and the authors’ perspectives on the available evidence in the field of extracorporeal sFLC removal, focusing in particular on the selection of eligible patients, technological aspects of the different devices, and timing for treatment initiation.
Production and intracorporeal catabolism of sFLC
In normal immunoglobulin synthesis, plasma cells produce an excess of sFLC that are released into the circulation. Normally sFLC are filtered by the glomerulus and endocytosed and metabolized by the proximal tubules, while a smaller portion is metabolized by the reticuloendothelial system. In physiological conditions, k and λ sFLC have a half-life of 2–4, and 3–6 h, respectively, increasing up to 3 days in patients with no renal function. Therefore, reticuloendothelial clearance can be calculated as 1.6 × 10−4/min [10–12].
sFLC are relatively small protein molecules (κ 25 kD and λ 50 kD), with similar concentrations in serum, the extravascular compartment, and tissue edema fluid, similar to other molecules of the same size [13]. Thus, the intravascular compartment may contain only 15–20 % of the total amount, with the greatest portion of sFLC distributed in the extravascular compartment. To describe sFLC kinetics, Hutchison et al. in 2007 [14] proposed a two-compartment mathematical model of FLC production, distribution, and removal in multiple myeloma. In this model, constant sFLC flow between intra- and extravascular compartments was reported 2.15 × 10−2/min, while constant FLC flow between extra and intravascular compartments was 4.3 × 10−3/min. Thus, the authors could simulate sFLC removal in different settings, demonstrating that, even with a 100 % tumor killing on day 1 of chemotherapy, the isolated reticuloendothelial removal of sFLC would take at least 15 days in a patient with 10 g/l sFLC. On the other hand, extracorporeal removal alone, without efficient tumor killing, could not reduce sFLC concentrations due to high production by the tumoral mass and rapid rebound between compartments [14].
Cast nephropathy treatment
Precipitating factors
Patients presenting with CN usually have been exposed to precipitating factors, including medications (e.g. non-steroidal anti-inflammatory agents, and antibiotics) and underlying conditions such as dehydration, iodinated contrast media exposure, and hypercalcemia, which exert either a direct tubule-interstitial toxicity or increase the concentration of sFLC in the distal nephron. In such circumstances, these medications should be withdrawn and underlying conditions appropriately treated, aiming at recovery of renal function. The mainstay of therapy is volume repletion, correction of metabolic acidosis, and maintenance of a high urinary volume, tailoring the use of diuretics to the patient’s needs [15].
Chemotherapy in patients with renal injury
Recent epidemiological data of newly diagnosed MM showed that the frequency of AKI was unchanged over different time periods, while both hematological response and overall survival of patients with severe renal failure significantly improved over the past decade [16]. This improvement was attributed to the recent introduction of novel highly active chemotherapeutic agents.
Dimopoulos et al. [17] recently evaluated in an observational study the role of the novel agents targeting the myeloma clone in its bone marrow microenvironment, namely thalidomide, bortezomib and lenalidomide, in the management of MM patients presenting with kidney damage. This analysis showed that all drugs induced a significant improvement in renal function with superiority of bortezomib in comparison to thalidomide and lenalidomide (renal recovery in 77, 55 and 43 % of cases respectively). In a multivariate analysis, bortezomib-based therapies were independently associated with a higher probability of renal response, and renal response occurred faster than with other drugs, the median time to renal response being 1.3 months vs 2.7 and 6 months for thalidomide and lenalidomide-treated patients respectively.
A bortezomib-containing regimen is thus considered the treatment of choice for MM patients presenting with AKI [18]. Advantages of bortezomib include rapid anti-MM activity as well as its unique effect on renal tubular cells. Bortezomib potently inhibits NF-kB pathways reducing the release of local inflammatory cytokines and induces anti-apoptotic pathways specific for tubular cells [19]; moreover, bortezomib metabolism is unaffected by renal function so it can be safely administered at full doses to patients with renal function impairment [20, 21]. In a sub-study of the VISTA trial comparing velcade (bortezomib)-melphalan-prednisone (VMP) with melphalan-prednisone (MP), the complete response rates were significantly better with VMP than MP. In the VMP arm there were no observed differences in response rates across cohorts based on the severity of renal impairment [22]. In the HOVON-65/GMMG-HD4 trial even patients with a baseline serum creatinine ≥2 mg/dl who were randomized to receive bortezomib, adriamycin and dexamethasone (PAD) versus vincristine, adriamycin and dexamethasone (VAD) had a significantly longer 3-year overall survival (74 % vs. 34 %, respectively) [23]. Although these trials did not include dialysis-dependent AKI, it is reasonable to extrapolate these results to the AKI-dialysis dependent population affected by CN.
Indications for extracorporeal removal of sFLC
Patient stratification and selection
All patients with plasma cell dyscrasias and renal disease should be stratified according to the type of renal involvement. Only CN should be regarded as a myeloma-defining event [24] while all other forms of renal injury represent unique disease states not always caused by MM [25, 26]. Identification of CN is now possible by combining several techniques including urinalysis, serum and urine electrophoresis/immunofixation, sFLC levels, and kidney biopsy [4, 27]. Proteinuria analysis (urinary electrophoresis with immune-fixation, Bence Jones proteinuria, and albuminuria quantification) [28, 29] can be used to guide the management of a patient with renal injury and a monoclonal protein [4] (Table 1). Where diagnostic uncertainty remains, assessment of histology is essential. In the past, some concerns were raised about the safety of biopsy in MM patients due to presumed coagulation abnormalities. Nevertheless, several case series reported that the procedure is generally safe with the same rate of adverse events as the general population [29–31]. The importance of renal biopsy goes beyond its diagnostic value, since it may also provide evidence that further treatment may be of no or rather limited value if renal parenchymal damage is deemed to be extensive and/or irreversible [32]. A recent report [31] correlated baseline renal pathological findings with kidney outcomes in a large cohort of MM patients with CN showing that the presence of numerous casts and diffuse tubular atrophy is associated with poor renal prognosis.
Patient selection criteria for consideration of initiation of extracorporeal removal of sFLC are summarized in Table 2.
Type, onset, and degree of kidney injury
Only highly suspected or biopsy-proven CN should be considered for extracorporeal sFLC removal to reduce the rate of cast formation and prevent further kidney damage. All other forms of sFLC-mediated nephropathies do not represent an indication for sFLC clearance. Since CN is usually an acute event, time of onset is one selection criterion. In addition, the decision to treat the patient should also take into account the level of pre-existing renal function and renal biopsy characteristics since previous renal failure and presence of renal sclerosis negatively affect renal recovery [31, 32].
The available evidence also supports initiating sFLC removal in those patients in whom dialysis is already indicated either to treat AKI or to control volume and electrolyte disorders. Some authors [33, 34] recommend broader indications, proposing intervention for all patients with evidence of progressive AKI stage 3 [Kidney Disease Improving Global Outcomes (KDIGO) 2012], independently of estimated glomerular filtration rate (eGFR) values, in order to remove the burden of sFLC. This aspect is highly controversial; therefore, we suggest to use common dialysis indications for starting extracorporeal removal therapies.
Chemotherapy
Extracorporeal sFLC removal must be considered as a supportive treatment to chemotherapy; therefore, patients should be eligible for onco-hematological treatment with the aim of disease remission. Without chemotherapy, extracorporeal sFLC removal is ineffective since the amount of released sFLC cannot be efficiently cleared by any available technique if production is not rapidly blunted.
sFLC basal levels
Cast nephropathy is usually associated with sFLC levels >500–1000 mg/l. In the presence of sFLC < 500 mg/l, diagnosis of CN should be carefully reconsidered and chemotherapy alone should be sufficient to obtain a rapid reduction of sFLC without the need of extracorporeal removal.
Time between AKI and cast nephropathy diagnosis
Any delay in diagnosis and treatment greatly reduces the probability of kidney recovery. When diagnosis of MM is delayed after severe AKI onset, patients will likely not benefit from extracorporeal treatments. In these cases, renal biopsy can give a better understanding of the degree of damage and chronicity [31, 32]. Moreover, if AKI is associated with recurrent MM, the possibility of renal recovery is reduced in comparison to de novo myeloma. In these cases, each situation should be individually assessed for the need for extracorporeal treatment.
Dialysis timing and dose
Timing of initiation and duration of extracorporeal treatment
The Authors’ opinion is that extracorporeal treatment must be started rapidly in the very first days if correction of common precipitating causes of renal failure has not resulted in recovery of renal function. Treatment should be continued as long as chemotherapy significantly affects sFLC production [33–35]. For this reason, sFLC concentrations should be used as an indicator of tumor killing activity of chemotherapy and a guide to treatment duration. Nevertheless, treatments can be interrupted if signs of renal recovery are observed independently of changes in sFLC levels.
Treatment scheduling
According to the theoretical two compartment model, sFLC are distributed mainly in the extravascular compartment [14]. Unlike other molecules with a similar distribution, sFLC have some unusual characteristics. The sFLC production rate is not constant since it depends on the activity of the neoplastic disease (which varies greatly between patients) and the effectiveness of chemotherapy. Nevertheless, given a theoretical high degree of production and a large volume of distribution, extracorporeal sessions should be either regular (4 h) or long/extended (8 h) on a daily basis for the first 7–12 days of treatment. Such schedules are the most commonly reported in retrospective studies on sFLC removal [14]. Currently ongoing clinical trials are using daily long (6–8 h) sessions within the first 12 days, except for chemotherapy days. Recently, Zannetti et al. [34] also reported efficacy of sFLC reduction using a regular dialysis schedule (three times a week) combined with bortezomib-based therapy. Notably, this was a prospective study with no control group, and a direct comparison with other treatments schedules is lacking.
Goals of treatment
The main treatment goal is to achieve the highest reduction of sFLC and to minimize the effects of rebound between treatment sessions. The degree of sFLC reduction in the first 21 days of treatment is linearly predictive of renal recovery suggesting that the reduction of sFLC is a good target on which to adjust treatment intensity and length [35]. For this reason, if no response is seen (according to sFLC levels), withdrawal of extracorporeal therapy should be considered after 21 days. Contrarily, if significant sFLC reduction is achieved (at least >60 % reduction) but renal function does not recover it is reasonable to stop specific sFLC removal therapies and convert to standard dialysis treatments. It is known that renal recovery may occur months after chemotherapy initiation. Extracorporeal treatments should be interrupted when signs of renal recovery (i.e. restoral of active diuresis or appearance of polyuria, interdialytic spontaneous reduction of serum creatinine) appear independently of sFLC reduction.
Available tecniques for extracorporeal removal of sFLC
sFLC are relatively small protein molecules (κ 25 kD and λ 50 kD) that accumulate in patients as renal function declines and are included in the extensive list of uremic toxins [36]. Due to their molecular weight, typical high flux dialyzers are unable to remove these molecules as shown by both in vitro and in vivo experiments [14, 37]. Therefore, extracorporeal clearance of sFLC can only be achieved through dialyzers with either higher molecular cut-off values (high cut-off and/or plasma exchange dialyzers) or with specific adsorption properties. No studies have compared the efficacy of different treatments, and information about clearance or percentage removal are not always reported. We have summarized the available information, to the best of our knowledge, about the efficacy of different devices (Table 3), while in Table 4 we describe the clinical evidence for each type of reported treatment.
Plasmapheresis
Until recently, plasmapheresis was the only extracorporeal technique used in CN treatment to remove sFLC through complete plasma substitution. Several retrospective observational studies and randomized controlled trials (RCTs) on its efficacy are available, but the quality of the data is poor and inadequate to draw definitive conclusions. Three RCTs were performed between 1988 and 2005 [38–40] in patients with AKI of varying degrees due to MM. Zucchelli et al. [38] found plasmapheresis to be effective in improving total and renal survival, while Johnson et al. [39] showed only an advantage on renal recovery in more severe renal failure. In the study by Clark et al. [40], the largest to date, no positive effects of plasmapheresis were observed on overall or renal survival. Similar results were obtained by other investigators in a retrospective analysis in single center [41], while opposite data are reported from other institutions [42]. A new RCT (the Myeloma Renal Impairment Trial, MERIT) was designed in 2008 to enroll 280 patients to better elucidate the role of plasmapheresis in CN. The study closed due to difficult recruitment. After enrolment of only 79 patients, negative results were reported in abstract form [43]. Besides limits in the studies’ design and statistical power, the main limitation of the RCTs is the absence of evaluation of plasma-exchange efficacy. In fact, sFLC assays became available only recently, making it impossible to determine in these initial trials the adequacy of sFLC reduction combining chemotherapy and extracorporeal removal. In 2008, Leung et al. [33] performed a retrospective study in which effectiveness of plasmapheresis was tested in biopsy proven CN and sFLC levels were available. Interestingly, this study showed that the vast majority of the patients resolved their renal disease when the sFLC levels were reduced by 50 % or more. In patients without CN, renal recovery occurred independently of reductions in sFLC levels. Although retrospective, this study provided for the first time information to guide treatments. Another important limitation regarding plasmapheresis studies is that the drug regimens used to treat MM did not include the more effective proteasome inhibitor bortezomib. Therefore, translation of those results into the current clinical practice is not advisable. Moreover, plasmapheresis is a short-lasting treatment and its efficacy is limited to the intravascular compartment that may contain only 15–20 % of the total sFLC, theoretically limiting its efficacy. Altogether, these data suggest that plasmapheresis cannot be recommended since it does not provide renal replacement and efficacy may be limited by great inter-treatment rebounds.
High cut-off dialyzers
In recent years, improvements in dialyzer technology have led to filters with increased pore size and sieving coefficients approximating that of the glomerular capillary wall. Such membranes have high permeability for substances in the molecular weight range of 15–45 kDa with retention of larger proteins with molecular weights greater than 60 kDa. These dialyzers were initially designed to remove inflammatory cytokines in septic shock but can also efficiently remove molecules such as sFLC. Hutchison et al. first described high cut-off (HCO) membrane properties in vitro and in vivo compared to other high flux membranes in clinical use [14]. The results indicated that the new membrane had improved clearance of sFLC through both diffusive and convective mechanisms. The in vitro clearance was reported to be 35.1 ml/min (range 7.5–50.9) and 32.2 ml/min (range 19.1–45.9) for k and λ sFLC, respectively. There are several reports on the use of HCO dialysis in CN [34, 35, 44–51]. In an international retrospective analysis, Hutchison et al. reported data from 67 patients with MM dialysis-dependent renal failure in nine countries [35]. Almost 60 % of patients underwent kidney biopsy and CN was found in 87 % of cases. Eighty-five percent of patients were treated with different regimens including dexamethasone in combination with novel agents (58 % received bortezomib). Extended (≥4 h) HCO dialysis was performed daily in 97 % of patients (median number of sessions was 11, range 3–45). A significant rate of renal recovery was observed, with dialysis independence in 63 % of patients. Logistic regression analysis indicated that only a sustained reduction in sFLC concentrations by days 12 and 21 significantly increased the probability of renal recovery. In fact, the probability of achieving dialysis independence increased linearly with increasing levels of reduction in sFLC concentrations by day 12. Compared to patients who achieved no reduction in serum FLC by day 12, a reduction of 75 % had an odds ratio of 52 for dialysis independence. Zannetti et al. published an observational prospective study in which all-consecutive patients (n = 21) with AKI and MM were treated with bortezomib-based regimens together with intermittent HCO dialysis (minimum of six treatments in 2 weeks); the presence of CN was confirmed by biopsy in 71 % of patients [34]. In this population, dialysis independence was reached in 76 % of patients at a median time of 32 days; moreover, the 3-year progression-free survival was 76 % and the 3-year overall survival rate was 67 %. In contrast with the previous report, HCO dialysis was performed in both dialysis-dependent patients as well as those with less severe AKI. More recently, a single center retrospective experience reported superiority of HCO treatments in 42 patients in comparison to standard dialysis. A sustained sFLC response was detected in a significantly higher proportion of HCO-HD patients (83.3 %) compared to conventional HD patients (29.4 %; p = 0.007). The corresponding rates of renal recovery were 64.3 and 29.4 %, respectively (Chi squared test, p = 0.014) suggesting superiority of this approach in comparison to standard therapy [50]. Although these studies differ in terms of populations and chemotherapy regimens, the results are similar and support the concept proposed by Leung et al. [33] that monitoring of sFLC is essential to guide treatment and to define the role of extracorporeal therapies. Two RCTs are now investigating the role of HCO membranes in this setting [52, 53]. In both studies, dialysis-dependent patients with biopsy proven CN are treated with bortezomib-based therapies and either with HCO dialysis or standard dialysis for AKI. According to Clinicaltrial.gov, the recruitment status of the Eulite study [52] is unknown, while the recruitment of the Myre study [53] is over but the study is still ongoing (results should be presented in September 2017).
Treatment with HCO membranes has some drawbacks, including loss of albumin during treatment and high cost. Due to albumin loss these devices are mainly used in standard hemodialysis since convection with hemofiltration would significantly increase protein removal. Therefore, patients may require supplementation with human albumin exposing them to potential risks and increased cost. Although HCO dialysis seems to be a more efficient method to remove sFLC, no studies have properly compared different types of dialyzers and the superiority of a single membrane has not been demonstrated. Recently, Rousseau-Gagnon et al. [51] reported that hemodiafiltration with a heat sterilized high-flux polyphenylene hemofilter could reduce only κ FLC similarly to HCO treatments. These results were derived from a retrospective comparison between HCO treatments obtained in just two patients versus polyphenylene treatments in ten patients. Efficacy was measured by the percent reduction of sFLC levels; no clearance data were reported. Since the reduction rate can be influenced by patient variability, these results need to be confirmed in a properly designed study.
sFLC removal through adsorption
In addition to HCO dialysis and plasmapheresis, there are additional reports on the efficacy of other dialytic techniques that mainly remove sFLC through a combination of convection and/or adsorption.
HFR-SUPRA
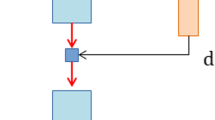
Hemodiafiltration with endogenous reinfusion (HFR) (BellCo, Mirandola, Italy) is a technique that combines convection, diffusion, and adsorption in a single treatment. Its use has been recently modified with the introduction of a higher cut-off membrane as shown in Fig. 1. HFR-SUPRA uses a dual chamber dialyzer. The first chamber contains a “Super High Flux” polyphenylene membrane with a molecular weight cut-off of 42 kD, through which convection is performed. In the second chamber, there is a membrane of low permeability where diffusion is carried out. The ultrafiltrate obtained in the first chamber passes through a resin cartridge where adsorption occurs and is then reinfused before the second chamber. This technique has been used in hemodialysis patients due to its high capacity of protein-bound toxin adsorption while not removing albumin [54]. The molecular weight cut-off of the first membrane allows sFLC removal through convection into the ultrafiltrate. The resin cartridge has significant affinity for sFLC [54] although the overall efficacy and adsorption limits have been poorly studied. In two small clinical series [55, 56], the reported removal rate was 51 % (range 38–63) with clearances from 3.2 to 11.5 ml/min for λ sFLC and from 4.9 to 15.3 ml/min for κ sFLC. Due to the small number of treated patients (n = 7) no clinical considerations can be drawn about the overall clinical utility of this treatment.
PMMA-EAD
Another technique recently described is enhanced adsorption dialysis (EAD) with PMMA BK-F 2.1 m2 (Toray Inc., Tokyo, Japan) dialyzers. This method is based on PMMA adsorption properties. Due to its symmetric and homogeneous structure, PMMA can adsorb molecules with a wide range of molecular weights, including sFLC. Hutchison et al. measured the capacity of EAD to adsorb up to 2 g of sFLC during 4-h in vitro treatment [57]. Overall results were poor with a 23 % reduction of sFLC levels, likely due to fast saturation of the membrane adsorption capacity. Therefore, to increase adsorption efficacy a new circuit was designed that allows the use of two PMMA membranes in parallel (Fig. 2). The double-filter circuit uses two filters with Y-connectors for filter attachment and equal distribution of dialysis fluid. In two reports on the new circuit, the removal capacity in a single session increased to 33.1 % for κ and 53.1 % for λsFLC [57, 58]. A preliminary report [59] in 12 patients showed good renal recovery with dialysis independence at 1 year of 78 %. Further studies are awaited to better assess the clinical applicability of this treatment modality.
CPFA
Coupled plasma filtration adsorption (CPFA) is an extracorporeal blood purification method most commonly used in septic patients due to its high efficacy in adsorbing inflammatory mediators. CPFA consists of filtration, adsorption and hemofiltration (Fig. 3). During the filtration phase, plasma is separated from blood using a plasma filter (MICROPES 0.45 m2 polyethersulfone). This separated plasma then passes through a nonselective hydrophobic styrene resin cartridge with a macroporous structure characterized by an average bead diameter of 75 µm, an average pore diameter of 30 nm and a surface area of 700 m2/g. Blood is subsequently reconstituted and dialyzed in a post-dilution mode through a synthetic, high-permeability, 1.4 m2 polyethersulfone hemofilter. An in vitro study showed good efficacy of MDR3resins in adsorbing sFLC without albumin loss [60]. Clinical data on a series of eight patients reported a mean sFLC reduction of 41.4 ± 5.7 % after a single CPFA session of 5 h [61]. The acute effect of CPFA treatment was also investigated in four patients with dialysis-dependent AKI and light chain MM who underwent 2–7 CPFA treatments of 5-h duration [62]. Similar removal rates of sFLC were observed. Nevertheless, strong evidence supporting CPFA use is still lacking and larger studies are needed to assess its clinical utility.
Conclusions
In this position paper, we have critically reviewed the role of sFLC extracorporeal removal during AKI in MM. In recent years, three major developments have changed the approach to this clinical condition. First, the ability to routinely and reliably measure sFLC levels in order to determine the efficacy of therapy; second the improvement in dialyzer technology providing new devices that can effectively remove sFLC; and finally, the availability of highly effective non genotoxic drugs that can induce a rapid reduction of tumor burden and sFLC production.
Despite limited data and several controversial aspects, the authors propose that the current knowledge of the disease allows us to identify which subset of patients could benefit from sFLC removal and how to guide these treatments. Specifically, treatments should only be used in patients with CN who are eligible for onco-hematological chemotherapy preferably based on the proteasome inhibitor bortezomib with the aim of sFLC rapid reduction. Several studies described in this review demonstrate effective removal of sFLC in patients with CN. The greatest experience is with HCO dialyzers (Theralite, Gambro-Baxter, Deerfield, IL, USA), which proved to be effective in experimental models and in vivo in various clinical settings [33–35]. In accordance with these results, the International Myeloma Working group recently indicated HCO treatment in combination with anti-myeloma therapy for patients with myeloma with AKI as a result of CN with grade B evidence [63].
More recently, newer techniques have been developed to combine effective sFLC removal with albumin retention and lower cost. The most promising systems appear to be enhanced adsorption dialysis (EAD) [57, 58] with polymethylmethacrylate dialyzers (Toray Inc., Japan) and HFR-supra (BellCo, Italy) [55, 56]. EAD and HFR-supra are in fact easy techniques with reduced cost (€100 and €150 per treatment, respectively, compared to €800/treatment for HCO) but, unfortunately, the available evidence is derived from a small number of patients usually treated in a single institution, for which no strong conclusions can be drawn.
Based on the available evidence, the Authors’ opinion is that a reasonable approach to cast nephropathy treatment would be a combination of different techniques based on using more efficient dialyzers on the first day of therapy (such as HCO dialysis) when sFLC levels are the highest, and subsequently changing to other techniques (EAD, HFR) when levels of sFLC start to decline with bortezomib-based chemotherapy [55]. We emphasize that such an approach should be guided by ongoing monitoring of sFLC levels that can clearly indicate if the treatment strategy is effective. While waiting for the results of ongoing RCTs, we cannot state that the addition of extracorporeal sFLC removal to standard bortezomib-based chemotherapy is superior to chemotherapy alone. Nevertheless, it is reasonable that such treatments can be used in patients who already need dialysis either for AKI or to control volume and electrolyte disorders.
References
Dimopoulos MA, Terpos E, Chanan-Khan A et al (2010) Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 28:4976–4984
Gaballa MR, Laubach JP, Schlossman RL, Redman K, Noonan K, Mitsiades CS, Ghobrial IM, Munshi N, Anderson KC, Richardson PG (2012) Management of myeloma-associated renal dysfunction in the era of novel therapies. Expert Rev Hematol 5:51–66
Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA (2012) Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res 18:2145–2163
Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, Herrera GA, Lachmann H, Sanders PW, International Kidney and Monoclonal Gammopathy Research Group (2011) The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 8(1):43–51
Abbott KC, Agodoa LY (2001) Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 56(3):207–210
Uttervall K, Duru AD, Lund J, Liwing J, Gahrton G, Holmberg E, Aschan J, Alici E, Nahi H (2014) The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One 9(7):e101819. doi:10.1371/journal.pone.0101819
Rodrigues L, Neves M, Sá H, Gomes H, Pratas J, Campos M (2014) Severe acute kidney injury and multiple myeloma: evaluation of kidney and patient prognostic factors. Eur J Intern Med 25:652–656
Tsakiris DJ, Stel VS., Finne P, Fraser E, Heaf J, de Meester J, Schmaldienst S, Dekker F, Verrina E, Jager KJ (2010) Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA registry study. Nephrol Dial Trasplant 25:1200–1206
Reule S, Sexton DJ, Solid CA, Chen SC, Foley RN (2013) End stage renal disease due to multiple myeloma in the United States, 2001–2010. J Am Soc Nephrol 27(5):1487–1494
Miettinen TA, Kekki M (1967) Effect of impaired hepatic and renal function on Bence Jones protein catabolism in human subjects. Clin Chim Acta 18:395–407
Wochner RD, Strober W, Waldmann TA (1967) The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med 126:207–221
Bradwell AR (2006) Serum Free light chain analysis, 4th edn. The Binding Site Ltd., Birmingham, pp 104–107
Takagi K, Kin K, Itoh Y, Enomoto H, Kawai T (1980) Human alpha 1-microglobulin in various body fluids. J Clin Pathol 33:786–791
Hutchison CA, Cockwell P, Reid S, Chandler K, Mead GP, Harrison J, Hattersley J, Evans ND, Chappell MJ, Cook M, Goehl H, Storr M, Bradwell AR (2007) Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 18:886–895
Walther C, Podoll AS, Finkel KW (2014) Treatment of acute kidney injury with cast nephropathy. Clin Nephrol 82(1):1–6
Dimopoulos MA, Delimpasi S, Katodritou E et al (2014) Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol 25:195–200
Dimopoulos MA, Roussou M, Gkotzamanidou M et al (2013) The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 27:423–429
Cohen C, Royer B, Javaugue V et al (2015) Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int 88:1135–1143. doi:10.1038/ki.2015.201
Sarközi R, Perco P, Hochegger K, Enrich J, Wiesinger M, Pirklbauer M, Eder S, Rudnicki M, Rosenkranz AR, Mayer B, Mayer G, Schramek H (2008) Bortezomib-induced survival signals and genes in human proximal tubular cells. J Pharmacol Exp Ther 327(3):645–656
Jagannath S, Barlogie B, Berenson JR, SUMMIT/CREST Investigators et al (2005) Bortezomib in recurrent and/or refractory multiple myeloma: initial clinical experience in patients with impaired renal function. Cancer 103:1195–1200
Chanan-Khan AA, Kaufman JL, Mehta J et al (2007) Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood 109:2604–2606
Dimopoulos MA, Richardson PG, Schlag R et al (2009) VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol 27:6086–6093
Scheid C, Sonneveld P, Schmidt-Wolf IGH et al (2014) Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-hd4 trial. Haematologica 99:148–154
Rajkumar SV, Dimopoulos MA, Palumbo A et al (2014) International Myeloma Working Group updated criteria for diagnosis of multiple myeloma. Lancet Oncol 15:e538–e548
Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G, Roussel M, Fervenza FC, Dispenzieri A, Kyle RA, Nasr SH, International Kidney and Monoclonal Gammopathy Research Group (2015) Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 87:698–711
L’Imperio V, Fabbrini P, Ferrario F, Pieruzzi F, Tosoni A, Brivio R, Pogliani EM, Pagni F (2015) Monoclonal gammopathy of renal significance: systemic involvement by benign condition. Kidney Int 88(1):200–202
Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R (2001) Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 47(4):673–680
Leung N, Gertz M, Kyle RA et al (2012) Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol 7(12):1964–1968
Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Lacy M, Dispenzieri A, Rajkumar SV, Kyle RA, Leung N (2012) Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis 59(6):786–794
Fish R, Pinney J, Jain P, Addison C, Jones C, Jayawardene S, Booth J, Howie AJ, Ghonemy T, Rajabali S, Roberts D, White L, Khan S, Morgan M, Cockwell P, Hutchison CA (2010) The incidence of major hemorrhagic complications after renal biopsies in patients with monoclonal gammopathies. Clin J Am Soc Nephrol 5(11):1977–1980
Ecotière L, Thierry A, Debiais-Delpech C, Chevret S, Javaugue V, Desport E, Belmouaz S, Quellard N, Kaaki S, Goujon JM, Fermand JP, Touchard G, Bridoux F (2015) Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant 31(1):64–72
Basnayake K, Cheung CK, Sheaff M, Fuggle W, Kamel D, Nakoinz S, Hutchison CA, Cook M, Stoves J, Bradwell AR, Cockwell P (2010) Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidney. J Clin Pathol 63(10):884–887
Leung N, Gertz MA, Zeldenrust SR et al (2008) Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 73(11):1282–1288
Zannetti BA, Zamagni E, Santostefano M et al (2015) Bortezomib-based therapy combined with high cut-off hemodialysis is highly effective in newly diagnosed multiple myeloma patients with severe renal impairment. Am J Hematol 90:647–652
Hutchison CA, Heyne N, Airia P, Schindler R, Zickler D, Cook M, Cockwell P, Grima D (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 27(10):3823–3828
Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) (2003) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 63:1934–1943
Cohen G, Rudnicki M, Schmaldienst S, Hörl WH (2002) Effect of dialysis on serum/plasma levels of free immunoglobulin light chains in end-stage renal disease patients. Nephrol Dial Transplant 17(5):879–883
Zucchelli P, Pasquali S, Cagnoli L, Ferrari G (1988) Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33(6):1175–1180
Johnson WJ, Kyle RA, Pineda AA, O’Brien PC, Holley KE (1990) Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 150(4):863–869
Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN, Canadian Apheresis Group (2005) Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med 143(11):777–784.
Movilli E, Jeannin G, Turina S, Scolari F, Cancarini G (2007) Plasma exchange in the treatment of acute renal failure of myeloma. Nephrol Dial Transplant 22(4):1270–1271
Burnette BL, Leung N, Rajkumar SV (2011) Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med 364(24):2365–2366
Madore F (2015) Plasmapheresis in cast nephropathy: yes or no? Curr Opin Nephrol Hypertens 24(2):177–182
Sinisalo M, Silvennoinen R, Wirta O (2012) High cut-off hemodialysis and bortezomib-based therapy to rescue kidneys in myeloma-dependent cast nephropathy. Am J Hematol 87:640
Tan J, Lam-Po-Tang M, Hutchison CA, de Zoysa JR (2014) Extended high cut-off haemodialysis for myeloma cast nephropathy in Auckland, 2008–2012. Nephrology (Carlton) 19(7):432–435
Marn Pernat A, Medved B, Gubenšek J, Premru V, Knap B, Buturovic-Ponikvar J, Ponikvar R (2016) Citrate extended high cut-off hemodiafiltration for renal recovery in patients with multiple myeloma. Ther Apher Dial 20(3):251–255
Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Hattersley J, Evans ND, Chappel MJ, Sampson P, Foggensteiner L, Adu D, Cockwell P (2009) Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol 4(4):745–754
Berni Wennekers A, Martín Azara MP, Dourdil Sahun V, Bergasa Liberal B, Ruiz Laiglesia JE, Vernet Perna P, Alvarez Lipe R. (2016) Thirteen treated of acute renal failure secondary to multiple myeloma with high cut off filters. Nefrologia 36(4):418–426
Heyne N, Denecke B, Guthoff M, Oehrlein K, Kanz L, Häring HU, Weisel KC (2012) Extracorporeal light chain elimination: high cut-off (HCO) hemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol 91(5):729–735
Gerth HU, Pohlen M, Görlich D, Thölking G, Kropff M, Berdel WE, Pavenstädt H, Brand M, Kümpers P (2016) Impact of high-cut-off dialysis on renal recovery in dialysis-dependent multiple myeloma patients: results from a case-control study. PLoS One 11(5):e0154993
Rousseau-Gagnon M, Agharazii M, De Serres SA, Desmeules S (2015) Effectiveness of haemodiafiltration with heat sterilized high-flux polyphenylene HF dialyzer in reducing free light chains in patients with myeloma cast nephropathy. PLoS One 10(10):e0140463
Hutchison CA, Cook M, Heyne N et al (2008) European trial of free light chain removal byextended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials 9:55–62
ClinicalTrials.Gov (2015) Studies in patients with multiple myeloma and renal failure due to myeloma cast nephropathy (MYRE). https://clinicaltrials.gov/ct2/show/NCT01208818
Testa A, Dejoie T, Lecarrer D, Wratten M, Sereni L, Renaux JL (2010) Reduction of free immunoglobulin light chains using adsorption properties of hemodiafiltration with endogenous reinfusion. Blood Purif 30:34–36
Pasquali S, Iannuzzella F, Corradini M, Mattei S, Bovino A, Stefani A, Palladino G, Caiazzo M (2015) A novel option for reducing free light chains in myeloma kidney: supra-hemodiafiltration with endogenous reinfusion (HFR). J Nephrol 28(2):251–254
Pendón-Ruiz de Mier MV, Alvarez-Lara MA, Ojeda-López R, Martín-Malo A, Carracedo J, Caballero-Villarraso J, Alonso C, Aljama P (2013) Effectiveness of haemodiafiltration with ultrafiltrate regeneration in the reduction of light chains in multiple myeloma with renal failure. Nefrologia 33(6):788–796
Fabbrini P, Sirtori S, Casiraghi E, Pieruzzi F, Genovesi S, Corti D, Brivio R, Gregorini G, Como G, Carati ML, Viganò MR, Stella A (2013) Polymethylmethacrylate membrane and serum free light chain removal: enhancing adsorption properties. Blood Purif 35(Suppl 2):52–58
Santoro A, Grazia M, Mancini E (2013) The double polymethylmethacrylate filter (DELETE system) in the removal of light chains in chronic dialysis patients with multiple myeloma. Blood Purif 35(Suppl 2):5–13
Fabbrini P, Sirtori S, Pieruzzi F, Stella A, Vigano MR (2014). Acute cast nephropathy in multiple myeloma: effects of intensive polymethylmethacrylate-enhanced adsorption dialysis on renal survival. J Am Soc Nephrol 25:586 A (abstract, available at http://www.asn-online.org/education/kidneyweek/archives/)
Mancini E, Sestigiani E, Gissara Z, Palladino G, Santoro A (2011) Light chain removal by means of adsorption in the extracorpeal treatment of myeloma-induced cast nephropathy. XLVIII ERA-EDTA Congress, Prague, Czech (abstract available at http://www.abstracts2view.com/era_archive/view.php?nu=ERA11L_1648)
Pasquali S, Mancini E, Mambelli E, Santoro A (2009) Studio cinetico di rimozione delle catene leggere: confronto fra plasmaferesi tradizionale, plasmaferesi a cascata con resine adsorbenti, emodialisi mediante una membrana ad alto cut-off. Giorn Ital Nefrol S-47:S89 (Abstract)
Pasquali S, Mancini E, de Sanctis LB, Mambell Ei, Wratten M, Santoro A (2008) Coupled plasmafiltration adsorption: a new technology for free light chain removal. XLV ERA-EDTA congress, Stockholm, 2008 (abstract available at http://www.abstracts2view.com/era_archive/view.php?nu=ERA08L_1571)
Meletios A, Dimopoulos, Pieter Sonneveld, Nelson Leung et al (2016) International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol 34(13):1544–1557
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
Informed consent
No informed consent was obtained.
Research involving human participants and/or animals
This Position Paper include authors’ opinions on previous published researches and no new unpublished specific research involving human participants and/or animals has been done.
Rights and permissions
About this article
Cite this article
Fabbrini, P., Finkel, K., Gallieni, M. et al. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the Onconephrology Work Group of the Italian Society of Nephrology. J Nephrol 29, 735–746 (2016). https://doi.org/10.1007/s40620-016-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0347-9