Abstract
Multiple myeloma (MM) is a clonal B-cell cancer of proliferating plasma cells. It represents nearly a tenth of all hematologic malignancies. Renal dysfunction is present in 25–50 % of newly diagnosed MM patients, about 9 % of which needs hemodialysis (HD). Cast nephropathy is determined by an overflow of filtered serum-free light chains (sFLC) in the proximal tubule that largely overwhelms its endocytic capacity. Bortezomib-based regimens, including high-dose dexamethasone, are recommended as first-choice therapy. In addition, kidney exposure to sFLC may be reduced with extracorporeal sFLC removal. Plasma exchange has been for long time the only extracorporeal technique used in cast nephropathy, but it is now not recommended for sFLC removal in patients with MM-associated AKI. In recent years, other techniques such as HD with new generation high cutoff dialyzers showed their efficacy in sFLC removal and should now be considered for a more rapid reduction of sFLC levels in combination with bortezomib-based therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 General Principles
Multiple myeloma (MM) is a clonal B-cell cancer of proliferating plasma cells. It represents nearly a tenth of all hematologic malignancies [1]. Renal dysfunction is among the most common complications in patients with active MM, together with hypercalcemia, osteolytic lesions, and anemia [2]. Kidney injury in plasma cells dyscrasias may be extremely heterogeneous. However, only cast nephropathy should be regarded as a myeloma-defining event since all other histological forms should be regarded as different entities related to a monoclonal serum-free light chain (sFLC) production [3].
Renal dysfunction is present in 25–50 % of newly diagnosed MM patients, about 9 % of which needs hemodialysis (HD) [4]. Half of patients with MM may develop renal injury during the course of the disease. Kidney dysfunction can be reversed in approximately 50 % of patients, but the remaining patients will have some degree of persistent chronic kidney disease (CKD). Patients with acute kidney injury (AKI) have higher early and overall mortality. Evidence is accumulating suggesting that kidney function is tightly related to myeloma cell mass: accordingly, CKD is more common in patients with a large tumor burden [5].
Since the recovery of renal function is associated with a dramatic improvement in survival, MM with severe renal dysfunction at presentation deserves a prompt and aggressive treatment, with the following key objectives [6]:
-
Removal or prevention of those factors possibly aggravating renal injury. Maximizing urine output in not anuric patients by optimizing intravascular volume, avoiding loop diuretics, correcting acidosis and hypercalcemia [7], and avoiding intravenous radiological contrast media or other nephrotoxic drugs.
-
Reduced exposure of the kidneys to sFLC. Effective chemotherapy is the cornerstone of treatment in patients with myeloma-related AKI. Hematologic response usually translates into renal improvement. Bortezomib, an ubiquitin-proteasome inhibitor, may counteract the harmful effects of FLCs on the kidney through a rapid reduction of sFLC (due to the reduction of myeloma cells) and a reduction of kidney inflammation due to the blockade of NF-κB activation. Bortezomib does not need dose adjustment in patients with renal failure. The International Myeloma Working Group (IMWG) [8] recommended the use of bortezomib-based regimens, including high-dose dexamethasone, as first choice. The combination of bortezomib with melphalan and prednisone may be used in elderly patients with renal disease. In patients with mild-to-moderate renal impairment, lenalidomide may be used as an alternative, adjusting the dose according to renal function. Several studies reported reversal of myeloma-associated renal injury, also in a 20–30 % of patients on dialysis who become dialysis independent following treatment [6].
Removal of sFLC with extracorporeal techniques such as plasma exchange has been also described as an adjunctive treatment to further reduce kidney exposure to sFLC and, possibly, to improve renal outcome in patients with MM. However, as discussed below, the impact of extracorporeal sFLC removal (with any technique) on survival is unclear.
2 Main Evidence
There is only a small single-center randomized controlled trial (RCT) reporting a reduction in mortality with plasma exchange in patients with MM-associated AKI [9]. Zucchelli et al. [10] randomized 29 patients to receive either peritoneal dialysis or plasma exchange in addition to hemodialysis (HD) and found a significantly reduced 1-year mortality in the plasma exchange/HD group. However, this study is rather old and therapy of MM has profoundly changed since its publication. Moreover, the combination of plasma exchange with HD and the comparison with a different dialysis technique (peritoneal dialysis) make it difficult to attribute the observed benefits to plasma exchange per se [9]. Indeed, more recent studies of plasma exchange in patients with MM-associated renal impairment failed to show any difference in renal outcomes or survival [11, 12].
Nevertheless, new evidence is now available regarding the role of extracorporeal sFLC removal combined with chemotherapy in patients with MM-associated kidney injury [13, 14]. In summary, extracorporeal removal of sFLC is considered reasonable in current clinical practice if it is reserved to patients with cast nephropathy [13] and if it aims to a great reduction of sFLC (60 %) in a very short time (12–21 days from the beginning of treatment) [14, 15].
3 Pathophysiological Principles and Clinical Practice
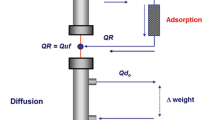
Cast nephropathy is determined by an overflow of filtered sFLC in the proximal tubule that largely overwhelms its endocytic capacity. The high amount of exceeding FLC in the tubular fluid leads to their accumulation within the proximal tubular cells and to intraluminal cast formation into distal tubules [13]. As mentioned, this state is usually induced by concomitant conditions such as hypovolemia, electrolyte/acid-base disturbances, and nephrotoxic drugs, which can impair tubular function.
Therefore, treatment should aim at preventing or removing such precipitant factors, at reducing sFLC production (with anti-myeloma drugs), and, reasonably, at removing sFLC through extracorporeal techniques.
3.1 Extracorporeal sFLC Removal
Since sFLC molecular weight is 25 and 50 kDa for κ and λ chains, respectively, even high/superflux dialyzers can barely remove these molecules [14]. Therefore, extracorporeal clearance of sFLC can only be achieved through dialyzers with either higher cutoff (high cutoff and/or plasma exchange dialyzers) or specific adsorption properties.
Plasma exchange has been for a long time the only extracorporeal technique used in cast nephropathy to achieve an effective removal of sFLC through a complete plasma substitution. Three RCTs [10, 11, 16] were performed, two of which [11, 16] found no benefit of plasma exchange on overall survival. It should be underlined that all these studies suffer from several methodological limitations: plasma exchange efficacy was not measured since sFLC assays were not available, patients included had a very wide range of renal failure, and the cause of renal failure was not clearly determined. Furthermore, novel chemotherapeutic agents which have proven effective in the treatment of MM were not used at the time of these studies, and this makes impossible to translate their results into the modern clinical practice.
However, plasma exchange has some conceptual disadvantages. The treatment is short and its efficacy is limited to the intravascular compartment, which may contain only 15–20 % of the total sFLC, due to their high volume of distribution. Moreover, AKI patients often need renal function replacement which is not provided by plasma exchange.
In recent years, other extracorporeal techniques showed their efficacy in sFLC removal [15, 17, 18]. In particular, the new generation of high cutoff dialyzers can provide effective removal of sFLC by either diffusion or convection. Different treatment schedules have been proposed, mainly consisting of daily long dialysis sessions in order to remove as much sFLC as possible. Two RCTs, the EuLITE trial in the UK and Germany [19] and the MYRE trial in France (NCT01208818), are now trying to determine the actual role of these devices, but no results are currently available. All other techniques are anecdotally reported in small case series and, to our knowledge, no RCTs are currently ongoing.
Finally, a new type of high cutoff membrane has been introduced in clinical practice. The Ultraflux EMiC2 dialysis filter (Fresenius, Bad Homburg v.d.H., Germany) was developed to increase the clearance of middle-sized molecules such as cytokines. The filter has a molecular weight cutoff of about 40 kDa (only 5 kDa less than ordinary high cutoff membranes) and allows a lower loss of larger molecules such as albumin or coagulation factors. These characteristics may be of great clinical interest since intensive dialysis with rapid reduction of sFLC but without loss of albumin should be considered as the best goal of extracorporeal therapy. No data have been published so far on this topic. However, our preliminary observations in few patients are encouraging.
4 Conclusion
Plasma exchange is now not recommended for sFLC removal in patients with MM-associated AKI. However, new extracorporeal therapies, especially high cutoff dialysis, should now be considered for a more rapid reduction of sFLC levels in combination with bortezomib-based therapies. RCTs are needed in order to better clarify the indications and the impact on relevant outcomes of such new techniques for sFLC removal. Meanwhile, we recommend a careful patient selection since only dialysis-dependent patients with cast nephropathy seem to be really eligible to extracorporeal removal of sFLC, and treatment should be tailored on the basis of sFLC levels and efficacy of chemotherapy.
Clinical Summary
Strategy | Indications | Main evidence | Notes |
|---|---|---|---|
Extracorporeal removal of serum-free light chains (sFLC) | Multiple myeloma (MM)-associated AKI | The only evidence of a survival benefit comes from an old, small RCT in which plasma exchange in addition to hemodialysis was compared with peritoneal dialysis Other studies of plasma exchange in this clinical setting were inconclusive | Plasma exchange is now not recommended in patients with MM-associated AKI Therapy of MM has profoundly changed in the last decades. Newer extracorporeal techniques for sFLC removal (especially high cutoff dialysis) seem to be promising in addition to modern anti-myeloma drugs (adequate RCTs are needed) |
References
Kyle RA, Rajkumar SV (2004) Multiple myeloma. N Engl J Med 351(18):1860–1921
Knudsen LM, Hippe E, Hjorth M et al (1994) Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur Haematol 53(4):207–212
Rajkumar SV, Dimopoulos MA, Palumbo A et al (2014) International Myeloma Working Group updated criteria for diagnosis of multiple myeloma. Lancet Oncol 15(12):e538–e548
Kyle RA, Gertz MA, Witzig TE et al (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78(1):21–33
Blade J, Fernandez-Llama P, Bosch F et al (1998) Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 158(17):1889–1893
Davenport A, Merlini G (2012) Myeloma kidney: advances in molecular mechanisms of acute kidney injury open novel therapeutic opportunities. Nephrol Dial Transplant 27(10):3713–3718
Mhaskar R, Redzepovic J, Wheatley K et al (2010) Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev (5):CD003188
Dimopoulos MA, Terpos E, Chanan-Khan A et al (2010) Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 28:4976–4984
Landoni G, Bove T, Székely A et al (2013) Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth 27(6):1384–1398
Zucchelli P, Pasquali S, Cagnoli L, Ferrari G (1988) Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33:1175–1180
Clark WF, Stewart AK, Rock GA et al (2005) Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med 143(11):777–784
Gupta D, Bachegowda L, Phadke G et al (2010) Role of plasmapheresis in the management of myeloma kidney: a systematic review. Hemodial Int 14:355–363
Leung N, Gertz MA, Zeldenrust SR et al (2008) Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 73(11):1282–1288
Hutchison CA, Cockwell P, Reid S et al (2007) Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo study. J Am Soc Nephrol 18:886–895
Hutchison CA, Heyne N, Airia P et al (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 27(10):3823–3828
Johnson WJ, Kyle RA, Pineda AA et al (1990) Treatment of renal failure associated with multiple myeloma plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 150(4):863–869
Fabbrini P, Sirtori S, Casiraghi E et al (2013) Polymethylmethacrylate membrane and serum free light chain removal: enhancing adsorption properties. Blood Purif 35(Suppl 2):52–58
Pasquali S, Iannuzzella F, Corradini M et al (2015) A novel option for reducing free light chains in myeloma kidney: supra-hemodiafiltration with endogenous reinfusion (HFR). J Nephrol 28(2):251–254
Hutchison CA, Cook M, Heyne N et al (2008) European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials 9:55
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Paternoster, G., Fabbrini, P., Attolico, I. (2016). Extracorporeal Removal of Serum-Free Light Chains in Patients with Multiple Myeloma-Associated Acute Kidney Injury. In: Landoni, G., Pisano, A., Zangrillo, A., Bellomo, R. (eds) Reducing Mortality in Acute Kidney Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-33429-5_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-33429-5_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33427-1
Online ISBN: 978-3-319-33429-5
eBook Packages: MedicineMedicine (R0)