Abstract
Introduction
Elevated fibroblast growth factor-21 (FGF-21) levels are related to carotid intima-media thickness (CIMT), a well-established marker of atherosclerosis. Acromegaly has also been linked to increased CIMT. There has been no data considering the association between FGF-21 levels and atherosclerosis in acromegaly patients. This study aimed to evaluate FGF-21 levels and CIMT in acromegalic patients in relation to atherosclerotic complications.
Design
Case–control study.
Materials and methods
The study group included 70 acromegaly patients and 72 healthy volunteers from the Department of Endocrinology and Metabolism Disease, Marmara University Medical School. FGF-21, growth hormone, insulin-like growth factor I, lipids, glucose, insulin levels were assessed. CIMT was measured from the common carotid artery wall on B-mode ultrasound.
Results
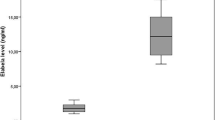
Median FGF-21 levels were significantly lower in the acromegaly group than in the control group. CIMT was higher in acromegaly patients compared to controls. Although there was no correlation between FGF-21 levels and CIMT in patients with acromegaly, a positive correlation was found between high-density lipoprotein-cholesterol and FGF-21 levels. Glucose metabolic markers were the determining factors of the FGF-21 levels in acromegaly patients.
Conclusion
Our study is the first to examine the relationship between serum FGF-21 levels and atherosclerosis in acromegaly patients. The lower serum FGF-21 levels in acromegaly subjects might be associated with the improving effects of growth hormone on liver fat. Acromegaly was linked to higher CIMT, but there was no correlation between FGF-21 levels and CIMT. The role of FGF-21 in acromegaly as a marker of atherosclerosis requires additional research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a progressive disease usually caused by a pituitary adenoma, characterized by the increased growth hormone (GH) [1] and insulin-like growth factor 1 (IGF-1) [2]. The excess GH or IGF-1 leads to several complications, such as diabetes mellitus or glucose intolerance, hypertension, and atherosclerosis [2]. Cardiovascular disease is the leading cause of morbidity and mortality in acromegaly due to a higher prevalence of emerging risk factors such as glucose tolerance, hypertension, dyslipoproteinaemia [3,4,5]. Studies showed an increased prevalence of subclinical atherosclerosis in patients with acromegaly by investigating a valuable marker of early atherosclerosis; ultrasound measurement of carotid intima-media thickness (CIMT) [4]. The mechanism of functional and morphological vascular changes in acromegaly is associated with elevated GH/IGF-1 beyond the established risk factors [3, 5]. GH excess plays a role in endothelial dysfunction; high levels of GH/IGF-1 could directly, detrimentally affect the vascular wall [3].
Fibroblast growth factor-21 (FGF-21) is a hormonal regulator of hepatic and systemic lipid and glucose homeostasis, predominantly secreted from the liver via the regulation of the transcription factor peroxisome proliferator-activated receptor α (PPARα) [6]. FGF-21 increases energy expenditure, lipolysis, hepatic fatty acid oxidation and improves insulin sensitivity in muscle, liver, and fat according to in vitro and animal models [6, 7]. Subsequently, FGF-21treatment increases weight loss, enhances glucose metabolism, and reduces liver fat [6].
Despite the metabolically beneficial effects of FGF-21in vitro and animal data, human data show a noticeable increase in FGF-21 in the presence of obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD) potentially due to compensatory mechanisms and/or FGF-21 resistance [6, 7]. Elevated FGF-21 levels have also been associated with subclinical atherosclerosis, consequently coronary heart disease (CHD) [7, 8]. However, the data on the relation between FGF-21 and atherosclerotic diseases, independent of established risk factors, were conflicting in humans [7].
GH is also a metabolic hormone with regards to its role in regulating glucose and lipid metabolism. Both GH and FGF-21 are stimulated in response to fasting and bring to bear their actions on lipolysis [9]. Nevertheless, the molecular interaction among these hormones remains uncertain, and it is mainly analyzed by animal studies [9, 10]. GH stimulates lipolysis, causing free fatty acid (FFA) release from adipocytes. FFA enhances hepatic FGF-21 production by activating the peroxisome proliferator-activated receptor α (PPARα) [9]. Furthermore, FGF-21 gene transcription in the liver is induced by GH through a mechanism that involves the signal transducer and activator of transcription 5 (STAT5) [10]. The inhibition of Janus kinase 2 (JAK2)-STAT5 signaling in the liver by FGF-21 suggests a novel negative feedback loop, preventing excessive production of IGF-I, and also counter-regulates the effects of GH on lipid and glucose metabolism [10].
Contrary to the animal studies suggesting a positive effect of GH on FGF-21 production, human data demonstrated no reduction in FGF-21 levels despite significantly reduced GH and IGF-1 levels in GH-deficient patients [11]. Moreover, the replacement of long-term GH in these patients normalized IGF-1 levels and improved metabolic balance but did not increase FGF-21 levels [11]. Another data suggested that growth hormone-releasing hormone (GHRH) agonists decreased serum FGF-21 levels by reducing liver fat in humans [6].
A limited number of studies evaluated the FGF-21 levels in acromegaly [12, 13]. Although FGF-21 role on atherosclerosis in other endocrine disorders has been investigated [14, 15], the association between FGF-21 levels and atherosclerosis in acromegaly patients remains unclear. In this study, we assessed the FGF-21 levels and their association with CIMT in patients with acromegaly.
Materials and methods
The study group involved 70 patients with acromegaly divided into 2 subgroups according to disease activity: active acromegaly (AA), controlled acromegaly (CA). Seventy-two subjects were assigned to the control group (CG). All participants were recruited from the Department of Endocrinology and Metabolism Disease, Marmara University Medical School. Blood samples were achieved to evaluate FGF-21, GH, IGF-1, lipids, glucose, and insulin levels. Detailed medical histories were obtained using a standardized questionnaire. The Local Ethics Committee approved the study protocol, and the patients signed informed consent.
Acromegaly was diagnosed based on recent guidelines [16]. Transsphenoidal surgery was the first treatment choice. Adjuvant gamma knife radiosurgery (GKS) was performed if required. GH values at or below 0.4 μg/L after an oral glucose tolerance test and normal age-related IGF-1 levels were characterized as remission after surgery and/or irradiation. Biochemical control was described as normal IGF-1 levels under medication [16]. Both groups were classified as ‘controlled disease’ at remission and biochemical control for at least 3 months. Elevated serum IGF-1 levels after surgery and/or radiosurgery despite medical treatment were defined as active disease. Fifty-three percent of patients had adjuvant GKS following transsphenoidal surgery, 45.7% of patients had surgery. Despite surgery and medical treatment, 22.8% of patients had elevated IGF-1 levels. Remission was achieved in 30% of patients. Regarding medical treatment; 37.1% of patients were on somatostatin receptors ligands (SRLs), 28.6% were on SRLs plus cabergoline, and 4.3% were on SRLs plus pegvisomant treatment after surgery and/or radiosurgery in whom remission could not have been achieved.
Prediabetes and type 2 diabetes were classified in line with American Diabetes Association-ADA criteria [17]. Hypertension and dyslypidemia were diagnosed according to the latest guidelines [18, 19].
CG were selected from the patients who applied to the internal medicine and endocrinology outpatient clinics of Marmara University Medical School with no clinical acromegaly symptoms and normal IGF-1 values that matched to patients with acromegaly for age.
Physical evaluation
Patients with acromegaly and control subjects were evaluated after overnight fasting. Anthropometric measurements and height, body weight, waist circumference (WC) and blood pressure were taken. Body mass index (BMI) was calculated as weight (in kg) divided by squared height (in m) (kg/m2). Blood pressure (BP) was measured on the right arm after the patient had been settled for at least 10 min.
Vascular ultrasound measurement
CIMT was measured between a double-line reflex pattern indicating the luminal-intimal and the medial-adventitial interfaces of the common carotid artery wall on a B-mode ultrasound [20] according to the Mannheim Consensus [21]. Three measurements were examined for each of the segments, and the mean was calculated. The same experienced operator performed all scans.
Laboratory evaluation
Serum glucose, total serum cholesterol (TCHOL), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), and triglyceride (TG) levels were measured enzymatically. Serum insulin was detected using chemiluminescent immunoassays. Hemoglobin A1C (HbA1c) was determined by an ion-exchange HPLC method. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated. A human FGF-21 enzyme-linked immunosorbent assay (ELISA) kit (standard range 31–2000 pg/ml, (Abnova, Taipei, Taiwan) was used to determine serum FGF-21 levels. Intra-assay and inter-assay coefficients of variation were 4.7% and 7.2%, respectively. Serum GH and IGF-1 levels were measured by the immulite solid-phase, enzyme-labeled chemiluminescent immunometric assay (Roche, Immulate, 2000). The GH intra-assay and inter-assay variation coefficients were 2.9–4.6% and 4.2–6.6%, respectively, the IGF-1 inter-assay variation coefficient was 3.0–7.6%.
Statistical analysis
All analyses were performed using commercial statistical software (version 22.0; IBM SPSS). Descriptive statistics were given as the median and range for continuous data and as percentages and frequency for categorical data. Continuous variables were analyzed for homogeneity of variance using the Kolmogorov–Smirnov test. The data with normal distribution were analyzed with the t test, while we analyzed the data with uneven distribution with the Mann–Whitney U test. The Chi-square test or Fisher’s exact test were used to analyze categorical data. We performed a Pearson correlation analysis between serum FGF-21 and IGF-1, GH, lipid levels, and CIMT. The determinant of serum FGF-21 level and independent factors of atherosclerosis in patients with acromegaly was assessed with multiple regression analysis. P values less than 0.05 were considered statistically significant.
Results
The general characteristics of the acromegaly and the control group are presented in Table 1. There was no significant difference between the groups with regards to age. Hypertension, hyperlipidemia, and diabetes mellitus rates were higher in acromegaly patients. Systolic BP (SBP) and diastolic BP (DBP) were also higher in acromegaly patients. BMI and WC were similar between the groups. Patients with acromegaly had higher HbA1c levels (p:0.02). While lipid profile did not show any difference among the groups, CIMT was higher in acromegaly patients than controls [0.59 (0.39–1.03) vs. 0.41 (0.28–0.80), p < 0.001]. Median FGF-21 levels were significantly higher in control group compared to acromegaly group [396.36 (79.52–1488.7) vs. 180.22 (85.87–843.12) pg/mL, p < 0.001, respectively].
According to the disease status, the characteristics of acromegaly patients are shown in Table 2. AA and CA groups showed no difference concerning gender. CA group tended to be older than AA group (p:0.06). Disease duration was longer in CA compared to AA (p:0.04). Although BMI was similar between the groups, WC was slightly higher in CA than AA (p:0.05). There was a moderate elevation in the rate of hypertension and dyslipidemia in CA compared to AA (p:0.05). However, the diabetes mellitus rate did not change between the groups (p:0.22). Glucose and lipid profiles were also similar among AA and CA groups despite moderately higher TCHOL in the CA group (p:0.05). FGF-21 levels and CIMT were not significantly different between controlled and active acromegaly patients (p:0.43; p:0.25, respectively).
There was no difference in FGF-21 levels [185 (87.3–304.5) vs. 178.6 (85.8–843.1) pg/mL] and CIMT (0.62 ± 0.14 vs. 0.64 ± 0.15 cm) between smoker and non-smoker acromegaly patients.
Correlation analysis of FGF-21 levels with CIMT, anthropometric, metabolic, and laboratory parameters is shown in Table 3. Although there was no correlation between FGF-21 levels and CIMT in acromegaly patients (p:0.38), a positive correlation was found between HDL-c and FGF-21 levels (p < 0.001). Considering anthropometric parameters, WC was positively correlated with FGF-21 (p:0.02). No correlation was found between IGF-1 and FGF-21 levels (p:0.18). In the control group, DBP, TCHOL, TG levels were negatively associated with FGF-21. Fasting glucose and fasting insulin levels were not correlated with FGF-21 in both groups.
Multiple regression analysis, establishing the influencing factors of plasma FGF-21 levels, is shown in Table 4. Model 1 included the whole study group to establish the influencing factors of plasma FGF-21 levels. Acromegaly was the only determining factor of the FGF-21 levels in the whole group. Model 2 involved acromegaly patients. HDL-c (β coefficient: 3.164, p:0.01), glucose (β coefficient: − 3.333, p:0.02), insulin (β coefficient: − 29.424, p:0.02), HbA1c (β coefficient: − 35.101, p:0.02) and HOMA-IR (β coefficient:128.084, p:0.01) were the determinants of FGF-21 levels in acromegaly patients. Regarding CIMT in multiple regression analysis, both models were also applied (Table 5). In model 1, age (β coefficient: 0.005, p:0.01), and presence of acromegaly (β coefficient: 0.173, p:0.001), were the influencing factors of CIMT in the whole study group. In model 2, age (β coefficient: 0.008, p:0.001) was the only determining factor of CIMT in the acromegaly group.
Discussion
Acromegaly is related to increased cardiovascular mortality, attributed to the concomitant atherosclerosis risk factors such as hypertension, dyslipoproteinaemia, glucose intolerance [4]. Previous data showed increased CIMT indicating endothelial dysfunction, a valuable marker of atherosclerosis in acromegaly patients [4, 5]. Several studies evaluated the mechanisms by which acromegaly induces vascular changes. Boysan et al. suggest that early atherosclerosis in patients with acromegaly may result from insulin resistance and direct vascular effects of GH and/or IGF-1 [5]. Further studies showed detrimental and direct effects of excess GH/IGF-I levels on the vascular wall, generating endothelial dysfunction [3]. IGFs are mitogenic to vascular smooth muscle cells [22], and constant IGF-1 infusion stimulates vascular smooth muscle cell proliferation after balloon catheter injury in the intima-media of rat aorta [23]. Furthermore, IGF-1 treatment increases the transcription and the expression of intercellular adhesion molecule-1[24] which is typically increased in endothelial dysfunction [25].
Elevated FGF-21 levels also have been linked to CIMT, in recent studies [7, 8]. The limited data showed conflicting results considering FGF-21 levels in acromegaly patients. To the best of our knowledge, this is the first study evaluating serum levels of FGF-21 in acromegaly patients with relation to atherosclerosis. We showed that serum FGF-21 levels were lower in acromegaly subjects than control group. There was a significant association between FGF-21 levels and the presence of acromegaly. Though a study found increased levels of FGF-21, the other one showed no significant differences in FGF-21 concentrations in acromegaly patients compared to controls [12, 13].
Although in animal data, FGF-21 reduces body weight, improves glucose metabolism, decreases liver fat [26], human data show a clear increase in FGF-21 in NAFLD that was associated with FGF-21 resistance [27]. Braun et al. showed that FGF-21 levels decreased after administration of a GHRH agonist, which was attributed to improving effects of GHRH agonist on liver fat [6]. This data suggest that increased endogenous GH pulsatility via GHRH agonist might contribute to reduction in FGF-21 resistance by affecting receptor expression or inflammation markers, causing liver fat reduction [10]. However, there has been no data indicating the effect of GH on FGF-21 receptor activity so far [10]. Our findings might be associated with reduced FGF-21 resistance via the improving effects of GH on liver fat. Also, we found a positive correlation between HDL-c and FGF-21 level in acromegaly patients. HDL-c, glucose, insulin, HbA1c and HOMA-IR were the determinants of FGF-21 levels in acromegaly patients according to multiple regression analysis.
Increased CIMT represent the earliest functional and structural vascular changes in atherogenesis and is associated with classical cardiovascular risk factors [3]. Studies suggest that acromegaly patients are associated with increased CIMT due to endothelial dysfunction [3, 5]. Similarly, we showed increasd CIMT in acromegaly patients independent of established risk factors for atherosclerosis. Age and presence of acromegaly were associated with CIMT in the whole study group. Age was an independent risk factor for increased CIMT in another study [7]. Kartal et al. showed that active acromegaly patients had higher CIMT than matched controls and inactive ones [5]. We found no significant difference regarding CIMT between AA and CA patients.
Previous studies on the association between FGF-21 and atherosclerosis have shown inconsistent results [28, 29]. High-serum FGF-21 levels were related to adverse lipid profiles in CHD patients, representing a paradoxical increase in CHD patients due to a compensatory response or resistance to FGF-21 [28]. Conversely, FGF-21 levels were associated with carotid atherosclerosis in women, independent of established risk factors including adverse lipid profiles and C-reactive protein [7]. Another study assessed the relationship between serum FGF-21 levels and atherosclerosis in patients without non-alcoholic fatty liver disease, and examined whether baseline FGF-21 could predict occurrence of atherosclerotic cardiovascular disease in a 7-year prospective cohort [8]. FGF-21 was significantly higher in patients with subclinical carotid atherosclerosis and baseline FGF-21 was significantly elevated in those who developed ischemic heart disease in the follow-up [8]. However, another study showed significantly lower serum FGF-21 concentrations in patients with subclinical atherosclerosis [30].
There has been no data considering the association between FGF-21 levels and atherosclerosis in acromegaly patients thus far. Our data showed no correlation between FGF-21 levels and CIMT in patients with acromegaly.
In an analysis of 141 DM subjects, the presence of carotid artery plaque was associated with an elevation in serum FGF-21 levels [31]. In contrast, other study that compared 60 CHD subjects with 129 BMI-matched controls, demonstrated that serum FGF-21 levels were not correlated with CHD, despite a significant association between FGF-21 levels and various established cardiovascular risk factors, such as lipid profiles, insulin resistance, and metabolic syndrome [29]. Similarly, FGF-21 was related to glucose, insulin, HOMA-IR, and HbA1c in the present study despite the lack of correlation between FGF-21 and CIMT.
Some limitations of our study should be disclosed:
-
1.
The cross-sectional design of the study disallowed the determination of a causative relationship. Further studies are needed to demonstrate the precise relationship between atherosclerosis and FGF-21 in patients with acromegaly.
-
2.
The imbalance of gender between acromegaly and the control group was another limitation of the study. However, sex did not influence CIMT, whereas age was the influencing factor of CIMT in multiple regression analyses in previous studies in line with our results [7, 8].
Conclusions
Our study is the first to examine the relationship between serum FGF-21 levels and atherosclerosis in acromegaly patients. We found lower serum FGF-21 levels in acromegaly subjects compared to the control group. There was a significant association between FGF-21 levels and the presence of acromegaly. This result may be associated with the improving effects of growth hormone on liver fat, where the primary regulation of FGF-21 takes place. The presence of acromegaly was associated with higher CIMT. Although there was no correlation between FGF-21 levels and CIMT, a significant correlation of FGF-21 existed with HDL-c. Glucose metabolic markers were the determining factors of the FGF-21 in acromegaly patients.
The role of FGF-21 in acromegaly patients as a beneficial marker or target of atherosclerotic processes calls for additional research.
Change history
25 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40618-022-01867-7
References
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz096 (Epub 2019/10/14)
Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM et al (2019) Acromegaly. Nat Rev Dis Primers 5(1):20. https://doi.org/10.1038/s41572-019-0071-6 (Epub 2019/03/23)
Brevetti G, Marzullo P, Silvestro A, Pivonello R, Oliva G, di Somma C et al (2002) Early vascular alterations in acromegaly. J Clin Endocrinol Metab 87(7):3174–3179. https://doi.org/10.1210/jcem.87.7.8643 (Epub 2002/07/11)
Rizzo M, Montalto G, Rizvi AA, Christ ER (2012) The role of elevated growth hormone on the increased atherosclerosis in patients with acromegaly. Angiology 63(7):492–494. https://doi.org/10.1177/0003319712436578.PubMedPMID:WOS:000308721000002
Kartal I, Oflaz H, Pamukcu B, Meric M, Aral F, Ozbey N et al (2010) Investigation of early atherosclerotic changes in acromegalic patients. Int J Clin Pract 64(1):39–44. https://doi.org/10.1111/j.1742-1241.2008.01750.x.PubMedPMID:WOS:000272655800008
Braun LR, Feldpausch MN, Czerwonka N, Torriani M, Grinspoon SK, Stanley TL (2017) Fibroblast growth factor 21 decreases after liver fat reduction via growth hormone augmentation. Growth Horm IGF Res 37:1–6. https://doi.org/10.1016/j.ghir.2017.10.002 (Epub 2017/10/17)
Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH et al (2013) Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol 33(10):2454–2459. https://doi.org/10.1161/ATVBAHA.113.301599 (Epub 2013/07/28)
Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li Y et al (2020) Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events. J Am Heart Assoc 9(11):e015226. https://doi.org/10.1161/JAHA.119.015226 (Epub 2020/05/21)
Chen W, Hoo RL-C, Konishi M, Itoh N, Lee P-C, Ye H-Y et al (2011) Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem 286(40):34559–34566
Yu J, Zhao LD, Wang AH, Eleswarapu S, Ge XM, Chen DW et al (2012) Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology 153(2):750–758. https://doi.org/10.1210/en.2011-1591.PubMedPMID:WOS:000299928200024
Lundberg J, Höybye C, Krusenstjerna-Hafstrøm T, Bina H, Kharitonenkov A, Angelin B et al (2013) Influence of growth hormone on circulating fibroblast growth factor 21 levels in humans. J Intern Med 274(3):227–232
Yurekli BS, Kutbay NO, Aksit M, Suner A, Simsir IY, Seckiner S et al (2019) Acromegaly is associated with high fibroblast growth factor-21 levels. J Endocrinol Invest 42(1):53–60. https://doi.org/10.1007/s40618-018-0885-1.PubMedPMID:WOS:000454270400007
Halupczok-Zyla J, Jawiarczyk-Przybylowska A, Skrzypski M, Strowski MZ, Bolanowski M (2017) Fibroblast growth factor 21 in patients with acromegaly. Exp Clin Endocrinol Diabetes 125(10):649–654. https://doi.org/10.1055/s-0043-115647 (Epub 2017/09/21)
Xiao F, Lin M, Huang P, Zeng J, Zeng X, Zhang H et al (2015) Elevated serum fibroblast growth factor 21 levels in patients with hyperthyroidism. J Clin Endocrinol Metab 100(10):3800–3805. https://doi.org/10.1210/jc.2015-1797 (Epub 2015/08/05)
Xiao Y, Liu L, Xu A, Zhou P, Long Z, Tu Y et al (2015) Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 14:72. https://doi.org/10.1186/s12933-015-0229-9 (Epub 2015/06/07)
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A et al (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700 (Epub 2014/10/31)
American DA (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. https://doi.org/10.2337/dc14-S081 (Epub 2013/12/21)
Zeitouni M, Sabouret P, Kerneis M, Silvain J, Collet JP, Bruckert E et al (2021) 2019 ESC/EAS guidelines for management of dyslipidaemia: strengths and limitations. Eur Heart J Cardiovasc Pharmacother 7(4):324–333. https://doi.org/10.1093/ehjcvp/pvaa077 (Epub 2020/07/12)
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (Epub 2018/08/31)
Naqvi TZ, Lee M-S (2014) Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC: Cardiovasc Imaging 7(10):1025–1038
Touboul P-J, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N et al (2012) Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). Cerebrovasc Dis 34(4):290–296
Ferns GA, Motani AS, Anggard EE (1991) The insulin-like growth factors: their putative role in atherogenesis. Artery 18(4):197–225 (Epub 1991/01/01 PubMed PMID: 1714710)
Bornfeldt KE, Arnqvist HJ, Capron L (1992) In vivo proliferation of rat vascular smooth muscle in relation to diabetes mellitus insulin-like growth factor I and insulin. Diabetologia 35(2):104–108. https://doi.org/10.1007/BF00402540 (Epub 1992/02/01)
Balaram SK, Agrawal DK, Edwards JD (1999) Insulin like growth factor-1 activates nuclear factor-kappaB and increases transcription of the intercellular adhesion molecule-1 gene in endothelial cells. Cardiovasc Surg 7(1):91–97. https://doi.org/10.1016/s0967-2109(98)00044-1 (Epub 1999/03/12)
Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ (1992) Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann NY Acad Sci 667:324–331. https://doi.org/10.1111/j.1749-6632.1992.tb51633.x (Epub 1992/12/04)
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G et al (2009) Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58(1):250–259. https://doi.org/10.2337/db08-0392 (Epub 2008/10/09)
Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM et al (2010) Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139(2):456–463. https://doi.org/10.1053/j.gastro.2010.04.054 (Epub 2010/05/11)
Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S et al (2010) Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE 5(12):e15534. https://doi.org/10.1371/journal.pone.0015534 (Epub 2011/01/06)
Lee Y, Lim S, Hong ES, Kim JH, Moon MK, Chun EJ et al (2014) Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol (Oxf) 80(1):57–64. https://doi.org/10.1111/cen.12134 (Epub 2013/01/03)
Basurto L, Gregory MA, Hernandez SB, Sanchez-Huerta L, Martinez AD, Manuel-Apolinar L et al (2019) Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol 124:110624. https://doi.org/10.1016/j.exger.2019.05.013 (Epub 2019/06/04)
An SY, Lee MS, Yi SA, Ha ES, Han SJ, Kim HJ et al (2012) Serum fibroblast growth factor 21 was elevated in subjects with type 2 diabetes mellitus and was associated with the presence of carotid artery plaques. Diabetes Res Clin Pract 96(2):196–203. https://doi.org/10.1016/j.diabres.2012.01.004 (Epub 2012/02/02)
Funding
This study did not receive any specific grants from any funding agencies in the public, commercial, or nonprofit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
The Local Ethics Committee approved the study protocol (protocol no: 09.2014.0043).
Informed consent
Patients signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uygur, M.M., Dereli Yazıcı, D. & Gogas Yavuz, D. Low serm Fibroblast Growth Factor-21 levels is not associated with Carotid intima-media thickness in acromegaly patients. J Endocrinol Invest 45, 1405–1412 (2022). https://doi.org/10.1007/s40618-022-01775-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01775-w