Abstract
Purpose
Fibroblast growth factor-21 (FGF-21) is a member of fibroblast growth factor family. Both growth hormone (GH) and FGF-21 take place in the regulation of glucose and lipid metabolism. We aimed to investigate FGF-21 levels in acromegaly which is characterized by excess GH levels and is associated with comorbidities and altered body composition.
Methods
We studied 43 subjects (21 females and 22 males, mean age of 50.0 ± 12.8) with acromegaly. The control group consisted of 40 gender- and age-matched subjects (25 females and 15 males, mean age of 48.8 ± 8.8). Acromegaly patients were classified into two groups; active acromegaly (AA; n = 26) and controlled acromegaly (CA; n = 17). Metabolic, anthropometric and laboratory values of subjects were recorded. FGF-21 level was measured by ELISA assay.
Results
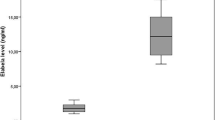
Median FGF-21 levels were significantly higher in acromegaly group compared to control group (85.5 vs. 59.0 pg/mL, p = 0.02, respectively). In the multiple regression model, FPG, A1c, HOMA-IR, glucose intolerance, BMI, visceral fat, hs-CRP, presence of hypertension, dyslipidemia and acromegaly were included as independent variables to explain variability of plasma FGF-21 levels in whole study group. The presence of acromegaly was the only determinant of increased FGF-21 levels in the whole study group (β coefficient = 0.253, p = 0.006).
Conclusion
FGF-21 levels were increased significantly in acromegaly group. Increased FGF-21 levels were significantly and independently associated with the state of acromegaly. Acromegaly may also be a FGF-21 resistance state independent from insulin resistance, glucose intolerance, obesity, hypertension and dyslipidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly caused commonly by a pituitary adenoma is characterized by high levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1). Acromegaly is a rare disease with an annual incidence of 3 or 4 per million [1]. Increased GH causes insulin resistance and leads to impaired glucose tolerance and diabetes mellitus (DM) which are seen in 15–38% of acromegaly patients. It is also associated with hyperlipidemia (HL) and hypertension (HT) [2].
Fibroblast growth factor-21 (FGF-21) is a member of the FGF family. FGF-21, mainly secreted by the liver, acts as an endocrine regulator unlike the other members of FGF family which have mitogenic activities [3]. It has a role in energy hemostasis, glucose and lipid metabolism. In monkeys, when recombinant FGF-21 was given, glucose and lipid profiles were improved [4]. It was shown that serum levels of FGF-21 were increased in obesity [5]. Besides, FGF-21 levels were found to be high in patients with impaired glucose tolerance, type 2 DM, metabolic syndrome and cardiovascular diseases [6]. FGF-21 binds to its receptor, FGF receptors (FGFR), to mediate its effects and needs also ß-Klotho (BKL) as a co-receptor for forming FGF-21/BKL/FGFR complex [7, 8]. FGF-21 expression is controlled by peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the liver and PPAR-gamma in the adipocytes [9, 10].
As we know, acromegaly is associated with insulin resistance and diabetes. FGF-21 is also increased in obesity and diabetes. Although body weight increases in acromegaly, lean body mass increases in greater amount. Both GH and FGF-21 take place in the regulation of glucose and lipid metabolism. So, it could be difficult to predict the serum levels of FGF-21 in such a clinical condition in which there is excess GH and associated comorbidities and altered body composition. So, we aimed to investigate FGF-21 levels in acromegaly and to compare FGF-21 levels with control group. Also, we aimed to determine the parameters that were associated with serum levels of FGF-21 in the study group.
Materials and methods
Subjects
We studied 43 subjects (21 females and 22 males, mean age of 50.0 ± 12.8) with acromegaly. Acromegaly patients who were followed-up in Ege University Endocrinology Clinic were recruited for this study. Acromegaly was diagnosed according to the criteria as follows: failure of suppression of serum GH concentration below 1 ng/mL after 75-g oral glucose tolerance test (OGTT) together with fasting serum IGF-1 concentrations above the normal ranges for age and gender [2]. Acromegaly patients were classified into two groups; active acromegaly (AA; n = 26) and controlled acromegaly (CA; n = 17). Controlled acromegaly was defined as GH below 1.0 ng/mL on a 75-g OGTT or random GH level was below 1.0 ng/mL and IGF-1 values were in the reference range for age and gender [1, 2]. Only IGF-1 value was taken into account to determine disease activity in patients on Pegvisomant therapy [11]. The control group consisted of 40 gender- and age-matched subjects (25 female and 15 male, mean ages of 48.8 ± 8.8). There was no renal or liver dysfunction in any of the subjects. Written informed consent was obtained from all participants. The study was approved by Ege University Local Ethic Committee.
Detailed medical history was recorded including demographic data, duration of the acromegaly, smoking history, comorbidities, use of medication, surgery and radiotherapy history. Physical examination was performed for each subject. Duration of acromegaly disease was 60 months (4–396) as median (min–max). Thirty-seven of 43 (86.0%) patients were treated with surgery, 6 of 43 (13.9%) patients were on primary medical therapy, 2 (4.6%) were treated with stereotactic radiosurgery, 1 (2.3%) with conventional radiotherapy. As far as medical therapy was concerned, 31 of acromegaly patients were on medical therapy. Nineteen patients were on dopamine agonist, 29 of them on somatostatin analog, 6 of them on pegvisomant therapy. Twelve of 43 (27.9%) acromegaly patients had hypopituitarism which was treated with hormone therapies. Out of 43, 5 acromegaly patients had one hormone deficiency and 7 acromegaly patients had more than one hormone deficiency.
Hypertension is defined as systolic blood pressure (SBP) is ≥ 140 mmHg or diastolic blood pressure (DBP) is ≥ 90 mmHg or if there is current use of antihypertensive medication. Dyslipidemia is defined as serum total cholesterol level is ≥ 200 mg/dL; serum triglyceride level is ≥ 150 mg/dL; serum low-density lipoprotein-cholesterol (LDL-c) level is ≥ 130 mg/dL; serum high-density lipoprotein-cholesterol (HDL-c) level is < 40 mg/dL for male, < 50 mg/dL for female subjects or if there is statin use for treatment of dyslipidemia [12]. There was no subject who was taking fenofibrate treatment. There were 4 subjects taking atorvastatin treatment in acromegaly group. Glucose intolerance consisted of both prediabetes and type 2 diabetes mellitus. Prediabetes and type 2 diabetes were defined according to American Diabetes Association-ADA criteria [13]. In control group, all subjects with glucose intolerance were on diet without oral antidiabetics (OAD). In acromegaly group, out of 43, 12 patients were taking OAD, only 2 patients were on multiple insulin injections therapy. No subject was taking pioglitazone as OAD therapy. The control group was similar to acromegaly group as far as hypertension and dyslipidemia were concerned.
Anthropometric and laboratory measurements
Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2). Waist circumference was measured at the midpoint between the inferior costal margin and the superior border of the iliac crest on the mid-axillary line. Total body fat, trunk fat, fat mass and lean body mass were measured using Tanita TBF-215 Body Composition Analyzer device based on Bioelectric Impedance Analysis (BIA) method. For the visceral fat measurement, Tanita Viscan AB-101 device was used.
Blood samples were collected after overnight fasting for serum lipid profile, fasting blood glucose, creatinine, liver function tests, HbA1c, fasting insulin, high sensitive-C reactive protein (hs-CRP). Blood samples stored at − 80 °C immediately. The estimate of insulin resistance was calculated using the Homeostatic Model Assessment–Insulin Resistance (HOMA-IR) index, with the following formula: insulin resistance = fasting plasma insulin (in micro units per milliliter) × fasting plasma glucose (in millimoles per liter)/22.5.
Serum samples for GH and IGF-1 analyses were obtained early in the morning after an overnight fasting. Serum IGF-1 levels were measured with an immunoradiometric assay using a Beckman-Coulter Immunotech kit. Age- and gender-matched normal reference ranges were used. Serum GH levels were measured with an Immulite2000 (Siemens) autoanalyzer via chemiluminescence method. Serum FGF-21 levels were determined using a commercial available ELISA kit (Aviscera Bioscience, Santa Clara, CA, USA). Range of measurement was 15.6–2.000 pg/mL. The intra-assay and inter-assay coefficients of variation were 4–6 and 8–12%, respectively [14].
Statistical analysis
The Statistical Packages for Social Sciences SPSS version 21.0 for Mac was used for the data analysis. Continuous variables were presented as mean ± standard deviation (SD) or median (min–max) according to distribution pattern. Categorical variables were presented as numbers and percentages. Variable distribution was assessed by the Kolmogorov–Smirnov normality test. Student’s t test or Mann–Whitney U test were used for comparison of two groups according to variable distribution. When AA, CA and control group were compared, one-way ANOVA followed by Bonferroni’s post hoc comparison test or Kruskal–Wallis U test were used. Categorical variables were compared by the Chi square test. Correlation analyses were performed using Spearman’s coefficient. Multiple linear regression analysis (stepwise method) was used to explain the variability of FGF-21 levels in whole study group and in acromegaly group. Before the regression analysis, natural log transformation of the data was performed where appropriate. All independent variables in the multiple linear regression analysis were tested for multicollinearity. A p value of less than 0.05 was accepted as statistically significant.
Results
Demographical, clinical and laboratory parameters of acromegaly and control subjects are shown in Table 1. There were no significant differences between the two groups as regards to age and gender. Hypertension and hyperlipidemia rates were similar between acromegaly and control groups. Glucose intolerance were observed in a higher rate in acromegaly group (p = 0.002). There were 14 (32.5%) prediabetic subjects in acromegaly group and 13 (32.5%) prediabetic subjects in control group. While there were 17 subjects with diabetes in acromegaly group, only 2 (5%) subjects had diabetes in control group. Although body weight and BMI were significantly higher in acromegaly group, lean body mass was also remarkably higher in acromegaly group (p = 0.002). While total body fat as percentage didn’t change between the groups, visceral fat was higher in the acromegaly group (Table 1).
Median FGF-21 levels were significantly higher in acromegaly group compared to control group (85.5 vs. 59.0 pg/mL, p = 0.02, respectively). FGF-21 levels did not show significant difference between AA, CA and control groups (data not shown) and no significant difference was observed between AA and CA groups (p = 0.817). Glucose intolerance, dyslipidemia, hypertension rates were similar between AA and CA groups (Table 2). Anthropometric parameters were similar between AA and CA groups (p > 0.05). GH and IGF-1 levels were significantly higher in AA group compared with CA group. There was significant difference as regards duration of acromegaly, hypopituitarism and medical therapy usage rate between groups (Table 2).
Correlation analysis
Correlation analysis of FGF-21 with metabolic, anthropometric and laboratory parameters was shown in Table 3. FGF-21 was significantly associated with A1c and FPG in whole study group analysis (p = 0.020, r = 0.256 and p = 0.022 r = 0.254, respectively) (Fig. 1a, b). FGF-21 was not found to be correlated with A1c and FPG in acromegaly and control groups according to separate group analyses. FGF-21 levels were not associated with the GH and IGF-1 levels in the acromegaly group (p = 0.575 and p = 0.285, respectively).
Regression analysis
In the multiple regression model, FPG, A1c, HOMA-IR, glucose intolerance, BMI, visceral fat, hs-CRP, presence of hypertension, dyslipidemia and acromegaly were included as independent variables to explain variability of plasma FGF-21 levels in whole study group as model 1 (Table 4). Visceral fat was taken into analysis standing for the anthropometric parameter in addition to the BMI. In acromegaly, as lean body mass increases, BMI is also expected to increase. We preferred to use visceral fat for the analysis. Besides, when we used the same model for the total body fat as percentage, the results didn’t change. The presence of acromegaly was the only determinant of increased FGF-21 levels in whole study group (β coefficient = 0.253, p = 0.006). The other parameters in the model didn’t explain the variability in FGF-21 levels in whole study group. In model 2, variability in FGF-21 was investigated in acromegaly group (Table 4). Stepwise regression analysis showed that presence of glucose intolerance was the significant parameter for increased FGF-21 levels in acromegaly group (β coefficient = 0.286, p = 0.042) (Table 4). The levels of GH and IGF-1 were not found to be significant parameters for increased FGF-21 levels in acromegaly group.
Discussion
We have showed for the first time that serum FGF-21 levels were found to be higher in acromegaly subjects when compared with control group. Increased FGF-21 levels were significantly and independently associated with the presence of acromegaly.
Chen et al. [15] demonstrated that GH administration caused increase in hepatic expression of FGF-21 and also caused an increase in circulating FGF-21 concentrations in mice. They suggested that increased FGF-21 levels acted as a negative regulator to end the GH effect on lipolysis in which there was an increase of free fatty acid levels in adipose tissues through activation of lipolysis [16]. When they gave niacin as a lipolysis inhibitor together with GH, the same inducing effect of GH on FGF-21 was not observed [15]. It was stated that FGF-21 increase after GH injection was through the activation of PPAR-alpha by free fatty acid [17, 18]. Chen et al. [15] also verified those findings with the study in which they treated the hepatocyte cells with PPAR-alpha antagonist (GW6471) and they showed that the same effect of GH on FGF-21 mRNA expression was not seen. It was suggested that PPAR-alpha was important in the hepatic induction of FGF-21 by GH-mediated lipolysis. When they investigated GH-induced lipolysis in FGF-21 knock-out mice, free fatty acid was higher in concentration in them compared to wild type mice [15]. So, it could be said that both GH and FGF-21 have an effect on lipolysis. The increase of FGF-21 levels in acromegaly could be as a compensatory mechanism to balance the effect of excess GH on metabolism leading to negative feedback loop system between GH and FGF-21.
Brooks et al. [19] measured FGF-21 levels together with FGF-21 mRNA, FGFR and BKL in mice to find out whether GH excess was also a FGF-21 resistance state. Brooks et al. [25] showed that male mice with increased GH had increased circulating FGF-21. But, FGFR mRNA did not increase as expected in resistance state. They suggested that FGF-21 could increase as regards to increase in liver size. Berryman et al. [20] showed that 6 month mice with excess GH had increased liver weight, but Brooks et al. [19] didn’t study the liver size in their study. We also couldn’t speculate anything related to the association of liver size with increased FGF-21 levels. The other explanation stated by Brooks et al. [19] was that FGF-21 may have not been eliminated only by the kidney, because it was known that FGF-21 levels are increased in acute and chronic kidney diseases [21]. In our study, no difference was found between acromegaly and control groups as regards to glomerular filtration rate. It was an interesting find from the study of Brooks et al. [19] that FGF-21 levels did not decrease in GH deficient mice. When there is a change in GH dynamics, metabolic factors like obesity or insulin resistance might not be unique factors affecting FGF-21 levels. Although acromegaly is associated with diabetes mellitus and insulin resistance we found that increased FGF-21 levels were only associated with the presence of acromegaly independent from those metabolic disorders. In the light of those studies, we could speculate that FGF-21 levels were increased as a compensatory response to the effects of excess GH.
After it was shown that GH increased FGF-21mRNA levels in animals, Lundberg et al. [22] observed that the FGF-21 levels also increased in healthy humans with GH treatment. It was figured out FGF-21 levels increased up to 3.7-fold after 3 weeks of GH injection in healthy males [22]. In our study, there was no significant difference in FGF-21 levels as regards to gender (data not shown).
Recently, Halupczok–Żyła et al. [23] found that there was no significant difference in FGF-21 levels between acromegaly and control groups. When they analyzed FGF-21 levels in acromegaly group with regards to disease activity, the results did not show statistical significance. They observed positive correlation between FGF-21 and GH, IGF-1 in acromegaly group. As opposed to this, Inagaki et al. [24] showed that chronic exposure of mice to FGF-21 inhibited janus kinase 2 (JAK2)-signal transducer and activator of transcription 5 (STAT5) signaling leading to low IGF-1 levels. GH resistance and low IGF-1 levels seen in anorexia nervosa may be explained with this mechanism in which FGF-21 was increased as a fasting induced hormone [25]. We also observed a negative relationship between FGF-21 levels and IGF-1 levels in acromegaly group, but this association didn’t show any statistical significance. FGF-21 levels did not change significantly between AA and CA groups. The small number of patients in AA and CA groups might not be enough to show the significant difference.
It is the fact that FGF-21 levels are increased in insulin resistance, diabetes, dyslipidemia [4, 6]. In our study, FGF-21 was associated with FPG and HbA1c in whole group correlation analysis. On the other hand, FGF-21 levels were only associated with the presence of acromegaly independent from metabolic parameters according to the multiple linear regression analysis. In acromegaly, while body weight increases, lean body mass also increases in amount, but there is still insulin resistance [26]. In our acromegaly group, body weight and lean body mass were increased compared to control group, but visceral fat was higher in acromegaly group. It could be difficult to predict the levels of FGF-21 in acromegaly patients who have altered body composition together with insulin resistance. However, FGF-21 was found to be significantly higher in acromegaly independent from BMI and visceral fat in our study.
There are several limitations to our study. It was not possible to state casuality as the study had a cross-sectional design. Having a relatively small study sample was another limitation. So, there is a need for further studies with larger groups to demonstrate the precise relationship between GH and FGF-21.
Conclusion
In conclusion, FGF-21 levels were increased significantly in acromegaly group. Increased FGF-21 levels were significantly and independently associated with the presence of acromegaly. FGF-21 levels may be increased as a compensatory mechanism in acromegaly. Acromegaly may also be a FGF-21 resistance state independent from metabolic disorders like insulin resistance, glucose intolerance, obesity, hypertension and dyslipidemia. But, it is obvious that a much more complicated interaction exists between GH and FGF-21. There is a need for further studies to enlighten GH/FGF-21 interaction.
References
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK (2011) American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update: executive summary. Endocr Pract 17(4):636–646
Katznelson L, Laws ER, Melmed SJ, Molitch ME, Murad MH, Utz A, Wass JA (2014) Acromegaly: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 99(11):3933–3951
Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8(3):235–253. https://doi.org/10.1038/nrd2792
Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ (2007) The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148(2):774–781 (Epub 2006 Oct 26)
Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A (2008) Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57(5):1246–1253
Kim WJ, Kim SS, Lee HC, Song SH, Bae MJ, Yi YS, Jeon YK, Kim BH, Kim YK, Kim IJ (2015) Association between serum fibroblast growth factor 21 and coronary artery disease in patients with type 2 diabetes. J Korean Med Ssci 30(5):586–590
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM (2006) Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281(23):15694–15700. https://doi.org/10.1074/jbc.M601252200
Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M (2007) Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282(37):26687–26695
Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E (2007) Hepatic fibroblast growth factor 21 is regulated by PPARa and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5(6):426–437. https://doi.org/10.1016/j.cmet.2007.05.002
Wang H, Qiang L, Farmer SR (2008) Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 28(1):188–200. https://doi.org/10.1128/MCB.00992-07
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177
Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W (2013) Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol 12:124. https://doi.org/10.1186/1475-2840-12-124.ORI
American Diabetes Association (2015) Diagnosis and classification of diabetes mellitus. Diabetes Care 38:S8–S16
Akyildiz ZI, Polat S, Yurekli BS, Kocabas GU, Tuluce K, Tuluce SY, Kocabas U, Bozkaya G, Yuksel A, Nazli C (2015) Epicardial fat, body mass index, and triglyceride are independent contributors of serum fibroblast growth factor 21 level in obese premenopausal women. J Endocrinol Invest 38(3):361–366. https://doi.org/10.1007/s40618-014-0185-3 (Epub 2014 Oct 14)
Chen W, Hoo RL, Konishi M, Itoh N, Lee PC, Ye HY, Lam KS, Xu A (2011) Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem 286(40):34559–34566. https://doi.org/10.1074/jbc.M111.285965 (Epub 2011 Aug 17)
Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC (2003) Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol 149(6):511–519
Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, Meinus S, Reinecke F, Möhlig M, Weickert MO, Clemenz M, Pfeiffer AF, Kintscher U, Spuler S, Spranger J (2009) Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 58(7):1532–1538. https://doi.org/10.2337/db08-1775 (Epub 2009 Apr 28)
Mai K, Bobbert T, Groth C, Assmann A, Meinus S, Kraatz J, Andres J, Arafat AM, Pfeiffer AF, Möhlig M, Spranger J (2010) Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab 299(1):E126–E130. https://doi.org/10.1152/ajpendo.00020.2010 (Epub 2010 Apr 27)
Brooks NE, Hjortebjerg R, Henry BE, List EO, Kopchick JJ, Berryman DE (2016) Fibroblast growth factor 21, fibroblast growth factor receptor 1, and β-Klotho expression in bovine growth hormone transgenic and growth hormone receptor knockout mice. Growth Horm IGF Res 30–31:22–30. https://doi.org/10.1016/j.ghir.2016.08.003 (Epub 2016 Aug 24)
Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ (2004) Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res 14(4):309–318
Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, Wang X, Lin S, Feng W, Li X (2011) Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 6(4):e18398 (6)
Lundberg J, Höybye C, Krusenstjerna-Hafstrøm T, Bina HA, Kharitonenkov A, Angelin B, Rudling M (2013) Influence of growth hormone on circulating fibroblast growth factor 21 levels in humans. J Intern Med 274(3):227–232. https://doi.org/10.1111/joim.12112 (Epub 2013 Jul 24)
Halupczok-Żyła J, Jawiarczyk-Przybyłowska A, Skrzypski M, Bolanowski M, Strowski MZ (2017) Fibroblast growth factor 21 in patients with acromegaly. Exp Clin Endocrinol Diabetes 125(10):649–654. https://doi.org/10.1055/s-0043-115647 (Epub 2017 Sep 20)
Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA (2008) Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8(1):77–83
Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski (2010) Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab 95(1):369–374. https://doi.org/10.1210/jc.2009-1730 (Epub 2009 Nov 19)
Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ (2011) Growth hormone and adipose tissue: beyond the adipocyte. Growth Hormon IGF Res 21(3):113–123
Funding
This research did not receive any specific grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Ethical approval
The study was approved by Ege University Local Ethic Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Yurekli, B.S., Kutbay, N.O., Aksit, M. et al. Acromegaly is associated with high fibroblast growth factor-21 levels. J Endocrinol Invest 42, 53–60 (2019). https://doi.org/10.1007/s40618-018-0885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0885-1