Abstract
Introduction
Thyrotropin stimulating hormone (TSH) suppression in patients with differentiated thyroid cancer (DTC) aims to decrease the growth and proliferation of thyroid cancer cells. However, the effect of TSH-suppressive therapy on bone microarchitecture remains undefined.
Methods
Cross-sectional study including 43 women with DTC undergoing TSH-suppressive therapy (sTSH) compared to 20 women also on levothyroxine (LT4) therapy but with TSH in the low-normal range (nTSH) since the thyroid surgery. Bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry (DXA), and trabecular bone score (TBS) was evaluated using the TBS iNsigth software. Fracture risk assessed by FRAX, with and without TBS, was calculated. The relationship between suppressive therapy-related parameters and bone parameters was investigated.
Results
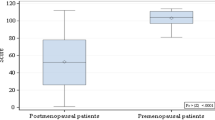
The TBS mean values were not significantly different in the sTSH and nTSH groups (1.273 ± 0.12 vs 1.307 ± 0.14, p = 0.7197). In both groups, postmenopausal women had degraded microarchitecture (TBS 1.216 ± 0.11 vs 1.213 ± 0.09, p = 0.9333), while premenopausal women had normal microarchitecture (1.328 ± 0.11 vs 1.401 ± 0.12, p = 0.195). The percentage of all postmenopausal women with degraded TBS was 54.7%, while the percentage of osteoporosis diagnoses was 16.1%. The TBS-adjusted FRAX-probability of fracture was similar in sTSH and nTSH groups. Body mass index (BMI) and menopausal status were the only variables associated with TBS and BMD.
Conclusion
Trabecular microarchitecture assessed by TBS was similar between women on long-term suppressive therapy in DTC and those on LT4 replacement therapy aiming at a TSH level within the low-normal reference range. Low TBS values were observed in postmenopausal women of both groups, suggesting that not only suppressed TSH levels but also a low-normal TSH is associated with deteriorated bone microarchitecture in postmenopausal women following total thyroidectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy worldwide, with an incidence that has increased by 300% over the last 3 decades [1]. After initial surgical treatment, suppressive therapy with levothyroxine (LT4) is often recommended, since the chronic reduction in levels of the thyrotropin-stimulating hormone (TSH) can inhibit the growth of residual neoplastic tissue and lead to regression of possible lymph node metastases [2]. However, there is a growing concern about the negative effects of prolonged maintenance of TSH suppression, especially the increased risk of osteoporosis and fractures [2, 3]. Thyroid hormones play a critical role in skeletal development, on the acquisition of peak bone mass and in maintenance of bone structure and strength. Thyrotropin, in turn, has a direct negative effect on bone remodeling, inhibiting bone resorption [4]. Consequently, several studies have shown a reduction in bone mineral density (BMD) and an increased risk of fractures among individuals with clinical and subclinical endogenous hyperthyroidism [2, 5]. In addition, the skeletal effects of subclinical hyperthyroidism associated with suppressive therapy of DTC are still controversial [6,7,8,9,10,11,12,13,14]. Likewise, the skeletal effects of different targets of TSH in the treatment of hypothyroidism are debatable, especially in postmenopausal women and the elderly [15,16,17].
Osteoporosis is characterized by low bone mass and the deterioration of the bone tissue microarchitecture [18]. Dual-energy X-ray absorptiometry (DXA) is the most commonly used test for the diagnosis of osteopenia and osteoporosis [19]. Although low BMD by DXA is a strong predictor of fracture risk, many fragility fractures occur in individuals with a BMD in the osteopenic or even normal range [20]. Moreover, the standard DXA test does not discriminate trabecular from cortical bone compartments and does not assess bone microarchitecture, which limits its accuracy in determining bone strength [21]. The trabecular bone score (TBS) is a texture index that evaluates pixel gray-level variations in the lumbar spine DXA image. It provides an indirect measure of bone architecture and is highly correlated with direct assessments of the trabecular microarchitecture [21]. TBS can predict incident major osteoporotic fractures and vertebral and hip fractures in women and men independently of lumbar spine BMD measurements and clinical risk factors [21, 22]. A high TBS value corresponds to a stronger bone with well-structured trabecular architecture and, therefore, with resistance to fracture; conversely, a low TBS value corresponds to a weaker bone, with degradation in the trabecular architecture and, therefore, increased susceptibility to bone fractures [23].
Studies that assess bone quality through TBS in DTC patients are scarce [24,25,26,27]. We aimed to compare the TBS of pre and postmenopausal women with a diagnosis of DTC, dividing them into two groups: one with long-term TSH-suppressive therapy and the other with chronic LT4 therapy and normal-low TSH levels since thyroid surgery. Clinical and laboratory parameters associated with suppressive therapy and their correlations with TBS and BMD were investigated.
Materials and methods
Subjects
In this cross-sectional study, all women diagnosed with DTC from 1999 to 2016 had their medical records reviewed. Subjects were eligible for inclusion if they had a total thyroidectomy due to DTC regardless of radioiodine therapy, age between 18 and 80 years and body mass index (BMI) between 15 and 37 kg/m2, since the TBS analysis is not recommended in patients out of this BMI range [28]. Exclusion criteria included bone metabolic diseases such as definitive hypoparathyroidism and hyperparathyroidism, renal failure, significant liver disease, rheumatic and neurological diseases, pregnancy and lactation, bone metastases, other malignancies and current or previous use of drugs that interfere with bone metabolism. From the 84 women evaluated, 21 were excluded due to TSH out of range (n = 7), BMI ≥ 37 kg/m2 (n = 3), and impossibility to perform the DXA test (n = 11). The final sample size available for analysis comprised 63 women.
All patients included in this study were treated with LT4 according to the initial risk stratification and response to therapy, as defined in the evidence-based guidelines [29]. Patients classified as intermediate risk or high risk, or those with persistent disease, received a dose of LT4 sufficient to suppress their TSH level below the normal TSH range (0.1–0.5 µUI/ml for mild suppression and < 0.1 µUI/ml for complete suppression). Those patients classified as low risk, who were not submitted to radioiodine therapy, and had negative tumor markers since the postoperative period received a dose of LT4 to maintain TSH levels in the low-normal range (0.5–2.0 µUI/ml) [3, 29].
Therefore, we defined two groups of women based on their mean TSH serum levels measured over an approximate period of 45 months. The suppressive therapy group (sTSH) was comprised of women with a mean TSH lower than 0.3 µUI/ml and free thyroxine (FT4) within the reference range. The nonsuppressive group (nTSH) included women with mean TSH equal to or greater than 0.3 µUI/ml, and FT4 within the reference range.
Trabecular bone score and BMD were analyzed using DXA, which was performed between January and December of 2017 in a single center. This study was approved by the ethics and research committees of Federal University of Minas Gerais (UFMG) and Instituto Mário Penna and all subjects signed an informed consent form.
Clinical and anthropometric parameters and biochemical analysis
Clinical characteristics including the average dose of LT4 per kilogram (kg) of weight, age at menopause, smoking, current calcium and vitamin D supplementation, diagnosis of diabetes mellitus, previous osteoporotic fractures and family history of hip fracture were recorded for all participants. Postmenopausal status was defined as the cessation of menstruation for more than 1 year. Weight and height were measured on a calibrated scale, with light clothing, and without shoes. Body mass index was calculated by determining the ratio between weight and height squared (kg/m2). All TSH measurements available in the medical records since the thyroidectomy, performed at a 6-month interval, were analyzed, except for those taken during thyroid hormone withdrawal or following the use of recombinant human thyroid-stimulating hormone (rhTSH). FT4 levels have been described in multiples of the upper limit of the reference range since different immunoassays have been used over time. After an overnight fast, blood sampling were collected and the dosages of total calcium corrected for albumin, 25-hydroxyvitamin D (25OHD) and parathyroid hormone (PTH) were obtained within 6 months of the DXA test. The majority of TSH and FT4 measurements were performed by chemiluminescence assays (TSH, Immunotech S.A.S. to Beckman Coulter Company, Marseille, France; FT4, Beckman Coulter, Inc., Brea, CA, USA). The thyrotropin assay had an analytical sensitivity of 0.001 μUI/mL and a reference range of 0.38–5.33 μUI/mL, while FT4 had an analytical sensitivity of 0.20 ng/dl and a reference range of 0.89–1.76 ng/dl. Total calcium was measured by a colorimetric method (Beckman Coulter, Inc., Brea, CA, USA) with a reference range of 8.6–10.6 mg/dl and the values were corrected for albumin concentration. Serum intact PTH was analyzed using a chemiluminescence assay (Beckman Coulter, Inc., Brea, CA, USA), with reference range of 4.1–58.2 ng/dl. 25OHD was analyzed with a chemiluminescence assay (Abbott Ireland Diagnostic Division, Longford, Ireland) with an analytical sensitivity of 2.4 ng/dl.
Bone mineral density (BMD)
Areal bone mineral density of the femoral neck (FN-BMD), total hip (TH-BMD) and lumbar spine (LS-BMD) was evaluated by DXA (Discovery W Hologic Inc., Waltham, MA, USA, software version 3.3) and expressed in grams/cm2, T-score and Z-score. T-score was calculated using the National Health and Nutrition Examination Survey (NHANES) young women as reference and osteoporosis, osteopenia and normal BMD were defined according to the World Health Organization’s criteria [30]. In premenopausal women, a Z-score equal to or less than – 2 standard deviation (SD) was defined as low bone mass, while a Z-score above – 2 SD was defined as normal. According to the Official Positions of the International Society for Clinical Densitometry (ISCD), it were excluded from the LS-BMD analyses vertebrae that had more than one SD difference in BMD from the adjacent vertebra and/or those vertebrae clearly abnormal [28]. The variation coefficients for BMD of the LS, TH and FN in our center are 1.81%, 0.96% and 1.04%, respectively.
Trabecular bone score (TBS)
The TBS was analyzed in the same region of interest (ROI) of the LS-BMD, using the TBS iNsigth software (TBS iNsight, Medimaps, Switzerland, version 2.1.2.0). TBS, expressed in values without units of measurement, was evaluated as previously described [28]. TBS was used to classify the subjects’ risk of fracture as suggested by McCloskey et al. [22]. Accordingly, a TBS above 1.310 defined a low fracture risk (i.e., normal microarchitecture pattern), a TBS between 1.230 and 1.310 characterized an intermediate fracture risk (i.e., partially degraded pattern), and a TBS below 1.230 defined a high fracture risk (i.e., degraded pattern) [22, 31]. The variation coefficient for TBS in our center was 1.51%.
FRAX
The 10-year probability of hip fracture and major osteoporotic fractures (hip, clinical spine, distal forearm, and proximal humerus) was assessed using the FRAX (with BMD) model for Brazil (http://www.abrasso.org.br) [32]. FRAX and TBS-adjusted FRAX were calculated for all women with DTC aged more than 40 years.
Statistical analysis
Categorical variables were reported as numbers and percentages and assessed using Fisher’s exact test. Quantitative variables, expressed as the mean and SD or medians and interquartile ranges (p25—p75), were compared with Student’s t test or Mann–Whitney tests, respectively. The variables were tested for normality using the Shapiro–Wilk test. The correlation between clinical and laboratory parameters of suppressive therapy with TBS and BMD was tested using Spearman’s correlation. A kappa coefficient was used to test the reliability between TBS and BMD. Multivariate regression analysis was performed to assess the determinants independently associated with TBS and BMD. Variables were entered into the multivariate model when the p value was < 0.20 in univariate analysis. A p value < 0.05 was regarded as statistically significant.
For the sample size calculation, the comparison of means of TBS between the groups with and without TSH suppression was used, considering an alpha error of 0.05, a statistical power of the bilateral test of 80% and a projected ratio of 2.5 of the sample sizes of the two groups. The required calculated sample was 17 patients for the control group and 42 patients for the case group [33].
Results
Subject characteristics
Sixty-three women with DTC, 32 of whom were premenopausal (aged 21–55 years) and 31 postmenopausal (45–71 years), were divided in 2 groups (43 in the sTSH and 20 in the nTSH) and included in this study. Their clinical, laboratory and densitometric characteristics are shown in Table 1. The groups were similar in age, BMI, age at menopause, previous osteoporotic fracture, family history of hip fracture, calcium and vitamin D supplementation, years since menopause, frequency of diabetes mellitus and smoking. The mean numbers of TSH measurements available in the sTSH and nTSH groups were 8.3 and 6.0, respectively. Lower serum TSH levels, higher multiple values of FT4 and higher doses of LT4 were present in the sTSH group, compared to nTSH. Serum levels of calcium, 25OHD and PTH did not differ between the groups. The mean follow-up period after total thyroidectomy was similar between the groups and the median duration of TSH-suppressive therapy was 4 years in the sTSH group.
TBS and BMD
The TBS and BMD (mean ± SD) of both groups are included in Table 1. The mean TBS was not significantly different in the sTSH and nTSH groups (1.273 ± 0.12 vs 1.307 ± 0.14, p = 0.7197). Similarly, using the TBS thresholds suggested by McCloskey et al. [22], the frequency of women with high, intermediate and low risk of fracture was not different between the sTSH and nTSH groups. In both groups, with and without suppression, premenopausal women had normal microarchitecture (1.328 ± 0.11 vs 1.401 ± 0.12, p = 0.195), while postmenopausal women had degraded microarchitecture (TBS 1.216 ± 0.11 vs 1.213 ± 0.09, p = 0.9333) (Table 2).
There were no significant differences in LS-BMD, FN-BMD, or TH-BMD, as well as in the T-score (postmenopausal) and Z-score (premenopausal) at all sites, between the sTSH and nTSH groups (Table 1). The rate of diagnosis of osteoporosis, osteopenia, or normal bone status among postmenopausal women was similar between the groups (p = 0.1554).
The TBS of all postmenopausal women (n = 31), from both groups, showed degraded microarchitecture in 17 (54.8%), partially degraded in another 6 (19.3%) and normal values in 8 (25.8%) patients. In contrast, osteoporosis was diagnosed in only 5 (16.1%), osteopenia in 13 (41.9%) and normal BMD in 13 (41.9%) postmenopausal women (Fig. 1). Among the 13 women classified in the osteopenic range, the TBS was classified as a high risk of fracture in 8 of them (61.5%) and as an intermediate risk of fracture in another 2 patients (6.45%). The agreement between the TBS and BMD classifications was weak but statistically significant (kappa = 0.253, p = 0.0261).
FRAX and TBS-FRAX
The 10-year probability of hip fracture and major osteoporotic fracture was assessed using FRAX with and without adjustment for TBS in all women aged more than 40 years. From the 47 women evaluated, 2 were excluded due to incomplete data. The risk of hip fracture and major osteoporotic fracture, with or without TBS, were similar between the sTSH and nTSH groups (Table 3). Overall, the probability of major osteoporotic fracture was greater when assessed by the TBS-adjusted FRAX than using FRAX without TBS.
Correlations
There were no significant correlations among TSH, FT4, and the duration of suppressive therapy with TBS and BMD in the premenopausal and postmenopausal women. The dose of LT4/weight did not correlate with TBS but was weakly correlated (r = 0.2455, p = 0.0480) with TH-BMD. However, this correlation disappeared when the model was adjusted for age and BMI (r = 0.0224, p = 0.8630).
Multiple linear regression analyses were performed to evaluate the independent association of clinical parameters with TBS and BMD. BMI and menopause status were the most significant predictors of TBS (β = – 0.0539, p = 0.0016 and β = – 0.0902, p = 0.0059), LS-BMD (β = 0.0420, p = 0.0411 and β = – 0.1347, p = 0.0020), FN-BMD (β = 0.0563, p = 0.0022 and β = – 0.0914, p = 0.0130) and TH-BMD (β = 0.0489, p = 0.0059 and β = – 0.0866, p = 0.0159), respectively. No significant associations of TBS or BMD with the parameters of TSH-suppressive therapy were found (Table 4). LS-BMD, FN-BMD, and TH-BMD were associated with the dose of LT4/weight in the univariate analysis, but not in the multivariate analysis.
Discussion
Total thyroidectomy followed by long-term TSH-suppressive therapy is the standard treatment of patients with DTC. However, there is growing concern about the negative effects of prolonged maintenance of a suppressed TSH on bone quality and fracture risk. The results of this study showed that women with DTC undergoing TSH-suppressive therapy do not present a significant deterioration of TBS relative to women on LT4 replacement therapy and low-normal TSH levels. This study was adequately powered to detect differences in TBS between the groups, and these negative findings support previous results of a longitudinal study showing preservation of TBS in women with long-term TSH-suppressive therapy compared to healthy controls [26]. Nevertheless, it is important to highlight that both groups, after menopause, showed deterioration of the trabecular bone assessed by TBS.
In this study, the average duration of suppressive therapy was 4 years. No correlation was found between TBS and the duration of TSH suppression, consistent with earlier reports [25, 27]. On the other hand, in another retrospective study, TBS was lower, despite the unchanged BMD, in women with a TSH suppression duration greater than 5 years compared to those whose duration was less than 3 years [24]. Our results have also shown that other parameters of TSH-suppressive therapy such as TSH and FT4 levels were not correlated with TBS, in agreement with other studies [24, 25, 27]. In a previous cross-sectional study with 648 healthy euthyroid postmenopausal women, the upper levels of FT4 within the normal reference range, but not TSH, was negatively correlated with TBS after adjusting for age, BMI, and BMD [34].
Menopausal status and BMI were the only variables correlated with both BMD and TBS. An average dose of LT4 per kilogram of weight was positively associated with LS-BMD (p = 0.0043), FN-BMD (p = 0.0022), and TH-BMD (p = 0.0013) in the univariate analysis, but not in the multivariate analysis. This unexpected association may have been influenced by the BMI, since both the LT4 dose and the BMD are positively associated with body weight and BMI. In fact, the positive association between the LT4 dose and BMD disappeared in the multivariate analysis, whereas the association between BMD and BMI remained significant. Obese individuals have higher areal and volumetric BMD measurements and better parameters of bone microarchitecture [35]. On the other hand, BMI was negatively correlated with TBS. Previous studies using TBS software version 2.x have also found a negative correlation between TBS and waist circumference, visceral fat mass and BMI on Hologic scanners [36,37,38]. This can be explained by the excessive soft tissue in the abdomen, overlying the region of interest, reducing the TBS [21].
The mean BMD, T-score, and Z-score at all sites were similar between the groups, even when considering the subgroup of postmenopausal women. This result is in line with previous studies that have shown a preserved BMD in postmenopausal women on TSH-suppressive therapy [24,25,26]. However, in a recently published meta-analysis of 11 controlled cross-sectional studies, TSH suppression therapy was associated with a lower BMD of the hip and lumbar spine in postmenopausal women, but not in premenopausal women [13]. In another study, osteoporosis following thyroidectomy was diagnosed three times more often in TSH-suppressive therapy women compared to those with no suppressed TSH [10]. In the only randomized controlled study of LT4 treatment aiming at different TSH targets, suppression of TSH after thyroidectomy caused a significant deterioration of the LS-BMD within 1 year after surgery, particularly in women aged ≥ 50 years [9]. Likewise, in a prospective study, TSH-suppressive therapy caused bone loss predominantly in postmenopausal women and exclusively in the early post-thyroidectomy period [39]. The divergence of results concerning skeletal changes during TSH-suppressive therapy is probably due to studies with small samples, retrospective designs and a short time of exposure to low TSH levels, insufficient for bone changes to occur. Furthermore, the years since menopause differ across the studies, and in many of them, these data are not reported, which may explain the discrepant results in the bone evaluation of postmenopausal women [40].
Consistent with previous studies, we identified a weak agreement between the TBS and BMD T-score classifications [24, 28]. In the entire study population, the mean TBS was normal in premenopausal women but markedly reduced in postmenopausal women. The percentage of postmenopausal women with degraded TBS was 54.7%, while the percentage of osteoporosis diagnoses was only 16.1%. Among women diagnosed with osteopenia (41.9%), more than half showed degraded TBS, a known risk factor for fragility fractures in postmenopausal women [22, 31, 41].
The effect of TSH-suppressive therapy on fracture risk remains uncertain, with previous studies demonstrating mixed results [14]. To our knowledge, only one study evaluated risk fracture assessed by FRAX in 78 DTC women (78% postmenopausal) and TSH-suppressive therapy. They found no difference in FRAX-probability of fracture between DTC women and a cohort of 120 euthyroid women, matched for age, menopausal status, and BMI [42]. Similarly, we found no significant difference in the FRAX-probability of hip and major fracture risk between the sTSH and nTSH groups, even after adjusting FRAX for TBS. Of note, in the entire study population, the risk of major osteoporotic fracture as assessed by FRAX was greater when adjusted by TBS than using FRAX alone.
Serum TSH in healthy populations is higher in women and it increases with age, particularly after the age of 70 years. In 2013, Vadiveloo et al. [43] measured the TSH distribution among 153,127 people of different age groups. The median TSH for people with negative thyroid antibodies was 2.0 µUI/ml and the 97.5th percentile was 6.14 µUI/ml, which increased by 1.96 µUI/ml across the age range [43]. Other studies observed similar results, including a Brazilian study [44]. In contrast, in our study, the nTSH group had a median TSH of 0.800 µUI/ml and a 75th percentile of 1.208 µUI/ml, notably lower levels than those usually described in healthy postmenopausal women in epidemiological studies. This observation could explain our finding of a high frequency of low TBS among postmenopausal women regardless of the study group. In fact, in postmenopausal women with DTC, LT4 replacement aiming at TSH levels in the low-normal range may cause a negative effect on the skeleton similar to that caused by TSH suppression. Further studies are needed to assess whether LT4 replacement in postmenopausal women and DTC, aiming at TSH levels between 0.5 and 2.0 µUI/ml, could increase the fracture risk.
Similarly, large cross-sectional studies with healthy women have demonstrated that low-normal TSH was associated with a low BMD and an increased risk of osteoporosis, compared to those with high-normal TSH [45,46,47]. Leader et al. [48], in a population-based historical prospective cohort study, observed that TSH in the low-normal range (0.35–1.6 mUI/l) was associated with an increased risk of hip fractures in healthy euthyroid women > 65 years, compared with intermediate (1.7–2.9 mUI/l) and high-normal TSH levels (3.0–4.2 mUI/l) [48]. In another study, higher TSH levels across the normal range were protective and reduced the fracture risk [49]. Moreover, previous studies have shown that subclinical hypothyroidism is associated with a higher BMD-FN [50] and a lower risk of osteoporosis in postmenopausal women [46, 51], without increasing the risk of bone fractures [50]. Physiologically, postmenopausal women with DTC are subject to the combined effect of estrogen deficiency and low TSH levels, leading to increased bone resorption. Therefore, our results and previous studies suggest that TSH may play a role in bone preservation in postmenopausal women, and maintaining chronically low-normal TSH levels in this group with DTC may not be a good choice.
Only one study has investigated the effect of subclinical hypothyroidism treatment on TBS [17]. One hundred and seventeen individuals > 65 years with persistent subclinical hypothyroidism and no history of previous thyroidectomy or thyroid disease were randomized to treatment with placebo or LT4. TSH was 6.4 ± 2.0 µUI/ml before randomization, and it evolved to 3.2 ± 1.5 µUI/ml at 1 year in the treatment arm. There was no significant difference between baseline TBS values and those measured after 1 year of follow-up in both groups (– 1.3%: 95% CI – 3.1 to 0.6, p = 0.19) [17]. Although this study demonstrates that treatment with LT4 for 1 year was safe for the bone health of subclinical hypothyroidism in elderly, it cannot be excluded that lower TSH levels may have deleterious effects on the skeleton.
The current study has some limitations. The cross-sectional design does not allow for establishing a cause–effect relationship between TSH-suppressive therapy and its skeletal effects on the microarchitecture and bone mineral density. The duration of TSH-suppressive therapy was, perhaps, insufficient to cause alterations in bone parameters. Another limitation is the lack of radiological images of the spine to assess morphometric vertebral fractures during TSH suppression therapy. In addition, we have a relatively small patient population and the absence of a healthy control group matched for age, BMI, and age at menopause.
Our study has several strengths. To our knowledge, this is the first study to compare TBS between two groups of women with DTC, one with chronic TSH-suppressive therapy and the other on LT4 replacement to keep TSH in the low-normal range since the thyroid surgery, unlike other studies that compared suppressive therapy with a healthy control group. The average TSH of the nTSH group was maintained in the range recommended by the main guidelines for thyroid cancer. However, these values are notably lower than those described in epidemiological studies of healthy postmenopausal women, which may explain the degraded TBS in more than half of the postmenopausal women in the entire group. Despite the small sample, this study was adequately powered to detect differences in TBS between the groups.
Conclusions
Our results showed that trabecular microarchitecture assessed by TBS is similar between women on long-term suppressive therapy in DTC and those on LT4 replacement therapy aiming at a TSH level within the low-normal reference range. Of note, we found that the TBS was degraded in more than 50% of the postmenopausal women with thyroid cancer on LT4 therapy, an abnormality not appreciated by the BMD analyses. Therefore, not only suppressed TSH levels but also a low-normal TSH is associated with deteriorated bone microarchitecture in postmenopausal women. Moreover, the sole analysis of BMD by DXA may be insufficient to predict fracture risk in this population. In this group, we should consider possible skeletal damage and avoid overtreatment when possible. This negative effect was not observed in the group of premenopausal women, as they had a normal TBS. Whether the TBS should be incorporated into the bone evaluation routine of postmenopausal women on LT4 replacement therapy is a matter of debate. Additional studies involving a larger number of participants and evaluating fracture risk are needed to define the skeletal effects of TSH-suppressive therapy and different target TSH ranges in individuals with differentiated thyroid cancer.
References
Seib CD, Sosa JA (2019) Evolving understanding of the epidemiology of thyroid cancer. EndocrinolMetabClin North Am 48(1):23–35. https://doi.org/10.1016/j.ecl.2018.10.002
Biondi B, Cooper DS (2019) Thyroid hormone suppression therapy. EndocrinolMetabClin North Am 48(1):227–237. https://doi.org/10.1016/j.ecl.2018.10.008
Grani G, Ramundo V, Verrienti A, Sponziello M, Durante C (2019) Thyroid hormone therapy in differentiated thyroid cancer. Endocrine 66:43–50. https://doi.org/10.1007/s12020-019-02051-3
Bassett JH, Williams GR (2016) Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev 37:135–187. https://doi.org/10.1210/er.2015-1106
Segna D, Bauer DC, Feller M, Schneider C, Fink HA, Aubert CE et al (2018) Association between subclinical thyroid dysfunction and change in bone mineral density in prospective cohorts. J Intern Med 283(1):56–72. https://doi.org/10.1111/joim.12688
Quan ML, Pasieka JL, Rorstad O (2002) Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature. J SurgOncol 79:62–69. https://doi.org/10.1002/jso.10043
Heemstra KA, Hamdy NA, Romijn JA, Smit JW (2006) The effects of thyrotropin-suppressive therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Thyroid 16:583–591. https://doi.org/10.1089/thy.2006.16.583
Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY (1996) Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J ClinEndocrinolMetab 81(12):4278–4289. https://doi.org/10.1210/jcem.81.12.8954028
Sugitani I, Fujimoto Y (2011) Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery 150(6):1250–1257. https://doi.org/10.1016/j.surg.2011.09.013
Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ et al (2015) Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid 25(3):300–307. https://doi.org/10.1089/thy.2014.0287
Mazziotti G, Formenti AM, Frara S, Olivetti R, Banfi G, Memo M et al (2018) High prevalence of radiological vertebral fractures in women on thyroid-stimulating hormone-suppressive therapy for thyroid carcinoma. J ClinEndocrinolMetab 103(3):956–964. https://doi.org/10.1210/jc.2017-01986
Papaleontiou M, Banerjee M, Reyes-Gastelum D, Hawley ST, Haymart MR (2019) Risk of osteoporosis and fractures in patients with thyroid cancer: a case-control study in U.S. Veterans Oncologist 24:1166–1173. https://doi.org/10.1634/theoncologist.2019-0234
Yoon BH, Lee Y, Oh HJ, Kim SH, Lee YK (2019) Influence of thyroid-stimulating hormone suppression therapy on bone mineral density in patients with differentiated thyroid cancer: a meta-analysis. J Bone Metab 26: 51–60. https://doi.org/10.11005/jbm.2019.26.1.51
Brancatella A, Marcocci C (2020) TSH suppressive therapy and bone. Endocr Connect 9:R158–R172. https://doi.org/10.1530/EC-20-0167
Vestergaard P, Rejnmark L, Mosekilde L (2005) Influence of hyper- and hypothyroidism, and the effects of treatment with antithyroid drugs and levothyroxine on fracture risk. Calcif Tissue Int 77(3):139–144. https://doi.org/10.1007/s00223-005-0068-x
Thayakaran R, Adderley NJ, Sainsbury C, Torlinska B, Boelaert K, Šumilo D et al (2019) Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ 366:l4892. https://doi.org/10.1136/bmj.l4892
Gonzalez Rodriguez E, Stuber M, Del Giovane C, Feller M, Collet TH, Löwe AL et al (2020) Skeletal effects of levothyroxine for subclinical hypothyroidism in older adults: a trust randomized trial nested study. J ClinEndocrinolMetabol. https://doi.org/10.1210/clinem/dgz058
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376. https://doi.org/10.1016/S0140-6736(18)32112-3
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795. https://doi.org/10.1001/jama.285.6.785
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE et al (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164:1108–1112. https://doi.org/10.1001/archinte.164.10.1108
Silva BC, Leslie WD (2017) Trabecular bone score: a new DXA-derived measurement for fracture risk assessment. EndocrinolMetabClin North Am 46:153–180. https://doi.org/10.1016/j.ecl.2016.09.005
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31:940–948. https://doi.org/10.1002/jbmr.2734
Martineau P, Silva BC, Leslie WD (2017) Utility of trabecular bone score in the evaluation of osteoporosis. CurrOpinEndocrinol Diabetes Obes 24:402–410. https://doi.org/10.1097/MED.0000000000000365
Moon JH, Kim KM, Oh TJ, Choi SH, Lim S, Park YJ et al (2017) The Effect of TSH suppression on vertebral trabecular bone scores in patients with differentiated thyroid carcinoma. J ClinEndocrinolMetab 102:78–85. https://doi.org/10.1210/jc.2016-2740
De Mingo Dominguez ML, Guadalix Iglesias S, Martin-Arriscado Arroba C, López Alvarez B, Martínez Diaz-Guerra G, Martinez-Pueyo JI et al (2018) Low trabecular bone score in postmenopausal women with differentiated thyroid carcinoma after long-term TSH suppressive therapy. Endocrine 62:166–173. https://doi.org/10.1007/s12020-018-1671-8
Kim EH, Jeon YK, Pak K, Kim IJ, Kim SJ, Shin S, et al (2019) Effects of thyrotropin suppression on bone health in menopausal women with total thyroidectomy. J Bone Metab 26: 31–8. https://doi.org/10.11005/jbm.2019.26.1.31
Iglesias SG, Dominguez MLM, Herrero EF, Martinez-Pueyo JI, Arroba CM, Diaz-Guerra GM et al (2019) Trabecular bone score and bone mineral density in patients with postsurgical hypoparathyroidism after total thyroidectomy for differentiated thyroid carcinoma. Surgery 165:814–819. https://doi.org/10.1016/j.surg.2018.10.034
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by Non-BMD DXA measures: the 2015 ISCD official positions Part 2: trabecular bone score. J ClinDensitom 18(3):309–330. https://doi.org/10.1016/j.jocd.2015.06.008
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated Thyroid cancer. Thyroid 26(1):1–133. https://doi.org/10.1089/thy.2015.0020
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis (1994) Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129. https://pubmed.ncbi.nlm.nih.gov/7941614
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769. https://doi.org/10.1002/jbmr.499
Radominski SC, Bernardo W, Paula AP, Albergaria BH, Moreira C, Fernandes CE et al (2017) Brazilian guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Rev Bras ReumatolEngl Ed 57(Suppl 2):452–466. https://doi.org/10.1016/j.rbre.2017.07.001
Rosner B (2016) Fundamentals of Biostatistics. Cengage Learning, Boston, USA
Hwangbo Y, Kim JH, Kim SW, Park YJ, Park DJ, Kim SY et al (2016) High-normal free thyroxine levels are associated with low trabecular bone scores in euthyroid postmenopausal women. OsteoporosInt 27(2):457–462. https://doi.org/10.1007/s00198-015-3270-3
Savvidis C, Tournis S, Dede AD (2018) Obesity and bone metabolism. Hormones (Athens) 17(2):205–217. https://doi.org/10.1007/s42000-018-0018-4
Langsetmo L, Vo TN, Ensrud KE, Taylor BC, Cawthon PM, Schwartz AV et al (2016) The association between trabecular bone score and lumbar spine volumetric bmd is attenuated among older men with high body mass index. J Bone Miner Res 31(10):1820–1826. https://doi.org/10.1002/jbmr.2867
Romagnoli E, Lubrano C, Carnevale V, Costantini D, Nieddu L, Morano S et al (2016) Assessment of trabecular bone score (TBS) in overweight/obese men: effect of metabolic and anthropometric factors. Endocrine 54(2):342–347. https://doi.org/10.1007/s12020-016-0857-1
Messina C, Buonomenna C, Menon G, Magnani S, Albano D, Gitto S et al (2019) Fat mass does not increase the precision error of trabecular bone score measurements. J ClinDensitom 22(3):359–366. https://doi.org/10.1016/j.jocd.2019.01.001
Kim MK, Yun KJ, Kim MH, Lim DJ, Kwon HS, Song KH et al (2015) The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone 71:101–105. https://doi.org/10.1016/j.bone.2014.10.009
Greendale GA, Huang M, Cauley JA, Liao D, Harlow S, Finkelstein JS et al (2020) Trabecular bone score declines during the menopause transition: the study of women’s health across the nation (SWAN). J ClinEndocrinolMetab. https://doi.org/10.1210/clinem/dgz056
Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R et al (2014) Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res 29:399–407. https://doi.org/10.1002/jbmr.2048
Vera L, Gay S, Campomenosi C, Paolino S, Pera G, Monti E et al (2016) Ten-year estimated risk of bone fracture in women with differentiated thyroid cancer under TSH-suppressive levothyroxine therapy. Endokrynol Pol 67(4):350–358. https://doi.org/10.5603/EP.a2016.0046
Vadiveloo T, Donnan PT, Murphy MJ, Leese GP (2013) Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J ClinEndocrinolMetab 98(3):1147–1153. https://doi.org/10.1210/jc.2012-3191
Rosario PW, Xavier AC, Calsolari MR (2010) TSH reference values for adult Brazilian population. Arq Bras EndocrinolMetabol 54(7):603–606. https://doi.org/10.1590/s0004-27302010000700003
Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS (2006) Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. ClinEndocrinol (Oxf) 64(1):86–90. https://doi.org/10.1111/j.1365-2265.2005.02422.x
Kim TH, Joung JY, Kang M, Choi SK, Kim K, Jang JY et al (2015) A modest protective effect of thyrotropin against bone loss is associated with plasma triiodothyronine levels. PLoS ONE 10(12):145292. https://doi.org/10.1371/journal.pone.0145292
Morris MS (2007) The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone 40(4):1128–1134. https://doi.org/10.1016/j.bone.2006.12.001
Leader A, Ayzenfeld RH, Lishner M, Cohen E, Segev D, Hermoni D (2014) Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but not men, over the age of 65 years. J ClinEndocrinolMetab 99(8):2665–2673. https://doi.org/10.1210/jc.2013-2474
Murphy E, Glüer CC, Reid DM, Felsenberg D, Roux C, Eastell R et al (2010) Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J ClinEndocrinolMetab 95(7):3173–3181. https://doi.org/10.1210/jc.2009-2630
Zhu H, Zhang J, Wang J, Zhao X, Gu M (2020) Association of subclinical thyroid dysfunction with bone mineral density and fracture: a meta-analysis of prospective cohort studies. Endocrine 67(3):685–698. https://doi.org/10.1007/s12020-019-02110-9
Lee K, Lim S, Park H, Woo HY, Chang Y, Sung E et al (2020) Subclinical thyroid dysfunction, bone mineral density, and osteoporosis in a middle-aged Korean population. OsteoporosInt 31(3):547–555. https://doi.org/10.1007/s00198-019-05205-1
Acknowledgements
We thank the densitometry service of the Jenny Faria de Andrade Institute, especially the doctors Olívio Brito Mallheiros and Carlyle Marques Barral and the assistants Maria do Socorro de Souza and Elizabeth Aparecida de Souza.
Funding
This research did not receive any specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Researches Ethics Committees of Federal University of Minas Gerais – UFMG—and Instituto Mario Penna.
Consent to participate and for publication
Informed consent was obtained from all participants and the document is attached.
Availability of data and material
Contact the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sousa, B.É.C.A., Silva, B.C., de Oliveira Guidotti, T. et al. Trabecular bone score in women with differentiated thyroid cancer on long-term TSH-suppressive therapy. J Endocrinol Invest 44, 2295–2305 (2021). https://doi.org/10.1007/s40618-021-01537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01537-0