Abstract
Objective
To investigate in a large sample of overweight/obese (OW/OB) children and adolescents the prevalence of prediabetic phenotypes such as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), and to assess their association with cardiometabolic risk (CMR) factors including hepatic steatosis (HS).
Methods
Population data were obtained from the CARdiometabolic risk factors in children and adolescents in ITALY study. Between 2003 and 2013, 3088 youths (972 children and 2116 adolescents) received oral glucose tolerance test (OGTT) and were included in the study. In 798 individuals, abdominal ultrasound for identification of HS was available.
Results
The prevalence of IFG (3.2 vs. 3.3%) and IGT (4.6 vs. 5.0%) was similar between children and adolescents. Children with isolated IGT had a 2–11 fold increased risk of high LDL-C, non-HDL-C, Tg/HDL-C ratio, and low insulin sensitivity, when compared to those with normal glucose tolerance (NGT). No significant association of IFG with any CMR factor was found in children. Among adolescents, IGT subjects, and to a lesser extent those with IFG, showed a worse CMR profile compared to NGT subgroup. In the overall sample, IGT phenotype showed a twofold increased risk of HS compared to NGT subgroup.
Conclusions
Our study shows an unexpected similar prevalence of IFG and IGT between children and adolescents with overweight/obesity. The IGT phenotype was associated with a worse CMR profile in both children and adolescents. Phenotyping prediabetes conditions by OGTT should be done as part of prediction and prevention of cardiometabolic diseases in OW/OB youth since early childhood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The decreasing age at onset of obesity has alarming public health implications [1, 2], since obesity-related metabolic abnormalities such as prediabetes and type 2 diabetes (T2D) are becoming increasingly more common at progressively younger ages [3]. Thus, it is important to identify those critical phases in obese youth, in whom diabetes may be prevented.
The onset of T2D is preceded by a long asymptomatic phase represented by the presence of impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), two distinct phenotypes of prediabetes with only partial overlap [3]. Although both subcategories increase the risk for diabetes, current evidence suggests that they may have different pathophysiologies and different consequences [4]. In particular, several cardiometabolic risk factors (CMRFs) are associated with both IFG and IGT, but it is unclear whether they occur more frequently in one state than the other [5].
Although current guidelines recommend that the screening for altered glucose metabolism should start at 10 years of age (or at the onset of puberty, if puberty occurs at younger age) [6], scattered data suggest that prediabetic conditions, such as IFG and IGT, are emerging among obese youths at younger ages [2, 7–18]. Therefore, the aim of the present study was to determine in a very large sample of Caucasian children (<10 years of age) and adolescents with overweight/obesity the prevalence of IFG and IGT and to assess the association of these phenotypes with CMRFs including liver steatosis. Indeed, intrahepatic fat accumulation is a common complication in pediatric obesity and is strongly associated with insulin resistance and impaired glucose regulation prior to the onset of overt diabetes [19, 20].
Subjects and methods
Participants
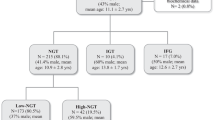
Population data were obtained from the CARdiometabolic risk factors in children and adolescents in ITALY (CARITALY) study [21], which includes data from 8639 children and adolescents (age below 10 years and ≥10 years, respectively, according to the World Health Organization’s definition of childhood and adolescence) [22] referred by general practitioners to secondary or tertiary care obesity centers for clinical evaluation and treatment. Eleven Italian centers participated to the study. Between 2003 and 2013, 3088 youths received oral glucose tolerance test (OGTT) and were included in the study. The exclusion criteria were: children and adolescents with recent history of acute infection, diabetes mellitus, secondary obesity, chronic diseases, malformations, and chronic use of drugs leading to metabolic disturbances. The study cohort comprised 972 (31%) children [mean age ± standard deviation (SD), 7.9 ± 1.2 years] and 2116 (69%) adolescents (mean age ± SD, 13.0 ± 2.1 years); 1509 were boys (49%). There were 344 (11%) overweight (OW), 1353 (44%) obese (OB), and 1391 (45%) morbidly obese (MOB) individuals.
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. In order to ensure data protection and confidentiality, data were anonymized before transmitting them to the coordinating center for analyses.
Measurement
Height and weight were measured in each center by the same investigator with standard methods. The pubertal status was assessed using Tanner criteria. Blood pressure (BP) was measured using aneroid sphygmomanometers with cuffs of appropriate size, according to standard procedures. Biochemical data were analyzed in each center after 12 h of fasting. Blood samples were drawn for detection of fasting plasma glucose (FPG) and insulin (FPI), total cholesterol, triglycerides (Tg), and high-density lipoprotein cholesterol (HDL-C). The low-density lipoprotein cholesterol (LDL-C) was calculated by using the Friedewald equation: total cholesterol − HDL-C − (Tg/5). Non-HDL-C was calculated by the following formula: total cholesterol − HDL-C. OGTT was performed using 1.75 g/kg of glucose up to a maximum of 75 g. Two-hour post-load glucose (2hPG) and insulin (2hPI) were analyzed. Insulin resistance (IR) was calculated by the homeostatic model assessment (HOMA-IR). Insulin sensitivity was calculated by the whole-body insulin sensitivity index (WBISI) with reduced time points according to the following formula: 10,000/√ (FPG × FPI × 2hPG × 2hPI). Although analyses were performed in different laboratories, all laboratories belong to the National Health System and are certified according to International Standards ISO 9000 (www.iso9000.it/), undergoing semiannual quality controls and inter-laboratory comparisons.
Ultrasound scan
Abdominal ultrasound scan was performed in 798 study subjects (369 boys, 46%) of whom 111 (14%) were OW, 366 (46%) OB, and 321 (40%) MOB. The rates of overweight, obese, and morbidly obese subjects undergoing ultrasound scan were not significantly different from those comprising the entire study population. An experienced operator identified hepatic steatosis with a standard method based on the appearance of hyperechoic liver parenchyma with tightly packed fine echoes and posterior beam attenuation. Hepatic steatosis was categorized as present or absent.
Definitions
OW, OB, and MOB were defined using the new international cutoffs of body mass index (BMI) provided by the International Obesity Task Force (IOTF) that captures the BMI ≥ 25, ≥30, and ≥35 kg/m2 at 18 years [23]. Isolated IFG was defined by FPG ≥ 100 mg/dl and 2hPG < 140 mg/dl; isolated IGT was defined by FPG < 100 mg/dl and 2hPG ≥ 140 mg/dl [6]. High LDL-C was defined by a cutoff ≥130 mg/l [24]. High non-HDL-C and Tg/HDL-C ratio were defined by a value ≥130 mg/l and ≥2.2, respectively [21]. IR was defined by 97th percentile of HOMA-IR for age and gender in normal-weight Caucasian children [25]. Low insulin sensitivity was defined by a value of WBISI ≤ 2.3 (which corresponded to the lower quartile of WBISI in our sample). High blood pressure (BP) was defined as systolic BP and/or diastolic BP ≥95th percentile for gender, age, and height [24].
Statistical analysis
Continuous variables are shown as means and SD. Categorical variables are reported as number (percentage) of participants with the characteristics of interest. Between-group comparisons were performed by ANOVA for continuous variables; post hoc analysis was performed using Bonferroni’s correction. 2 × 2 tables were used for categorical variables. The Chi-square or Fisher exact test, as appropriate, was used to compare proportions. Multiple logistic regression analysis was performed to estimate odds ratio of CMRFs adjusted for centers, gender, and morbid obesity both in children and adolescents. Statistical significance was set at P < 0.05. Data were analyzed using IBM SPSS Statistics version 20.0.
Results
Among the 3088 participants, 2837 had normal glucose tolerance (NGT), 101 (3.3%) isolated IFG, and 150 (3.8%) isolated IGT. Eight youths (0.26%) with combined IFG/IGT were excluded from the subsequent analyses.
The prevalence of both phenotypes of prediabetes was similar between the two age categories: isolated IFG was present in 31 (3.2%) of the 972 children and in 70 (3.3%) of the 2116 adolescents, while isolated IGT was present in 45 (4.6%) of the children and in 105 (5.0%) of the adolescents. The clinical features of the entire study population according to glucose homeostasis status (NGT, IFG, and IGT) are shown in Table 1. Age was similar among the three subgroups, whereas the prevalence of males was higher in the IFG subgroup compared to the other subgroups. Compared to NGT subgroup, IFG subjects showed higher values of HOMA-IR, LDL-C, non-HDL-C, and lower WBISI values, while IGT subjects had higher BMI and systolic BP, as well as higher values of HOMA-IR, LDL-C, non-HDL-C, Tg/HDL-C ratio, and lower WBISI values. Subjects with isolated IGT exhibited higher values of Tg/HDL-C ratio and diastolic BP and lower values of WBISI compared to the IFG subgroup.
IGT children as well as IGT adolescents showed a higher prevalence of elevated BP, LDL-C, non-HDL-C, Tg/HDL-C ratio, and low insulin sensitivity when compared, respectively, to NGT children and NGT adolescents (Fig. 1). Among the 798 subjects with abdominal ultrasound scan, the prevalence of hepatic steatosis was similar between NGT and IFG individuals (42 vs. 46%), whereas it was significantly higher in the IGT than the NGT subgroup (62 vs. 42%, P = 0.003) (Fig. 2). The odds ratios for CMRFs (adjusted for centers, age, gender, prepubertal status, and morbid obesity) showed that children with isolated IGT had a 2–11 fold increased risk of high LDL-C, non-HDL-C, Tg/HDL-C ratio, and low insulin sensitivity, when compared to those with isolated NGT (Table 2). No significant association of IFG with any of the CMRFs was found in children. Among adolescents, the IGT subgroup, and to a lesser extent the IFG phenotype, showed a worse cardiometabolic risk profile compared to subjects with NGT (Table 2). Given the low number of IFG subjects with hepatic steatosis (Fig. 2), the odds ratio for hepatic steatosis was estimated in the overall sample without stratification by age. The subgroup with IGT showed an odds ratio for steatosis of 2.27 (95% CI 1.26–4.09), P = 0.006, as compared to the subgroup with NGT. No significant difference was observed between NGT and IFG subjects.
Proportions of high blood pressure, LDL-C, non-HDL-C, Tg/HDL-C, insulin resistance (high HOMA-IR), and low insulin sensitivity (low WBISI) among children (left panel) and adolescents (right panel) with NGT (white bars), isolated IFG (gray bars), and isolated IGT (black bars). *P < 0.05, IFG versus NGT; † P < 0.05, IGT versus NGT; § P < 0.05, IGT versus IFG
Discussion
The present study demonstrates that the two prediabetes phenotypes, i.e., isolated IFG and isolated IGT, had a similar prevalence in a large sample of Caucasian children and adolescents with overweight/obesity. The American Diabetes Association (ADA) suggests that the prediabetes screening should start at the age of 10 years (or at the onset of puberty, if puberty occurs at younger age) in subjects with BMI >85th percentile for age and gender, and at least two additional risk factors for diabetes [6]. Since our aim was to determine the prevalence of IFG and IGT in a cross section of an OW/OB population, rather than in high-risk groups, we did not use the ADA screening recommendations. Using the ADA criteria would have failed to identify, in our cohort, 31 children with IFG and 45 children with IGT, meaning that 50% of all cases of IGT would have been missed. This issue is important considering the natural history of IGT in OW/OB children and adolescents. In a longitudinal follow-up (mean, 20.4 ± 10.3 months), Weiss et al. [26] showed that of the 33 OB youths initially classified as IGT, 30.3% remained IGT, while 24.1% progressed to diabetes, pointing out that IGT in OB children is indeed a transitional, “prediabetic” state. On the other hand, their data also indicated that OB children with IGT could revert to NGT (45.5%) on follow-up testing [26]. Similar results on the improvement of IGT have been reported by other studies with 1- to 5-year follow-up [27–29], underlining the good potential of recovery from impaired glucose metabolism in childhood with appropriate lifestyle intervention. Thus, even OB youths under the age of 10 years may require the most intensive intervention and careful consideration for T2D prevention.
One important finding of this study is that, in the overall sample of OW/OB children and adolescents, the IGT state was more strongly associated with CMRFs (including high BP, atherogenic dyslipidemia, low insulin sensitivity, and hepatic steatosis) than the IFG state. Our results confirm previous reports in adults indicating that these two prediabetes phenotypes represent two metabolically distinct categories [30]. In particular, our data are in line with the observations that isolated IFG and isolated IGT differ in the site of IR, although they both are insulin-resistant states [5]. The combination of hepatic IR and defective early-phase insulin secretion in isolated IFG results in excessive fasting hepatic glucose production accounting for fasting hyperglycemia. In contrast, in isolated IGT the defective late-phase insulin secretion, combined with moderate to severe muscle IR and hepatic IR, results in post-challenge hyperglycemia [5].
The association between IGT and atherogenic dyslipidemia (i.e., high levels of Tg/HDL-C ratio) has been previously described. In the Insulin Resistance Atherosclerosis Study involving a large adult population, Lorenzo et al. [31] demonstrated distinct lipid profiles in IFG and IGT. Isolated IFG was associated with higher apolipoprotein B, whereas isolated IGT was associated with higher values of Tg and Tg/HDL-C ratio, suggesting differences in cardiovascular risk between IFG and IGT subjects. In a previous study, we demonstrated that in normoglycemic OW/OB youths atherogenic dyslipidemia was associated with 2hPG levels still considered in the high normal range [32]. Interestingly, a longitudinal study showed that adolescents with elevated Tg/HDL-C ratio are prone to express a proatherogenic lipid profile in adulthood, independently of baseline BMI [33]. This profile was additionally worsened by weight gain [33]. An unexpected finding of our study was the more pronounced association between IGT and atherogenic dyslipidemia in children as compared to adolescents. These differences are difficult to interpret and are unlikely to be related to IR. Therefore, it is possible that other factors, such as low physical activity, incorrect dietary regimens, or both, may account for the stronger relationship in children than in adolescents between IGT and high LDL-C, high non-HDL-C, and high Tg/HDL-C ratio.
The strong association of hepatic steatosis with a distinct prediabetic phenotype such as IGT in OB youths is a novel finding, underlining the strong relationship between post-load hyperglycemia and fat accumulation in the liver [19, 20]. Although we are unable to explore the direction of this association [20], we speculate it may be related to the lower insulin sensitivity in IGT than IFG and NGT subjects. Longitudinal studies will clarify the issue. Thus, the association between obesity, altered glucose metabolism, and hepatic steatosis earlier in life urges to screen at younger ages youths at high risk for development of metabolic and cardiovascular diseases. Notably, several epidemiologic studies have recently suggested that nonalcoholic fatty liver disease may be an early predictor and a potentially important determinant of the development of both T2D [34] and cardiovascular disease [35].
In this study, IR was assessed through indirect markers such as HOMA-IR, whereas clamping studies are regarded as the gold standard for evaluating IR. However, HOMA-IR, developed for application in large epidemiologic investigations, is the most commonly used surrogate measure of insulin resistance. Compared to the glucose clamp technique, HOMA-IR has the same precision, but less accuracy. Using HOMA-IR makes it possible to study a large number of subjects and with a single glucose and insulin measurement in the fasting state [36].
In conclusion, based on results obtained in a very large sample of Italian youths with overweight/obesity, our study demonstrated a similar prevalence of isolated IFG and isolated IGT in children and adolescents. In addition, we showed that the IGT phenotype was associated with a worse cardiometabolic risk profile, including hepatic steatosis, as compared to the IFG and NGT subgroups. Our results suggest that phenotyping prediabetes conditions by OGTT should be done as part of prediction and prevention of cardiometabolic diseases in OW/OB youth since early childhood, but we acknowledge that its efficacy must be assessed in longitudinal clinical outcome studies.
References
Cunningham SA, Kramer MR, Narayan KM (2014) Incidence of childhood obesity in the United States. N Engl J Med 370(5):403–411
Shashaj B, Bedogni G, Graziani MP et al (2014) Origin of cardiovascular risk in overweight preschool children: a cohort study of cardiometabolic risk factors at the onset of obesity. JAMA Pediatr 168(10):917–924
Giannini C, Caprio S (2013) Progression of β-cell dysfunction in obese youth. Curr Diab Rep 13(1):89–95
Meyer C, Pimenta W, Woerle HJ et al (2006) Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29(8):1909–1914
Nathan DM, Davidson MB, DeFronzo RA et al (2007) American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30(3):753–759
American Diabetes Association (2016) Classification and diagnosis of diabetes. Diabetes Care 39(Suppl 1):S13–S22
Morrison KM, Xu L, Tarnopolsky M, Yusuf Z, Atkinson SA, Yusuf S (2012) Screening for dysglycemia in overweight youth presenting for weight management. Diabetes Care 35(4):711–716
Hagman E, Reinehr T, Kowalski J, Ekbom A, Marcus C, Holl RW (2014) Impaired fasting glucose prevalence in two nationwide cohorts of obese children and adolescents. Int J Obes 38(1):40–45
Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Zdravkovic D (2012) Type 2 diabetes mellitus and impaired glucose regulation in overweight and obese children and adolescents living in Serbia. Int J Obes 36(11):1479–1481
Ek AE, Rössner SM, Hagman E, Marcus C (2015) High prevalence of prediabetes in a Swedish cohort of severely obese children. Pediatr Diabetes 16(2):117–128
Hagman E, Arani PI, Fischer M et al (2014) Blood sugar levels are higher in obese young children in Sweden than in Poland. Acta Paediatr 103(11):1174–1178
Sinha R, Fisch G, Teague B et al (2002) Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346(11):802–810
Tester J, Sharma S, Jasik CB, Mietus-Snyder M, Tinajero-Deck L (2013) Gender differences in prediabetes and insulin resistance among 1356 obese children in Northern California. Diabetes Metab Syndr 7(3):161–165
Shalitin S, Abrahami M, Lilos P, Phillip M (2005) Insulin resistance and impaired glucose tolerance in obese children and adolescents referred to a tertiary-care center in Israel. Int J Obes 29(6):571–578
Brufani C, Ciampalini P, Grossi A et al (2010) Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatr Diabetes 11(1):47–54
Brufani C, Fintini D, Ciampalini P et al (2011) Pre-diabetes in Italian obese children and youngsters. J Endocrinol Invest 34(9):e275–e280
Invitti C, Guzzaloni G, Gilardini L, Morabito F, Viberti G (2003) Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care 26(1):118–124
Grandone A, Amato A, Luongo C, Santoro N, Perrone L, Miraglia Del Giudice E (2008) High-normal fasting glucose levels are associated with increased prevalence of impaired glucose tolerance in obese children. J Endocrinol Invest 31(12):1098–1102
Cali AM, De Oliveira AM, Kim H et al (2009) Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 49(6):1896–1903
D’Adamo E, Cali AM, Weiss R et al (2010) Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 33(8):1817–1822
Di Bonito P, Valerio G, Grugni G et al (2015) Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: the CARITALY study. Nutr Metab Cardiovasc Dis 25(5):489–494
Canadian Paediatric Society (2003) Age limits and adolescents. Paediatr Child Health 8(9):577
Cole TJ, Lobstein T (2012) Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 7(4):284–294
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute (2011) Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128(Suppl. 5):S213–S256
Shashaj B, Luciano R, Contoli B et al (2016) Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol 53(2):251–260
Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S (2005) Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 28(4):902–909
Körner A, Wiegand S, Hungele A et al (2013) Longitudinal multicenter analysis on the course of glucose metabolism in obese children. Int J Obes 37(7):931–936
Kleber M, Lass N, Papcke S, Wabitsch M, Reinehr T (2010) One-year follow-up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabet Med 27(5):516–521
Kleber M, deSousa G, Papcke S, Wabitsch M, Reinehr T (2011) Impaired glucose tolerance in obese white children and adolescents: three to five year follow-up in untreated patients. Exp Clin Endocrinol Diabetes 119(3):172–176
Festa A, D’Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM (2004) Differences in insulin resistance in nondiabetic individuals with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 53(6):1549–1555
Lorenzo C, Hartnett S, Hanley AJ et al (2013) Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab 98(4):1622–1630
Di Bonito P, Licenziati MR, Baroni MG et al (2014) High normal post-load plasma glucose, cardiometabolic risk factors and signs of organ damage in obese children. Obesity (Silver Spring) 22(8):1860–1864
Weiss R, Otvos JD, Sinnreich R, Miserez AR, Kark JD (2011) The triglyceride to high-density lipoprotein-cholesterol ratio in adolescence and subsequent weight gain predict nuclear magnetic resonance-measured lipoprotein subclasses in adulthood. J Pediatr 158(1):44–50
Li WD, Fu KF, Li GM et al (2015) Comparison of effects of obesity and non-alcoholic fatty liver disease on incidence of type 2 diabetes mellitus. World J Gastroenterol 21(32):9607–9613
Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesäniemi Y (2014) Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open 4(3):e004973
Bonora E, Targher G, Alberiche M et al (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23(1):57–63
Authors’ contribution
PDB, LP, CC, GV, and MGB made substantial contributions to the conception and design of the study and wrote and edited the manuscript. MM, MRL, CM, LP, EMdG, AM, FF, SL, GT, and CI collected, analyzed, and interpreted the data and wrote the manuscript. All authors reviewed and accepted the final version of the manuscript. PDB, GV, and MGB are guarantors of this work.
Funding
This work was funded by research grants from the Regione Autonoma della Sardegna (Grant RAS 2007 n.CRP-59453), and from the Foundation Banca d’Italia (Projects 2015), all awarded to Marco G. Baroni.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest relevant to this manuscript to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participating centers obtained informed consent from parents of subjects included in the study.
Additional information
See “Appendix” for a detailed list of CARITALY investigators, who belong to the Childhood Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology.
Appendix
Appendix
The CARdiometabolic risk factors in overweight and obese children in ITALY (CARITALY) Investigators:
D. Driul, Pediatric Unity, AOU Udine, Udine, Italy.
A. Grandone, Department of Woman, Child and General and Specialized Surgery, SUN, Naples, Italy.
M. Incani and M. G. Pani, Department of Medical Sciences, University of Cagliari, Cagliari, Italy.
Michela Tomat, Pediatric Unit, AOU Udine, Udine, Italy.
E. Sanguigno, Pediatric Unit, “S. Maria delle Grazie”, Pozzuoli Hospital, Naples, Italy.
L. Gilardini, IRCCS Istituto Auxologico Italiano, Department of Medical Sciences & Rehabilitation, Milan, Italy.
M. C. Pellegrin, Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy.
Rights and permissions
About this article
Cite this article
Di Bonito, P., Pacifico, L., Chiesa, C. et al. Impaired fasting glucose and impaired glucose tolerance in children and adolescents with overweight/obesity. J Endocrinol Invest 40, 409–416 (2017). https://doi.org/10.1007/s40618-016-0576-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0576-8