Abstract
Aims
Insulin resistance (IR) may develop very early in life being associated with occurrence of cardiometabolic risk factors (CMRFs). Aim of the present study was to identify in young Caucasians normative values of IR as estimated by the homeostasis model assessment (HOMA-IR) and cutoffs diagnostic of CMRFs.
Methods
Anthropometrics and biochemical parameters were assessed in 2753 Caucasians (age 2–17.8 years; 1204 F). Reference ranges of HOMA-IR were defined for the whole population and for samples of normal-weight and overweight/obese individuals. The receiver operator characteristic analysis was used to find cutoffs of HOMA-IR accurately identifying individuals with any CMRF among total cholesterol and/or triglycerides higher than the 95th percentile and/or HDL cholesterol lower than the 5th for age and sex, impaired glucose tolerance, and alanine aminotransferase levels ≥40 U/l.
Results
Overweight/obese individuals had higher HOMA-IR levels compared with normal-weight peers (p < 0.0001) at any age. HOMA-IR index rose progressively with age, plateaued between age 13 and 15 years and started decreasing afterward. HOMA-IR peaked at age 13 years in girls and at 15 years in boys. The 75th percentile of HOMA-IR in the whole population (3.02; AUROC = 0.73, 95 % CI = 0.70–0.75), in normal-weight (1.68; AUROC = 0.76, 95 % CI = 0.74–0.79), and obese (3.42; AUROC = 0.71, 95 % CI = 0.69–0.72) individuals identified the cutoffs best classifying individuals with any CMRF.
Conclusions
Percentiles of HOMA-IR varied significantly in young Caucasians depending on sex, age, and BMI category. The 75th percentile may represent an accurate cutoff point to suspect the occurrence of one or more CMRFs among high total cholesterol and triglycerides, low HDL cholesterol, and ALT ≥ 40 UI/l.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance (IR) denotes a condition of relative insensitivity of peripheral tissues (e.g., muscle, liver, and adipose tissue) to the effects of the hormone. IR plays a pivotal role in the development and progression of cardiometabolic risk factors (CMRFs) that, in association to obesity, somehow belong to the metabolic syndrome (e.g., impaired glucose tolerance, IGT; type 2 diabetes, dyslipidemia, hypertension, hepatic steatosis, and cardiovascular disease) [1].
In youth, IR is a physiological condition that favors body accretion and pubertal development. At the puberty, IR reaches its zenith and then declines to prepubertal values [2]. Age, gender, and ethnicity mostly influence IR [3–5]. In obese young individuals, IR may exceed physiological values, especially at the time of the puberty, putting the subject at significantly increased risk of developing CMRFs [6, 7].
Recognition of physiological versus not physiological conditions of IR in youth is of substantial clinical importance for identifying young individuals at increased cardiovascular risk, implementing preventive strategies and optimizing therapies to reduce the burden of cardiovascular disease associated with obesity and not physiological IR. Gold standard techniques for the estimation of IR are the euglycemic hyperinsulinemic clamp (EHC) and the frequently sampled intravenous glucose tolerance test. Both techniques are invasive, extremely labor-intensive, and time-consuming [8, 9].
The homeostasis model assessment of insulin resistance (HOMA-IR) is a proxy estimate of IR based upon the relationship between fasting glucose and insulin levels, with higher values of HOMA-IR representing more severe IR. It has been validated against the whole-body glucose uptake estimated by the EHC showing a high degree of correlation with the latter estimate [10].
Identifying normative values of HOMA-IR that are age-, gender-, and BMI-specific in pediatric populations would be of extreme value to accurately classify patients presenting not physiological IR [3, 11]. Studies have tried, but inconsistently, at identifying values of HOMA-IR suggestive of not physiological IR that poses these individuals at enhanced cardiometabolic risk [12, 13]. They have been conducted in small samples of patients without including normal-weight. As such, they are not informative of values of HOMA-IR in a general population [14] and of physiological variations of HOMA-IR across the age continuum from childhood to adolescence [2, 3].
Objectives of the present study were to draw age-, gender-, and BMI-specific percentiles of HOMA-IR in a large sample of Caucasians young individuals and to identify cutoffs of HOMA-IR accurately classifying patients at increased cardiometabolic risk.
Methods
Study population
Between July 2012 and 2013, 2573 Caucasian children and adolescents, 1369 males and 1204 females (age range 2.0–17.8 years) from the Metropolitan area of Rome (Italy) were referred from family pediatricians to the Bambino Gesù Children’s Hospital to participate in the study “Profiling the genetic risk of complex diseases in the Italian population” which aims to identify genetic profiles associated with increased risk of impaired glucose metabolism [15].
As secondary objective of the profiling study, the present investigation aimed at describing the distribution of HOMA-IR in the general pediatric Italian population and identifying cutoffs of HOMA-IR diagnostic of CMRFs.
Twelve family pediatricians, working in the six health districts of the Rome Municipality (two pediatricians for each district), enrolled the children and adolescents during the routine activity of growth monitoring (follow-up growth control visits). Children and adolescents, who were invited to participate to the study, were in good health and they were not following a weight loss diet or an intensive exercise program. Exclusion criteria were any chronic illnesses (i.e., allergies, inflammatory and autoimmune diseases, and endocrine disease), pregnancy, and use of contraceptives or other medications which can interfere with glucose metabolism. No child had known diagnosis of impaired glucose metabolism or diabetes at the time of the enrollment, and all children were healthy.
Information on family history of diabetes in the first-degree relatives was obtained by means of a short questionnaire completed by the parents.
The study was approved by the Ethics Committee of the Bambino Gesù Children’s Hospital. Written informed consent was obtained from the child’s parents/guardians, and patient’s data were treated to guarantee privacy.
Anthropometric measurements
Weight was measured with an approved scale (90/384/EEC, SECA) with precision of 50 g and periodic calibration. Children were weighed with minimal dress and weight recorded to the last 100 g. Height (without shoes) was measured with a Holtain’s stadiometer with precision of 0.1 cm and registered with approximation of 0.5 cm. The average of two measurements was used for both height and weight. Body mass index was calculated as weight in kilograms divided by height in meters squared (kg/m2) and BMI classes defined according to the International Obesity Task Force (IOTF) criteria [16]. Children were classified as normal weight if BMI was <85th percentile and overweight/obese if BMI was ≥85th percentile. Waist circumference (WC) was measured midway between the superior border of the iliac crest and the lower most margin of the ribs at the end of normal expiration.
Biochemical assays
All the participants were asked to refrain from intensive physical activity in the 3 days prior to the study. Fasting blood samples were drawn after 8–12 h fast. Plasma concentrations of triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and total cholesterol (mg/dl) were assessed by using colorimetric kits (Roche/Hitachi Modular systems P/S, Can 433, Milan, Italy). Alanine aminotransferase (ALT, normal range 10–37 UI/l), aspartate aminotransferase (ASP, normal range 10–65 UI/l), γ -glutamyltransferase (γ-GT, normal range <50 UI/l) and uric acid (UA, normal range 2.4–5.7 mg/dl) were measured by a radioimmunoassay method (ADVIA 1650 Chemistry System; Bayer Diagnostics). Glucose level was measured by the glucose oxidase technique (Cobas Integra, Roche) and insulin by a chemiluminescent immunoassay method (ADVIA Centaur analyzer; Bayer Diagnostics) on two fasting blood samples obtained at time points 0 and 5 min. Thyroid hormones were determined by antibody radioimmunoassay technique (reference range 6–18 pg/ml for free thyroxine, FT4 and 0.3–4.1 µU/ml for thyroid-stimulating hormone, TSH). To rule out celiac disease, anti-tissue transglutaminase antibodies (anti-tTG) determination was also performed. Intra-assay and inter-assay variations for the concentrations of these variables were <5 %.
All participants underwent a standard oral glucose tolerance test (OGTT) (1.75 g of glucose/kg body weight up to a maximum of 75 g). Glucose and insulin levels were measured during the OGTT at baseline and every 30 min for 120 min.
HOMA-IR was calculated as average on two blood samples (−5 and 0 min) as [fasting glucose (mg/dl) × fasting insulin (µU/ml)/405] [17]. Insulin sensitivity index (ISI) was calculated according to the formula by Matsuda and DeFronzo [ISI = 10,000/√(fasting glucose × fasting insulin) × (mean glucose × mean insulin)] [18].
Definition of metabolic abnormalities
Dyslipidemia was diagnosed as value of total cholesterol and/or triglycerides higher than the 95th percentile and/or HDL cholesterol lower than the 5th for age and sex according to the American Academy of Pediatrics [19]; impaired fasting glucose (IFG) as fasting glucose ≥100 mg/dl; impaired glucose tolerance (IGT) as 2-h glucose ≥140 mg/dl following the OGTT [20]; steatohepatitis was suspected in the presence of ALT > 40 U/l after ruling out other conditions causing abnormalities of liver enzyme according to a standardized protocol [21].
Statistical analysis
Statistical analysis was performed using Stata 12.1 (Stata Corp, College Station, TX, USA) together with the user-written oglm package. Continuous variables are reported as mean ± standard deviation (SD). Categorical variables are reported as number and percentage of subjects with the characteristics of interest. Between-group comparisons were performed with unpaired t test for continuous variables or with Fisher’s exact test for categorical variables. A p value ≤0.05 was considered significant. Correlations between continuous variables were analyzed using the Pearson’s correlation coefficients.
HOMA-IR distribution was tabulated for the values corresponding to the 3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentiles using a parametric procedure and plotted using the Cole’s LMS method which requires data normalization at each age using a Box–Cox power transformation. Hence, the centiles at each age can be summarized in terms of the Box–Cox power needed to make the distribution normal (called L), together with the median (M) and coefficient of variation (S) of the distribution. The fitting process ensures that values of L, M, and S change smoothly with age so that they can be represented as smooth curves plotted against age.
We estimated the accuracy of the 75th and the 90th percentile of HOMA-IR to identify patients with one or more CMRFs by calculating the area under the receiver operating characteristic curve (AUROC) [22] in the whole sample and in normal-weight and overweight/obese groups.
Results
Description of the sample
In the study population (n = 2573 children and adolescents; 53 % males; mean age 9.2 ± 3.6 years), 808 participants (31.4 %) had a BMI ≤ 85th percentile, and 1765 (68.8 %) were overweight/obese. No child was underweight (BMI ≤ 10th). Demographics, anthropometrics, and clinical characteristics of the sample as whole and in normal-weight versus overweight/obese groups are shown in Table 1. Overweight/obese individuals had levels of fasting insulin, triglycerides, ALT, and ASP significantly (p ≤ 0.05) higher and HDL cholesterol (p < 0.0001) values lower than normal-weight peers. Mean age, WC (p < 0.0001 for both), and birth weight (p = 0.001) were significantly higher in the overweight/obese group. Twenty individuals presented with impaired fasting glucose, four (0.5 %) in the normal-weight group and 16 (0.9 %) in the overweight/obese one (p = 0.09). Twenty-eight overweight/obese patients (1.6 %) presented with impaired glucose tolerance. High total cholesterol was found in 131 (16 %) normal-weight participants versus 226 (13 %) overweight/obese peers (p = 0.4). Low HDL cholesterol was significantly more prevalent in obese than in normal-weight participants (361, 20 vs. 32, 4 %; p = 0.003). Conversely, the percentages of participants with high triglycerides (80, 10 % vs. 398, 22 %; p = 0.006) and ALT ≥ 40 (45, 5.6 % vs. 230, 13 %; p = 0.002) were significant in overweight/obese individuals. The average ISI values were, respectively, 5.80 ± 2.46 and 3.86 ± 1.96 in the normal-weight and overweight/obese groups (p < 0.0001).
Distribution of HOMA-IR
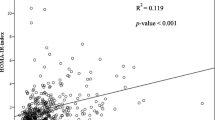
Overweight/obese individuals had higher HOMA-IR levels compared with normal-weight peers (p < 0.0001; Table 1). HOMA-IR values were higher in the overweight/obese individuals of any age as compared with normal-weight ones (Fig. 1). Table 2 reports HOMA-IR percentiles from the 3rd to 97th in normal-weight individuals grouped by sex and age. HOMA-IR index rose progressively with age, plateaued between age 13 and 15 years and started decreasing afterward (Fig. 1). HOMA-IR peaked at age 13 years in girls and at 15 years in boys (Table 2). In normal-weight females, HOMA-IR increased significantly (p < 0.05) from 2 to 3 years of age, from 4 to 5 years, and from 9 to 10 years. In normal-weight males, it rose significantly (p < 0.05) from 3 to 4 years of age, from 5 to 6 years, from 13 to 14 years, while it fell significantly (p = 0.04) from 16 to 17 years. In the normal-weight girls, HOMA-IR was significantly higher (p < 0.02) at ages 3, 5, 11, and 13 years old, compared with boys of the same age. Table 3 shows HOMA-IR distribution (from 3rd to 97th percentiles) in overweight/obese children and adolescents grouped by age and sex. HOMA-IR index gradually increased with age and peaked between 12 and 14 years in girls. On the contrary, there was no peak in overweight/obese boys.
HOMA-IR increased significantly (p < 0.05) from 2 to 3 years of age, from 6 to 7 years, from 9 to 10 years, and from 10 to 13 years of age in overweight/obese males. It significantly (p = 0.006) fell between 13 and 14 years in overweight/obese females. HOMA-IR was significantly higher (p < 0.01) in females ≤ 14 years old than in age-matched males, while it was significantly lower (p < 0.05) in females aged 15–17 years old compared with males of the same age.
HOMA-IR and CMRFs
In the overall population, HOMA-IR index was significantly correlated with age [0.14, 95 % CI 0.12–0.15, p < 0.001], BMI [0.13, 95 % CI 0.106–0.154, p < 0.001], WC [0.06, 95 % CI 0.053–0.074, p = 0.11], HDL cholesterol [95 % CI −0.03 (−0.039 to −0.025) p < 0.001], triglycerides [0.018, 95 % CI 0.015–0.022, p < 0.001], γGT [0.069, 95 % CI 0.052–0.086, p < 0.001], uric acid [0.23, 95 % CI 0.016–0.30, p < 0.001], and AST levels [0.004, 95 % CI 0.001–0.007, p = 0.001]. No significant correlation was found between HOMA-IR and cholesterol, 2-h glucose, 2-h insulin, FT4, TSH, and ALT levels. HOMA-IR index was significantly (p < 0.001) associated with age, BMI, WC, and uric acid when multiple linear regression analysis was performed.
The 75th percentile of HOMA-IR best identified the CMRF occurrence in the whole sample (Fig. 2 Panel a; HOMA-IR cutoff point 3.02; AUROC = 0.73, 95 % CI = 0.70–0.75; sensitivity = 46.3 %; and specificity = 82.6 %); in normal-weight (Fig. 2 Panel b; HOMA-IR 1.68; AUROC = 0.76, 95 % CI = 0.74–0.79; sensitivity = 47.4 %; and specificity = 84.9 %) and in overweight/obese individuals (Fig. 2 Panel c; HOMA-IR 3.42; AUROC = 0.71, 95 % CI = 0.69–0.72; sensitivity = 48.8 %; and specificity = 81.3 %). Once the accuracy of the 90th percentile of HOMA-IR was tested (4.42 for whole sample, 2.33 for normal-weight, and 4.98 for overweight/obese groups, respectively), the sensitivity of HOMA-IR to diagnose CMRFs decreased to 22.9, 34.6, and 38.2 %, while the specificity increased to 94.6, 95.2, and 96.1 %, respectively.
ROC curves showing the diagnostic accuracy of HOMA-IR to identify subjects with one or more CMRFs among low HDL cholesterol, high total cholesterol and/or triglycerides, impaired fasting glucose, blood pressure, and levels of ALT ≥ 40 UI/l in the whole sample (left, Panel a), in the normal-weight individuals (middle, Panel b), and in the overweight/obese peers (right, Panel c)
Discussion
We report age- and sex-specific percentiles of HOMA-IR in a very large population of normal-weight and overweight/obese young Caucasians and identify obesity-status-specific cutoff points of HOMA-IR that are diagnostic of the occurrence of one or more CMRFs among low HDL cholesterol, high total cholesterol and/or triglycerides, impaired glucose tolerance, and levels of ALT ≥ 40 UI/l.
Prior small sample size studies [2, 23–25] as well as the largest study by Lee et al. [3] in 1802 adolescents aged 12–19 years old from the National Health and Nutrition Examination Survey (NHNES) 1999–2002 found that values of HOMA-IR increased with increasing BMI in youth and that obese individuals have levels of HOMA-IR higher than normal-weight age-matched peers. Furthermore, overweight and obese patients with larger waist circumference are likely to present with greater values of HOMA-IR, being the waist circumference a reliable marker of increased visceral adiposity [26].
We demonstrate therewith and in keeping with a previous observation of us [6] that obesity starts affecting HOMA-IR very early in childhood. In the present series, we saw a progressively increase in HOMA-IR values in both sexes from 5 to 7 years of age even in normal-weight children. The EarlyBird study 26, a prospective cohort study of 235 healthy children aged 5–14 years, reported a progressive increase in HOMA-IR by 7 years of age onward in normal-weight children [2]. Importantly, both studies [2, 6] suggest that IR starts to increase years before the beginning of the pubertal development and findings from the present series confirm this observation. On the contrary, D’Annunzio et al. [27] did not observe any age-related difference of HOMA-IR values in 142 prepubertal normal-weight Italian children who were cross-sectionally evaluated. There is no doubt that the pubertal status influences the degree of insulin resistance. It has been demonstrated by using gold standard technique that glucose clearance decreases by ∼30 % in children from Tanner stage (TS) 2 to TS4 [28], reaches the nadir at TS3, and recedes to prepubertal levels afterward [27, 29]. In a cross-sectional study of 6132 Mexican healthy children and adolescents aged 6–18 years, Aradillas-Garcia et al. [5] observed a gradual increase in HOMA-IR from 6 to 13 years of age. The sex-specific and BMI-related increase in HOMA-IR with aging, observed in our series, very likely reflected the different tempo of pubertal development in normal-weight and obese individuals. HOMA-IR peaked at 13–14 years of age in normal-weight and at 12–14 years in overweight boys, while in girls it increased at 11–13 years of age in normal-weight ones and the peak anticipated to 9–10 years of age in overweight females. Since information on the pubertal stage of participants to the present study was lacking in most, we were unable to rule out the impact of puberty on variation of IR over time.
Our findings support evidence for a major effect of gender on insulin sensitivity. Mean values of HOMA-IR in girls were significantly higher than in boys at ages 3, 5, 11, and 13 years old in the normal-weight group, and at all ages from 2 to 14 years old in the overweight/obese group. This observation is in agreement with previous studies, demonstrating that prepubertal girls are more insulin resistant than boys [5, 30].
Data on gender-related differences in insulin sensitivity among adolescents (age ≥10 years) seem, conversely, to be not consistent. Two studies found girls to be more insulin resistant than males [31] [3]. Between age 13 and 15 years old, we found normal-weight girls to be more insulin resistant than males and on the contrary, overweight/obese girls to be more sensitive than males, being probably the result of the different interplay of the hormonal milieu and the adiposity at the time of puberty in males and females (Table 2). Although age, gender, and body adiposity cause physiological changes of IR that translate in a different distribution of HOMA-IR values in boys and girls of different age and with different adiposity, our findings demonstrate that values greater than 1.68 in normal-weight individuals cannot be defined as “physiological” and may pose the patient at an increased cardiovascular risk that deserve the physician’s assessment and intervention. Once considering overweight and obese individuals, the cutoff rises to 3.42. With limits and drawbacks above described, studies have attempted at identifying cutoff values of HOMA-IR diagnostic of a condition of not physiological IR based on the HOMA-IR distribution in the population they surveyed. Thresholds in the different studies were 4.39 in normal-weight normo-tolerant adolescents from the NHNES corresponding to SD above the mean of distribution in the sample [3]; 3.29 in a study of 84 children aged 6–13 years old from normal-weight to obese [32]; 3.16 in a study of 57 obese children and adolescents [14].
Even the NHNES, which was the largest and enclosed normal-weight individuals of different ethnicities, identified a threshold value of HOMA-IR based on distribution and not on the occurrence of CMRFs [3]. Overweight and obese individuals had significantly higher birth weight and more prevalent family history of type 2 diabetes than normal-weight mates, in keeping with the notion that IR develops early in life more often in offsprings of obese diabetic mothers [33].
The strength of the present investigation is the sample size, the wide age range, the participation of not only overweight and obese patients but also of a consistent number of normal-weight healthy individuals. This study is the first large-scale work in the Italian pediatric population and one of the largest reported in the scientific literature so far.
Lack of information on the pubertal development and follow-up data are major drawbacks. Additionally, it would be advisable to have information on children’s blood pressure, body composition, and markers of cardiometabolic derangement.
Participants were enrolled across all the health districts in the municipality of Rome to have a representative sample. However, almost two-thirds of participants in our sample were overweight/obese. Indeed, the recruitment process was subject to parental cooperation, and parents of overweight/obese children probably recognized the condition of enhanced health risk related to their child’ BMI status. This selection bias, while resulting in a not representative of a general population-based sample, is unlikely to have affected the HOMA-IR distribution in the two different normal-weight and overweight/obese groups.
We acknowledge that HOMA-IR is not as sensitive as the euglycemic clamp and the intravenous frequent sampling glucose tolerance test for determining insulin resistance/sensitivity. Therefore, we may have somewhat underestimated the prevalence of insulin resistance in our study population.
In summary, values of HOMA-IR vary by age, sex, and BMI categories. The percentile distribution can be a useful tool in the clinical practice to identify children and adolescents at increased cardiovascular risk who deserve further investigation and tighter follow-up. Threshold values of HOMA-IR vary based on the individual’s BMI, but the 75th percentile may represent an accurate value to suspect the occurrence of some CMRFs among low HDL cholesterol, high total cholesterol and/or triglycerides, impaired glucose tolerance, and levels of ALT ≥ 40 UI/l.
Abbreviations
- BMI:
-
Body mass index
- HDL:
-
High-density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein cholesterol
- IOTF:
-
International Obesity Task Force
- ALT:
-
Alanine aminotransferase
- ASP:
-
Aspartate aminotransferase
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- γ-GT:
-
Gamma-glutamyltransferase
- OGTT:
-
Oral glucose tolerance test
- IR:
-
Insulin resistance
- HOMA-IR:
-
Homeostasis model assessment for insulin resistance
- WC:
-
Waist circumference
- CMRFs:
-
Cardiometabolic risk factors
References
Kurl S, Zaccardi F, Onaemo VF, YoungJae S, Kauhanen J, Ronkainen K, Laukkanen JA (2014) Association between HOMA-IR, fasting insulin and fasting glucose with coronary heart disease mortality in nondiabetic men: a 20-year observational study. Acta Diabetol. doi:10.1007/500592-014-0615-x
Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ (2012) Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care 35(3):536–541. doi:10.2337/dc11-1281
Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG (2006) Prevalence and determinants of insulin resistance among US adolescents. Diabetes Care 29:2427–2432
Allard P, Delvin EE, Paradis G, Hanley JA, O’Loughlin J, Lavallèe C, Levy E, Lambert M (2003) Distribution of fasting plasma insulin, free fatty acids and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin Chem 49:644–649
Aradillas-Garcia C, Rodriguez-Moràn M, Garay-Sevilla ME, Malacara JJM, Rascon-Pacheco RA, Guerrero-Romero F (2012) Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. EJE 166:301–306
Shashaj B, Bedogni G, Graziani MP, Tozzi AE, DiCorpo ML, Morano D, Tacconi L, Veronelli P, Contoli B, Manco M (2014) The ORIGIN Study: cardiometabolic risk factors at the onset of obesity relationship to reduced insulin sensitivity and fatty liver. JAMA Pediatr 168(10):917–924. doi:10.1001/jamapediatrics.2014.900
Jasik CB, Lustig RH (2008) Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci 1135:265–279
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Bergman RN (1989) Towards physiological understanding of glucose tolerance-minimal model approach. Diabetes 38:1512–1527
Gungor N, Saad R, Janovsky J, Arslanian S (2004) Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents: a validation study. J Pediatr 144:47–55
Almeida CA, Pinho AP, Ricco RG, Pepato MT, Brunetti IL (2008) Determination of glycemia and insulinemia and the homeostasis model assessment (HOMA) in schoolchildren and adolescents with normal body index. Jorna de Pediatria 84:136–140
Tobisch B, Blatniczky L, Barkai L (2013) Cardiometabolic risk factors and insulin resistance in obese children and adolescents: relation to puberty. Pediatr Obes. doi:10.1111/j.2047-6310.2013.00202.x
Yin J, Li M, Wang Y, Cheng H, Zhao X, Mi J (2013) Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr 5:71. doi:10.1186/1758-5995-5-71
Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115:e500–e505
Luciano R, Barraco GM, Muraca M, Ottino S, Spreghini MR, Wietrzykowska Sforza R, Rustico C, Morino GS, Manco M (2015) Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. doi:10.1542/peds.2014-2391
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition of child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Daniels SR, Greer FR, the Committee on Nutrition (2008) Lipid screening and cardiovascular health in childhood. Pediatrics 122:198–208
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus et al (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–3167
Manco M (2011) Metabolic syndrome in childhood from impaired carbohydrate metabolism to nonalcoholic fatty liver disease. J Am Coll Nutr 30(5):295–303
Pepe M (2003) The statistical evaluation of medical tests for classification and prediction. Oxford University Press, New York
Bocca G, Ongering EC, Stolk RP, Sauer PJ (2013) Insulin resistance and cardiovascular risk factors in 3-to 5-year-old overweight or obese children. Horm Res Paediatr 80:201–206
Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S (2004) Obesity and metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374
Singh Y, Garg MK, Tandon N, Marwaha RK (2013) A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban indian adolescents. J Clin Res Pediatr Endocrinol 5(4):245–251
Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G (1997) Insulin resistance and hypersecretion in obesity. European group of the study of insulin resistance (EGIR). J Clin Invest 5:1166–1173
D’Annunzio G, Vanelli M, Pistorio A, Minuto N, Bergamino L, Iafusco D, Lorini R, the Diabetes Study Group of Italian Society for pediatric Endocrinology and Diabetes (ISPED) (2009) Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed 80:21–28
Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV (1986) Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 315:215–219
Ball GD, Huang TT, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ, Goran MI (2006) Longitudinal changes in insulin sensitivity, insulin secretion and beta-cell function during puberty. J Pediatr 148:16–22
Murphy MJ, Metcalf BS, Voss LD, Jeffrey AN, Kirkby J, Mallan KM, Wilkin TJ (2006) Girls at five years are intrinsically more insulin resistant than boys: the programming hypotheses revisited- the EarlyBird study (EarlyBird 6). Pediatrics 113:82–86
Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR (1999) Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 48:2039–2044
Hirschler V, Aranda C, de Lujàn Calcagno M, Maccalini G, Jadzinsky M (2005) Can waist circumference identify children with the metabolic syndrome? Arch Pediatr Adolesc 159(8):740–744. doi:10.1001/archpedi.159.8.7402005
Simental-Mendía LE, Castaneda-Chacón A, Rodríguez-Morán M, Guerrero-Romero F (2012) Birth-weight, insulin levels, and HOMA-IR in newborns at term. BMC Pediatrics 12:94. doi:10.1186/1471-2431-12-94
Acknowledgments
The work was supported by grants from the Italian Ministry of Health (RF-OPG-2008-1142374 to MM; RC 201302R003008 to MM; “Sviluppare profili genetici e trasferirli alla sanità pubblica, in Italia” To BD) and from the EC to MM and BD (FP7-ICT-2012-600932 MD PAEDIGREE). BS was supported by the Grant FP7-ICT-2012-600932 MD PAEDIGREE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study was performed in accordance with the Declaration of Helsinki as revised in 2008 and approved by the Ethical Committee of the Bambino Gesù Children’s Hospital.
Human and Animal Rights disclosure
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent disclosure
Informed consent was obtained from all parents or responsible guardians.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Shashaj, B., Luciano, R., Contoli, B. et al. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol 53, 251–260 (2016). https://doi.org/10.1007/s00592-015-0782-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0782-4