Abstract
Aims
To identify metabolic phenotypes at increased risk of impaired glucose tolerance (IGT) in Italian overweight/obese children (n = 148, age 5–10 years) and adolescents (n = 531, age 10–17.9 year).
Methods
Phenotypes were defined as follows: obesity by the 95th cut-points of the Center for Disease Control body mass index reference standards, impaired fasting glucose (fasting plasma glucose ≥100 mg/dl), high circulating triglycerides (TG), TG/HDL cholesterol ≥2.2, waist-to-height ratio (WTHR) >0.6, and combination of the latter with high TG or TG/HDL cholesterol ≥2.2.
Results
In the 148 obese children, TG/HDL-C ≥ 2.2 (OR 20.19; 95 % CI 2.50–163.28, p = 0.005) and the combination of TG/HDL-C ≥ 2.2 and WTHR > 0.60 (OR 14.97; 95 % CI 2.18–102.76, p = 0.006) were significantly associated with IGT. In the 531 adolescents, TG/HDL-C ≥ 2.2 (OR 1.991; 95 % CI 1.243–3.191, p = 0.004) and the combination with WTHR > 0.60 (OR 2.24; 95 % CI 1.29–3.87, p = 0.004) were associated with significantly increased risk of IGT. In the whole sample, having high TG levels according to the NIH National Heart, Lung and Blood Institute Expert Panel was not associated with an increased risk of presenting IGT.
Conclusions
TG/HDL-C ratio can be useful, particularly in children, to identify obese young patients at risk of IGT. Its accuracy as screening tool in a general population needs to be verified. The combination of TG/HDL-C ratio and WTHR > 0.6 did not improve prediction. Having high TG according to the NIH definition was not associated with increased risk of developing IGT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing prevalence of childhood obesity in westernized and developing countries adds new concerns for healthcare providers. Its impact on type 2 diabetes (T2D) is one major point [1].
When severe enough, obesity might anticipate the onset of pre-diabetes (i.e., impaired fasting glucose, IFG, and impaired glucose tolerance, IGT) to teen age and full-blown T2D to early adulthood. In the USA, 20–30 % of obese youth have “pre-diabetes” as a condition of marked insulin resistance (IR) and β-cell dysfunction [2]. IFG seems resulting from an impaired first-phase insulin secretion, indicating both dysfunctional β-cell activity and increased hepatic IR. IGT arises from impaired β-cell glucose sensitivity that leads to defective first-phase secretion in the long run [3]. By placing an increased demand on the β-cell to hypersecrete insulin, IR influences the progressive β-cell failure up to the development of IGT first, and T2D later on [4]. Obese children and adolescents of both genders show increased IR at any age [5]. General awareness of pre-diabetes and diabetes in youth has developed during the last decade. The American Diabetes Association (ADA) recommends screening at-risk adolescents (i.e., those with severe IR) using the fasting plasma glucose (FPG) or the oral glucose tolerance test (OGTT) every 2 years starting at 10 years of age or at the onset of puberty. The ADA specifies that if FPG is impaired (FPG ≥ 100 mg/dl), additional diagnostic testing with an OGTT is recommended [6]. Albeit preferred for practical reasons, the FPG is less sensitive than the OGTT because it cannot identify all the individuals with IGT that, worth mentioning, is the strongest predictor of T2D in youth [7]. Studies described FPG as poor predictor of IGT [2, 8–14]. Two of studies recommended adding serum triglyceride (TG) concentrations to FPG to improve the accuracy to detect IGT cases [11, 13]. One study investigated the ability of the product of FPG per TG to predict T2D. The performance of such product was, however, poorer as compared to the performance of 1- and 2-h plasma glucose [14].
Findings from the Early Bird study suggest that the decline of insulin sensitivity in children begins years before the puberty [15] and it is more severe in low-birth-weight children who rapidly gain body weight during the early postnatal years as found by the Avon longitudinal study of pregnancy and childhood study [16]. Smaller size at birth, lower IGF-I levels, and childhood height are associated with reduced compensatory insulin secretion [16]. The combination of severely increased IR and the impaired β-cell response may cause IGT even before 10 years of age in severely obese children [17]. Hence, the identification of phenotypes at increased risk of IGT in children (<10 years of age) might be useful in the clinical practice.
For this purpose, we tried to identify metabolic phenotypes among high abdominal adiposity, impaired fasting glucose (IFG), high TG, low HDL-C, or high TG-to-HDL cholesterol ratio (TG/HDL-C) that best predict IGT in a sample of 679 Italian obese children (age <10 years) and adolescents (age ≥10 years of age). In addition, phenotypes combining high TG or high TG/HDL-C to high abdominal adiposity were assessed. Abdominal adiposity was estimated as waist-to-height ratio (WTHR) instead of waist circumference to avoid use of specific population percentiles.
Research design and methods
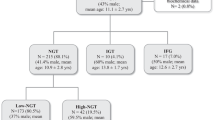
Between January 2005–December 2009, eight pediatric obesity services participated in a retrospective cross-sectional study, providing medical records of 679 outpatient obese children and adolescents who underwent clinical examination, fasting blood analysis, and OGTT. Inclusion criteria were: Caucasian race, age (5–17.9 years), and having complete data set as elsewhere described [18]. Exclusion criteria were: secondary obesity, chronic diseases, malformations and chronic use of drugs causing metabolic disturbances, and 2-h blood glucose >200 mg/dl (OGTT).
The study protocol was approved by the Childhood Obesity Group Review Board of the Italian Society for Paediatric Endocrinology and Diabetology (SIEDP). It conformed to the guidelines of the European Convention of Human Rights and Biomedicine for Research in Children. All measures were taken to ensure the confidentiality of families and children whose data were used. Patient’s personal and clinical data were anonymized before transmission and analysis. The directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of personal data was complied with for data storage and handling.
Anthropometry and blood pressure evaluation
Body weight and height were measured in each center by the same investigator, who was specifically trained in anthropometry according to SIEDP guidelines [18]; the average of the two closest measurements of height was used for the analysis; if a difference of 0.5 cm or more was found, a third measurement was taken. The BMI was calculated as weight divided by square of height (kg/m2). Waist circumference was measured using a flexible tape at the midpoint between the last rib and the iliac crest at minimal respiration when the participant was in a standing position. The WTHR was calculated (waist circumference in centimeters divided by height in centimeters). Blood pressure (BP) was measured according to standard procedures [19]. Briefly, BP was measured at the right arm in the sitting position after a 5-min rest, using an aneroid sphygmomanometer with an appropriately sized cuff; three readings were taken 2 min apart, and the average of the two last values was used in the analyses.
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the formula HOMA-IR = [glucose (nmol/L) * insulin (µU/mL)/22.5].
Biochemical parameters
Fasting TG were measured enzymatically, the HDL-C fraction was obtained after precipitation using a phosphotungstic reagent, and glucose was measured using a glucose oxidase method. Although analyses were performed in different laboratories, all centers belong to the Italian National Health system and are certified according to International Standards ISO 9000 (www.iso9000.it/), undergoing semi-annual quality controls and inter-laboratory comparisons; this contributes to limit the potential differences among laboratories.
Definition of phenotypes at increased risk of IGT
Obesity was defined by the 95th cut-points of the Center for Disease Control (CDC) BMI reference standards [20]. BMI values were transformed into standard deviation scores (SDS), based upon the CDC established normative curves [20].
The following cardiometabolic risk factors were considered: WTHR ≥ 0.6 as index of central adiposity [21]; high BP (HBP), defined by systolic (SBP) and/or diastolic (DBP) blood pressure ≥95th percentile for age, sex, and height [19]; high TG levels, defined using the cutoff ≥100 mg/dl (children 0–9 years) or ≥130 mg/dl (adolescents 10–19 years) as proposed by the Expert Panel Definition [19]; low HDL-C levels, defined by values <40 mg/dl [19]; TG/HDL-C ≥ 2.2 as a marker of atherogenic lipid profile [22, 23]; IFG as glucose ≥100 mg/dl; and IGT as 2 h glucose ≥140 mg/dl following the OGTT [6]. The hypertriglyceridemic WTHR phenotype was defined as TG ≥100 mg/dl (children 0–9 years) or ≥130 mg/dl (adolescents 10–19 years) and WTHR ≥ 0.60, while the combined dyslipidemic WTHR phenotype was defined as TG/HDL-C ≥ 2.2 and WTHR ≥ 0.60.
Statistical analysis
Continuous data are reported as means and standard deviations (SD), with categorical data as count and percentage. Variables were not normally distributed (except age, weight, and BMI-SDS). The general linear model was used to determine whether the means between IGT and no IGT groups differed, controlling by age, sex, and center. Contingency tables were used for categorical variables.
Odds ratio (OR) associated with IGT was calculated using multiple logistic analysis, controlled for major confounding factors (centers, gender, and BMI-SDS).
The Statistical Package of Social Sciences (SPSS, Chicago, IL, USA) for Windows software program release 18.0 was used. A p value <0.05 was considered significant.
Results
The whole sample included 302 males (44.5 %), 148 children (22.6 %), and 531 (77.4 %) adolescents. IGT was found in nine children (6.0 %) and 67 adolescents (12.6 %). Table 1 describes characteristics of obese patients with (n = 76; 11 %) and without IGT (n = 603; 89 %). Significant differences were observed in BMI and visceral adiposity, FPG, IR as estimated by the HOMA-IR, TG, TG/HDL-C ratio, SBP, and DBP (p ranging from 0.007 to <0.0001). IFG was found in six (8 %) IGT versus 28 (5 %) non-IGT patients (p = 0.2); WTHR >0.6 in 68 (90 %) IGT versus 367 (61 %) non-IGT subjects (p < 0.0001); high TG levels in 18 (24 %) versus 110 (18 %) (p = 0.25); low HDL-C in 27 (36 %) versus 160 (27 %) (p = 0.09); TG/HDL-C ≥ 2.2 in 46 (61 %) and 209 (35 %) (p < 0.0001); high BP in 37 (49 %) versus 245 (41 %), p = 0.2; the combination of the WTHR > 0.6 and high TG in 17 (22 %) and 66 (11 %) (p = 0.004); and the combination of WTHR > 0.6 and TG/HDL-C ≥ 2.2 in 40 IGT (53 %) and 136 non-IGT (23 %) patients (p < 0.0001). When comparing IGT versus non-IGT obese patients and taking into account the two classes of age (Table 2), the prevalence of high TG tended to be different in children but not in adolescents. The prevalence of both high TG levels and WTHR > 0.6 was significantly higher (p = 0.004) in children but not in adolescents (p = 0.06). Phenotypes of WTHR >0.6, TG/HDL-C ≥ 2.2, and their combination were significantly more prevalent in IGT children and adolescents than in non-IGT peers (p ranging from 0.03 to <0.0001).
Table 3 reports the ORs estimating the risk of IGT associated with different metabolic phenotypes in the whole population and in the two age groups (adjusted for centers, gender, and BMI-SDS).
Discussion
Findings of our study suggest that the TG/HDL cholesterol ≥2.2 is the phenotype best predicting the risk of IGT in the youth. It seems working better in children than in adolescents. The combination of the WTHR >0.6 to the TG/HDL cholesterol did not improve the performance of the latter. Of note, the hypertriglyceridemic phenotype, defined by the criteria of the NIH National Heart, Lung and Blood Institute Expert Panel, was not associated with an increased risk of IGT even when combined to the WTHR >0.6.
The prevalence of IGT and diabetes in young Italian patients affected by severe obesity is not so high [24] as reported in US obese youth by the SEARCH for Diabetes in youth study [25]. In our series, one adolescent solo was diagnosed with T2D (prevalence of 0.14 %) and excluded from the analysis. The prevalence of impaired glucose metabolism was significantly lower than that reported by the SEARCH study in white Americans [25]. These different figures in white youth may be due to differences in obesity rates between US and European Caucasians populations; in exposure to prenatal (nutritional cues and endocrine-disrupting chemicals in utero) and early postal factors [16]; and to frequent single nucleotide polymorphisms but with small phenotypic effect or relatively rare variants with larger effects whose frequencies vary also in people of same ethnicity (in this case all Caucasians) but different ancestry [26]. In front of such heterogeneity of causative factors, there is need of specifically population-tailored models predicting the risk of impaired glucose metabolism [27].
The TG/HDL-C ratio ≥2.2 has been very recently confirmed as accurate screening tool identifying IR, high blood pressure, metabolic syndrome, liver steatosis, higher carotid intima-media thickness, and concentric left ventricular hypertrophy in the 5505 children and adolescents from the “Cardiometabolic risk factors in overweight and obese children in Italy” (CARITALY) study [23]. Previously, Weiss et al. [28] demonstrated the association of a high TG/HDL-C in adolescence with development of a proatherogenic lipid profile later in young adulthood. In a study of 1452 obese youths, the TG/HDL-C ratio was associated with IR mainly in white obese boys and girls, but not in Hispanics and African-Americans individuals, again confirming the need of population-specific predictive model of risk [27]. Furthermore, these predictive models in young patients must take into account also pubertal development, which is well known to affect IR. Unfortunately, this information was missed in our series. However, we run all the regression models controlling for age.
As to other investigated phenotypes, previous studies tested the accuracy of FPG to predict IGT [29, 30]. Studies investigated the performance of risk phenotypes associated with the worsening of IR (i.e., greater whole body fatness as estimated by the BMI, visceral adiposity, high levels of fasting TG, and low levels of HDL-C, fasting state free fatty acids (FFAs), FFAs during an OGTT, and overnight FFAs among others) [31, 32]. Values of FPG ranging from 75 to 95 mg/dl in association with classes of TG as high as 80 mg/dl to 120 mg/dl were found to predict IGT with good accuracy [29]. In a clinic-based Canadian population of 256 obese children and adolescents (age ranging from 5 to 17 years of age) with a 13.9 % prevalence of IGT, a fasting TG concentration >1.17 mmol/l (104 mg/dl) was proposed as a simple clinical criterion for selecting obese children who deserve OGTT (71.4 % sensitivity, 64.1 % specificity for isolated IGT) [33].
The association of high circulating TG and waist circumference has been known for a long time as metabolic risk factor efficiently predicting cardiovascular disease and T2D in adults [34]. In the pediatric population, cutoff points of fasting TG and waist circumferences to define a combined phenotype of risk may result cumbersome since their values vary physiologically based on ethnicity, gender, and age. The use of the TG/HDL-C allows overcoming the use of specific population percentiles, being TG and HDL-C routinely estimated in the clinical practice, and also importantly economically affordable on large scale. It is worth noting that our results were obtained in a sample of obese patients enrolled in secondary healthcare centers and, they need to be verified in a general population.
In conclusion, findings of the present investigation support the reliability of the TG/HDL-C ratio to predict the risk of IGT in both obese children and adolescents. The risk of IGT associated with the TG/HDL-C ratio ≥2.2 was extremely high in children as compared to adolescents.
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- FBG:
-
Fasting blood glucose
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- HDL-C:
-
High-density lipoprotein cholesterol
- OGTT:
-
Oral glucose tolerance test
- T2D:
-
Type 2 diabetes
- TG/HDL-C:
-
Triglycerides-to-HDL cholesterol ratio
- WTHR:
-
Waist-to-height ratio
References
Misra A, Bhardwaj S (2014) Obesity and the metabolic syndrome in developing countries: focus on South Asians. Nestle Nutr Inst Workshop Ser 78:133–140
Savoye M, Caprio S, Dziura J et al (2014) Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care 37(2):317–324
Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383(9922):1084–1094
Defronzo RA (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58(4):773–795
Shashaj B, Luciano R, Contoli B et al (2015) Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. doi:10.1007/s00592-015-0782-4
American Diabetes Association (2004) Screening for type 2 diabetes. Diabetes Care 27:S11–S14
Weiss R, Dziura J, Burgert TS et al (2004) Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374
Cambuli VM, Incani M, Pilia S et al (2009) Oral glucose tolerance test in Italian overweight/obese children and adolescents results in a very high prevalence of impaired fasting glycaemia, but not of diabetes. Diabetes Metab Res Rev 25:528–534
Conwell LS, Batch JA (2004) Oral glucose tolerance test in children and adolescents: positives and pitfalls. J Paediatr Child Health 40:620–626
Greig F (2012) Which obese youth are at increased risk for type 2 diabetes? Latent class analysis and comparison with diabetic youth. Pediatr Diabetes 13:181–188
Love-Osborne K, Butler N, Gao D, Zeitler P (2006) Elevated fasting triglycerides predict impaired glucose tolerance in adolescents at risk for type 2 diabetes. Pediatr Diabetes 7:205–210
Tsay J, Pomeranz C, Hassoun A et al (2010) Screening markers of impaired glucose tolerance in the obese pediatric population. Horm Res Paediatr 73:102–107
Morandi A, Maschio M, Marigliano M et al (2014) Screening for impaired glucose tolerance in obese children and adolescents: a validation and implementation study. Pediatr Obes 9(1):17–25
Janghorbani M, Almasi SZ, Amini M (2015) The product of triglycerides and glucose in comparison with fasting plasma glucose did not improve diabetes prediction. Acta Diabetol 52(4):781–788
Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ (2012) Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care 35(3):536–541
Ong KK, Petry CJ, Emmett PM et al (2004) Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 47(6):1064–1070
Manco M, Spreghini MR, Luciano R et al (2013) Insulin sensitivity from preschool to school age in patients with severe obesity. PLoS ONE 8(7):e68628
Valerio G, Maffeis C, Balsamo A et al (2013) Severe obesity and cardiometabolic risk in children: comparison from two international classification systems. PLoS ONE 8(12):e83793
National Heart, Lung, and Blood Institute (2011) Expert Panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128(suppl 5):S237
Kuczmarski RJ, Ogden CL, Guo SS et al (2002) 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11(246):1–190
Khoury M, Manlhiot C, McCrindle B (2013) Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol 62:742–751
Di Bonito P, Moio N, Scilla C et al (2012) Usefulness of the high triglyceride-to-HDL cholesterol ratio to identify cardiometabolic risk factors and preclinical signs of organ damage in outpatient children. Diabetes Care 35:158–162
Di Bonito P, Valerio G, Grugni G et al (2015) Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: the CARITALY study. Nutr Metab Cardiovasc Dis 25(5):489–494
Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M (2012) Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol 166(1):107–114
Dabelea D, Mayer-Davis EJ, Saydah S et al (2014) Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311(17):1778–1786
Bonnefond A, Froguel P (2015) Rare and common genetic events in type 2 diabetes: What should biologists know? Cell Metab 21(3):357–368
Tanamas SK, Magliano DJ, Balkau B et al (2015) The performance of diabetes risk prediction models in new populations: the role of ethnicity of the development cohort. Acta Diabetol 52(1):91–101
Giannini C, Santoro N, Caprio S et al (2011) The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 34(8):1869–1874
Maffeis C, Pinelli L, Brambilla P et al (2010) Fasting plasma glucose (FPG) and the risk of impaired glucose tolerance in obese children and adolescents. Obesity (Silver Spring) 18(7):1437–1442
O’Malley G, Santoro N, Northrup V et al (2010) High normal fasting glucose level in obese youth: a marker for insulin resistance and beta cell dysregulation. Diabetologia 53(6):1199–1209
Morrison KM, Xu L, Tarnopolsky M, Yusuf Z, Atkinson SA, Yusuf S (2012) Screening for dysglycemia in overweight youth presenting for weight management. Diabetes Care 35(4):711–716
Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ (2015) Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol 52(2):277–284
Bailey DP, Savory LA, Denton SJ, Davies BR, Kerr CJ (2013) The hypertriglyceridemic waist, waist-to-height ratio, and cardiometabolic risk. J Pediatr 162(4):746–752
Lemieux I, Pascot A, Couillard C et al (2000) Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 102(2):179–184
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights disclosure
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Porta.
On the behalf of the Childhood Obesity Group of the Italian Society of Paediatric Endocrinology and Diabetology. See study members referred in “Appendix” section.
Appendix
Appendix
Childhood Obesity Group of the Italian Society of Paediatric Endocrinology and Diabetology: Nicola Corciulo, MD, Ospedale S. Cuore di Gesù Gallipoli, Lecce; Maria Rosaria Licenziati, MD, Dipartimento di Pediatria, AORN Santobono-Pausilipon, Napoli. Anita Morandi, MD, Pediatria ad Indirizzo Diabetologico e Malattie del Metabolismo, Università di Verona, Verona; Beatrice Moro, MD, Ospedale Civile Piove di Sacco, Padova; Alessandro Sartorio, MD, Divisione di Auxologia, Istituto Auxologico Italiano, Piancavallo, Verbania; Rita Tanas, MD, Azienda Ospedaliero-Universitaria Arcispedale S. Anna, Ferrara.
Rights and permissions
About this article
Cite this article
Manco, M., Grugni, G., Di Pietro, M. et al. Triglycerides-to-HDL cholesterol ratio as screening tool for impaired glucose tolerance in obese children and adolescents. Acta Diabetol 53, 493–498 (2016). https://doi.org/10.1007/s00592-015-0824-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0824-y