Abstract
Background
Parkinson’s disease (PD) represents the second most common neurodegenerative disease.
Objective
To evaluate the effects of dance therapy (DT) aimed at improving non-motor symptoms in PD.
Methods
Studies were performed through PubMed, Web of Science, The Cochrane Library, Embase, and Science Direct from inception to October 27, 2021. The data were screened independently by two reviewers, and the quality of the papers was assessed using the Cochrane manual. The included studies were randomized controlled trials and quasi-randomized controlled trials, reporting random-effects standardized mean differences, and 95% confidence intervals as the effect size. I2 statistics were used to assess heterogeneity. The main outcomes included the Montreal Cognitive Assessment Scale (MOCA), Baker Depression Scale (BDI), Parkinson’s Fatigue Scale (FPS-16), and Apathy Scale (AS). RevMan 5.3 software was integrated for meta-analysis.
Results
Nine literatures were analyzed for the meta-analysis with a total of 307 patients. Random effects showed that DT significantly improved cognitive of PD (MD = 1.50, 95% CI [0.52, 2.48], P = 0.0003; I2 = 51%). However, this meta-analysis demonstrated that dance therapy had no significance for improving depression (MD = − 1.33, 95% CI [− 4.11, 1.45], P = 0.35; I2 = 79%), fatigue (MD = 0.26, 95% CI [− 0.31, 0.83], P = 0.37; I2 = 0%), and apathy (MD = 0.07, 95% CI [− 2.55, 2.69], P = 0.96; I2 = 50%).
Conclusion
The meta-analysis suggests that dance can improve cognitive function in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a complex, chronic and progressive neurodegenerative disease in senior citizens that is expected to increase to 9.3 million by 2030 [1, 2]. PD patients may have dyskinesia, accompanied by symptoms such as bradykinesia, tremor, rigidity, and postural instability [3, 4]. Additional disability arises from the presence of non-motor symptoms (non-motor symptoms, NMS) such as anxiety, depression, cognitive function, and apathy [2, 4] with PD that was frequently overlooked by clinicians.
Despite the many side effects of drug treatment, people are still trying to understand the disease and find effective treatments for Parkinson’s [5, 6]. However, drug’s efficacy decreases with the progression of patients with Parkinson (patients with Parkinson, PwP) [5], and often larger doses are required to relieve non-motor symptoms. At the same time, some NMS, such as psychopaths (e.g., depression or anxiety), can be exacerbated or even induced by dopaminergic drugs and become more common and visible throughout the disease [7, 8]. In addition, many studies have demonstrated that the use of antipsychotics also increases mortality and morbidity in PD patients [9, 10]. Therefore, the researcher needs to seek alternative therapies to relieve their non-motor symptoms [6, 11, 12] and improve their quality of life.
Dance therapy (DT), as a clinical exercise method for neurological rehabilitation of Parkinson’s disease, is of great significance in improving balance, functional activity, dual-task walking, frozen gait, cognitive function, and sleep disorders [12,13,14]. In addition, a rhythmic dance could activate neurons that are advantageous to motor control, and the promotion of neural plasticity improves balance, movement, and cognition of the body with PD [15, 16]. Indeed, dancing is a feasible, acceptable, complex, and creative social activity [11] that can improve the attention and memory of PwP, and may have a positive effect on mild to moderate PD patients.
More and more attention has been paid to the influence of dance therapy on motor symptoms and non-motor symptoms of PD patients [11, 13]. However, previous studies have focused more on the effects of dance intervention on motor symptoms [13, 17] and few meta-analysis has focused on the impact of dance intervention on non-motor symptoms [11, 18]. This review was intended to evaluate the current evidence on the impact of dance therapy on non-motor symptoms in people with PD.
Materials and methods
The meta-analysis was considered under the Preferred Reporting Project for Systematic Reviews (PRISMA) guidelines [19].
Inclusion and exclusion criteria
The inclusion criteria are in line with the PICOS strategy [20].
1. Study design: The study design was randomized controlled trials (RCTs) or quasi-randomized controlled trials (quasi-RCTs). This meta-analysis was limited to papers published in English.
2. Participants: Patients of any age, sex, or disease stage who have been diagnosed with PD and received dance intervention.
3. Interventions and comparison: The inclusion criteria were discussed at least one association of dance or dance combined with other interventions as the interventions in the experimental group; In contrast, no intervention or non-dance intervention was used as the control group.
4. Outcomes: The Montreal Cognitive Assessment has sufficient psychometric properties as a screening tool for detecting mild cognitive impairment in Parkinson’s disease [21]. The baker Depression Scale (BDI) was used to examine the validity, reliability, and potential reactivity of patients with PD. The actual difference in total BDI was minimal at 3.3. BDI was an effective, reliable, and potentially reactive tool for assessing the severity of depression in PD patients [22]. PFS was suited for researchers and health care professionals to assess fatigue in patients with PD [23]. Compared with other scales, only AS was recommended for evaluating Parkinson’s apathy [24]. Therefore, the outcome measures included at least one of the Montreal Cognitive Assessment Scale (MOCA), Baker Depression Scale (BDI), Parkinson's Fatigue Scale (FPS-16), and Apathy Scale (AS).
Meanwhile, the following research was ruled out: (1) conference papers, abstracts, animal experiments, duplicate studies, systematic reviews, and meta-analyses, (2) unable to extract objective data or data loss, contact the author is still unable to obtain the original data of the study.
Literature retrieval strategy
Electronic literature was independently performed by two researchers (LLW, CJS) through PubMed, Web of Science, The Cochrane Library, Embase, and Science Direct from inception to October 27, 2021. Combinations of key terms including “Parkinson’s disease” OR “Parkinson” OR “parkinsonian disorders” OR “Lewy body Parkinson disease” AND “Dance Therapy” OR “dance” OR “dancing”. The specific search strategy for each database is given in Supplementary Appendix. Lists of references, systematic reviews, and meta-analyses were manually searched or referenced to identify other potential studies.
Selection and quality evaluation of research data
All the selected documents from the database were imported into EndNote X9, removed duplicates, and screened abstracts by two investigators independently (LLW, YW). The remaining full-text documents that meet the criteria were downloaded and examined. Data were independently extracted by two researchers (LLW, CJS) according to pre-set criteria, including first author, year of publication, country, age, sex, intervention type, experimental group, and control group, sample, follow-up time, PD duration, and outcome indicators. The quality of the qualified literature was evaluated using the Cochrane Collaboration Risk of bias tool (YCN, SH) [25]. If there were different opinions during the extraction and assessment process, the third researcher (JY) in the team was asked to discuss and decide whether to be included.
Statistical analysis
Statistical analysis of studies was calculated for meta-analyses using the RevMan version 5.3 software (The Cochrane Collaboration, Software Update, Oxford, UK). Chi square-based Q test and I2 statistics were performed for heterogeneity among studies [26]. All analyses were homogeneous (P > 0.1, I2 ≤ 50%) [27] and were analyzed using a fixed-effect model. Instead, use a random-effects model [28]. For continuous variables, standardized mean difference (SMD) or weighted mean difference (WMD) were used as effect scale indicators, and 95% CI was calculated. A funnel plot can be used to detect publication bias if more than ten articles meet the conditions of meta-analysis.
Results
Characteristics of the included research literature
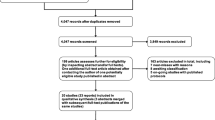
A total of 1090 papers were initially identified according to the pre-set retrieval strategy, including 278 in Web of Science, 192 in PubMed, 134 in The Cochrane Library, 401 in Embase, 78 in Science Direct, and 7 in other sources. After removing duplicates, 439 abstracts were independently screened by two evaluators (LLW, YW). After layer by layer screening, nine literature were finally analyzed for the meta-analysis [29,30,31,32,33,34,35,36,37] with a total of 307 patients. Detailed reasons for literature selection and exclusion were performed in Fig. 1.
Study characteristics and methodological quality
Nine trials were analyzed in this review, and the baseline characteristics of the patients are shown in Table 1. The selected studies included eight RCTs [29, 31,32,33,34,35,36,37] and one quasi-RCTs [30], and the eligible studies were published between 2013 and 2021. Research areas mainly include China, the United States, Italy, South Korea, Canada, the United Kingdom, and Japan. There were 307 cases in the papers, and the number of participants in the nine trials ranged from 13 to 80, with an average age of > 60 years; that is, the participants were mainly elderly. Moreover, there were more men than women (173 males versus 134 females). Additionally, dance interventions mainly include open-air fitness dancing, Turo (Qi Dance), tango, Sardinian folk dance, virtual reality dance, dance therapy, and dance. Finally, the duration of the intervention ranged from 5 to 12 weeks.
Quality assessments of the selected literature
The methodological quality of the included research literature is shown in Fig. 1. Of the nine studies included, six papers [30,31,32, 34,35,36] mentioned generation of random sequence, and none reported detailed information hidden in allocation. Although five studies [29,30,31,32, 36] mentioned blinding of participates and personnel, however, the specificity of the experiments made it impossible to determine whether blinding was performed throughout the entire process. Moreover, five studies [29,30,31, 34, 36] accomplished blinding of outcomes evaluators. In addition, seven [29,30,31, 33, 35,36,37] studies mentioned incomplete outcome data. All the papers [29,30,31,32,33,34,35,36,37] presented selective reporting of outcomes and reported unclear risk in other bias sources.
Forest plot comparing dance group and control
Cognition
Six RCTs [29, 33,34,35,36,37] with a total of 217 patients were used the MoCA (Fig. 2A) for assessment of cognition in the meta-analysis. A randomized effects meta-analysis showed that DT significantly improved cognition compared with no dance intervention (MD = 1.50, 95% CI [0.52, 2.48], P = 0.0003; I2 = 51%).
Depression
Six studies [29, 31, 32, 34,35,36] utilized BDI for the measurement of depression (Fig. 2B). This meta-analysis demonstrated that DT had no statistically significant effect on improving depression in patients with Parkinson’s disease (MD = − 1.33, 95% CI [− 4.11, 1.45], P = 0.35; I2 = 79%). A sensitivity analysis was performed by removing papers, respectively. After the exclusion of Michels et al. [34], the heterogeneity changed, and the results implied that DT was positive to the control on depression (MD = − 2.47, 95% CI [− 4.08, − 0.15], P = 0.04; I2 = 67%).
Fatigue
Two studies [29, 36] utilized PFS-16 for the measurement of fatigue (Fig. 2C). There may be no evidence of significant differences between the dance group and the control group (MD = 0.26, 95% CI [− 0.31, 0.83], P = 0.37). Heterogeneity was low among studies (I2 = 0%).
Apathy
Only two studies [30, 35] in this study used the AS to measure apathy (Fig. 2D). The result was not significant (MD = 0.07, 95% CI [− 2.55, 2.69], P = 0.96) with moderate heterogeneity (I2 = 50%).
Discussion
The purpose of this study is to collect the relevant studies on the current randomized controlled trials of dance therapy for the treatment of non-motor symptoms in PwP, to provide a theoretical basis for better studies on dance therapy for NMS in the future. In the qualitative synthesis of this review, a total of nine literature involving 307 patients with PD were identified, and the improvement effect of dance intervention on PD was evaluated according to four indicators of MOCA, BDI, PFS-16, and AS. The results showed that DT could have a positive impact on cognition levels among PD patients. However, data showed no differences in depression, fatigue, and apathy between the two groups. Therefore, future studies with more randomized controlled trials are needed to identify the effect of dance on non-motor symptoms.
Although the number of included papers is small, the earliest one included in this study was published in 2013, suggesting that DT as replacement therapy for non-motor symptoms of Parkinson’s disease is a relatively new area of research. The evidence has shown that DT has a positive impact on cognition, cognitive dual-tasking, and brain structure when compared to controls [17, 38,39,40]. It has been noted that there was increased attention and interest in non-motor symptoms, and it could improve physical, mental or mood state, cognitive ability, and social effects in PD [41,42,43,44,45,46]. In recent years, the PD patients to participate in the dance for a long time may be more practical than other forms of physical or mental exercise, because the research shows that PD patients participate in the dance is a very high degree of enthusiasm and persistence [13, 44, 45, 47]. Moreover, it has been found that dance, as a creative, social, and artistic alternative therapy [48] can relieve non-motor symptoms and improve the quality of life of PwP by reducing the depressive symptoms of Parkinson’s disease [49, 50]. Furthermore, animal studies have shown that music enhances spatial cognition due to increased expression of brain-derived neurotrophic factor (brain-derived neurotrophic factor, BDNF) in the dorsal hippocampus [51]. Overall, there was no evidence for dance therapy as a treatment for depression, fatigue, and apathy.
Nevertheless, the current meta-analysis showed that dance therapy was statistically significant in improving cognition in PD patients, which was not consistent with the previous study [51]. Some possible reasons are analyzed below. First, studies have shown that dance therapy has no statistical significance for non-motor symptoms such as depression, fatigue, and apathy in PD patients, which may be related to the complex pathophysiology of non-motor symptoms of Parkinson’s disease [6]. In terms of non-drug therapy, there is evidence that dance therapy is a relatively successful alternative [44], but many PD patients may prefer psychotherapy. As a result, it has been difficult for researchers to rigorously quantify the effect of dance therapy on non-motor symptoms [11]. Ruled out, in fact, the study [34], the dance therapy improved depression in Parkinson’s disease with statistical significance, but the evidence was lacking. Moreover, this study found that the dance intervention had no statistical significance on non-motor symptoms of depression, apathy, and fatigue in patients with Parkinson’s disease, which may be due to the short intervention time or insufficient dance intensity, and the small sample size.
Additionally, the study has several limitations. First, the randomized controlled trial of dance intervention for non-motor symptoms with PwP is a relatively new alternative therapy and lacks long-term follow-up data. Second, the sample size of our meta-analysis was unequal; the study of Michels et al. [34] demonstrated that the sample size was only 13. The overall sample size was too small and varied between studies, which may have affected the overall quality of the evidence. Third, the study included only English literature, which may increase the risk of publication bias.
Indeed, in the process of diagnosis and care of Parkinson’s disease, non-motor symptoms are often ignored by clinicians [52], so in future work, the study should carefully identify the risk factors of Parkinson’s disease, carefully assess the characteristics [6] of Parkinson’s non-motor symptoms. Research showed [44] that dance can affect every aspect of a person’s life, and the study needs to pay more attention to the physical, psychological, social, and emotional benefits of dance therapy in treating Parkinson’s disease. Randomized controlled trial on a much larger scale and of a much higher quality. At the same time, more attention should be pay to the real experience of PwP participating in dance intervention.
Conclusion
This study suggests that dance can improve cognitive function in patients with Parkinson’s disease. Although the mechanism of DT's effect on fatigue, depression, and apathy in Parkinson’s disease is unknown, we believe that dance intervention may be a promising alternative therapy in future clinical practice. In the future, dance RCTS for PD patients should follow the accepted standards of clinical trial methods, and the study of sample size and follow-up period should be enlarged. In addition, this study should also explore the most appropriate type of dance, intervention time, weekly intervention frequency, follow-up time, so as to achieve the best effect of dance therapy.
References
Dorsey ER, Constantinescu R, Thompson JP et al (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384–386. https://doi.org/10.1212/01.wnl.0000247740.47667.03
Berganzo K, Tijero B, González-Eizaguirre A et al (2016) Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Síntomas no motores y motores en la enfermedad de Parkinson y su relación con la calidad de vida y los distintos subgrupos clínicos. Neurologia 31:585–591. https://doi.org/10.1016/j.nrl.2014.10.010
Opara J, Małecki A, Małecka E et al (2017) Motor assessment in Parkinson’s disease. Ann Agric Environ Med 24:411–415. https://doi.org/10.5604/12321966.1232774
Berganzo K, Tijero B, González-Eizaguirre A et al (2016) Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurologia 31:585–591. English, Spanish. https://doi.org/10.1016/j.nrl.2014.10.010
Pereira APS, Marinho V, Gupta D et al (2019) Music therapy and dance as gait rehabilitation in patients with Parkinson disease: a review of evidence. J Geriatr Psychiatry Neurol 32:49–56. https://doi.org/10.1177/0891988718819858
Seppi K, Ray Chaudhuri K, Coelho M et al (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34:180–198. https://doi.org/10.1002/mds.27602 (Erratum in: Mov Disord (2019) 34:765)
Poewe W, Seppi K, Tanner CM et al (2017) Parkinson disease. Nat Rev Dis Primers 23:17013. https://doi.org/10.1038/nrdp.2017.13
Schaeffer E, Berg D (2017) Dopaminergic therapies for non-motor symptoms in Parkinson’s disease. CNS Drugs 31:551–570. https://doi.org/10.1007/s40263-017-0450-z
Weintraub D, Chiang C, Kim HM et al (2017) Antipsychotic use and physical morbidity in Parkinson disease. Am J Geriatr Psychiatry 25:697–705. https://doi.org/10.1016/j.jagp.2017.01.076
Weintraub D, Chiang C, Kim HM et al (2016) Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol 73:535–541. https://doi.org/10.1001/jamaneurol.2016.0031
Carapellotti AM, Stevenson R, Doumas M (2020) The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. PLoS One 15:e0236820. https://doi.org/10.1371/journal.pone.0236820
Bhalsing KS, Abbas MM, Tan LCS (2018) Role of physical activity in Parkinson’s disease. Ann Indian Acad Neurol 21:242–249. https://doi.org/10.4103/aian.AIAN_169_18
Dos Santos DM, Komeroski IG, Monteiro EP et al (2018) Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res 30:727–735. https://doi.org/10.1007/s40520-017-0836-2
van der Kolk NM, King LA (2013) Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord 28:1587–1596. https://doi.org/10.1002/mds.25658
Fan B, Jabeen R, Bo B et al (2020) What and how can physical activity prevention function on Parkinson’s disease? Oxid Med Cell Longev 2020:4293071. https://doi.org/10.1155/2020/4293071
Shanahan J, Morris ME, Bhriain ON et al (2015) Dance for people with Parkinson disease: what is the evidence telling us? Arch Phys Med Rehabil 96:141–153. https://doi.org/10.1016/j.apmr.2014.08.017
Kalyani HHN, Sullivan K, Moyle G et al (2019) Effects of dance on gait, cognition, and dual-tasking in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. https://doi.org/10.3233/JPD-181516
Zhang Q, Hu J, Wei L et al (2019) Effects of dance therapy on cognitive and mood symptoms in people with Parkinson’s disease: a systematic review and meta-analysis. Complement Ther Clin Pract. https://doi.org/10.1016/j.ctcp.2019.04.005
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
da Costa Santos CM, de Mattos Pimenta CA, Nobre MR (2007) The PICO strategy for the research question construction and evidence search. Rev Lat Am Enferm 15:508–511. https://doi.org/10.1590/s0104-11692007000300023
Hoops S, Nazem S, Siderowf AD et al (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73:1738–1745. https://doi.org/10.1212/WNL.0b013e3181c34b47
Visser M, Leentjens AF, Marinus J et al (2006) Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Mov Disord 21:668–672. https://doi.org/10.1002/mds.20792
Çilga G, Genç A, Çolakoğlu BD et al (2019) Turkish adaptation of Parkinson fatigue scale and investigating its psychometric properties. Int J Rehabil Res 42:20–25. https://doi.org/10.1097/MRR.0000000000000314
Leentjens AF, Dujardin K, Marsh L et al (2008) Apathy and anhedonia rating scales in Parkinson’s disease: critique and recommendations. Mov Disord 23:2004–2014. https://doi.org/10.1002/mds.22229
Higgins JPT, Thomas J, Chandler J et al (eds) (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. http://www.training.cochrane.org/handbook Accessed 28 July 2021
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Rodríguez I, Zambrano L, Manterola C (2016) Criterion-related validity of perceived exertion scales in healthy children: a systematic review and meta-analysis. Arch Argent Pediatr 114:120–128. https://doi.org/10.5546/aap.2016.eng.120
Frisaldi E, Bottino P, Fabbri M et al (2021) Effectiveness of a dance-physiotherapy combined intervention in Parkinson’s disease: a randomized controlled pilot trial. Neurol Sci. https://doi.org/10.1007/s10072-021-05171-9
Hashimoto H, Takabatake S, Miyaguchi H et al (2015) Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med 23:210–219. https://doi.org/10.1016/j.ctim.2015.01.010
Lee HJ, Kim SY, Chae Y et al (2018) Turo (Qi Dance) program for Parkinson’s disease patients: randomized, assessor blind, waiting-list control, partial crossover study. Explore (NY) 14:216–223. https://doi.org/10.1016/j.explore.2017.11.002
Lee NY, Lee DK, Song HS (2015) Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci 27:145–147. https://doi.org/10.1589/jpts.27.145
McKee KE, Hackney ME (2013) The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav 45:519–529. https://doi.org/10.1080/00222895.2013.834288
Michels K, Dubaz O, Hornthal E et al (2018) “Dance therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement Ther Med 40:248–252. https://doi.org/10.1016/j.ctim.2018.07.005
Rios Romenets S, Anang J, Fereshtehnejad SM et al (2015) Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med 23:175–184. https://doi.org/10.1016/j.ctim.2015.01.015
Solla P, Cugusi L, Bertoli M et al (2019) Sardinian folk dance for individuals with Parkinson’s disease: a randomized controlled pilot trial. J Altern Complement Med 25:305–316. https://doi.org/10.1089/acm.2018.0413
Wang G-Y (2019) Therapeutic efficacy of open-air fitness dancing with established training motions on cognitive dysfunction in patients with Parkinson’s disease. J Int Neurol Neurosurg 46:520–523. https://doi.org/10.16636/j.cnki.jinn.2019.05.010
Meng X, Li G, Jia Y et al (2019) Effects of dance intervention on global cognition, executive function and memory of older adults: a meta-analysis and systematic review. Aging Clin Exp Res. https://doi.org/10.1007/s40520-019-01159-w
Aguiar AS Jr, Castro AA, Moreira EL et al (2011) Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev 132:560–567. https://doi.org/10.1016/j.mad.2011.09.005
Yamazaki Y, Sato D, Yamashiro K et al (2017) Inter-individual differences in exercise-induced spatial working memory improvement: a near-infrared spectroscopy study. Adv Exp Med Biol 977:81–88
McNeely ME, Duncan RP, Earhart GM (2015) Impacts of dance on non-motor symptoms, participation, and quality of life in Parkinson disease and healthy older adults. Maturitas 82:336–341. https://doi.org/10.1016/j.maturitas.2015.08.002
Murray DK, Sacheli MA, Eng JJ et al (2014) The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener 3:5. https://doi.org/10.1186/2047-9158-3-5
Dereli EE, Yaliman A (2010) Comparison of the effects of a physiotherapist-supervised exercise programme and a self-supervised exercise programme on quality of life in patients with Parkinson’s disease. Clin Rehabil 24:352–362. https://doi.org/10.1177/0269215509358933
McGill A, Houston S, Lee RY (2014) Dance for Parkinson’s: a new framework for research on its physical, mental, emotional, and social benefits. Complement Ther Med 22:426–432. https://doi.org/10.1016/j.ctim.2014.03.005
Sharp K, Hewitt J (2014) Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev 47:445–456. https://doi.org/10.1016/j.neubiorev.2014.09.009
Volpe D, Signorini M, Marchetto A et al (2013) A comparison of Irish set dancing and exercises for people with Parkinson’s disease: a phase II feasibility study. BMC Geriatr 4:54. https://doi.org/10.1186/1471-2318-13-54
Bek J, Arakaki AI, Lawrence A et al (2020) Dance and Parkinson’s: a review and exploration of the role of cognitive representations of action. Neurosci Biobehav Rev 109:16–28. https://doi.org/10.1016/j.neubiorev.2019.12.023
Meekums B, Karkou V, Nelson EA (2015) Dance movement therapy for depression. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009895.pub2
Kiepe MS, Stockigt B, Keil T (2012) Effects of dance therapy and ballroom dances on physical and mental illness: a systematic review. Arts Psychother 39:9. https://doi.org/10.1016/j.aip.2012.06.001
Soh ES, Morris ME, McGinley JL (2011) Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 17:1–9. https://doi.org/10.1016/j.parkreldis.2010.08.012
Xing Y, Chen W, Wang Y et al (2016) Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res Bull 121:131–137
Chaudhuri KR, Prieto-Jurcynska C, Naidu Y et al (2010) The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord 25:704–709. https://doi.org/10.1002/mds.22868
Acknowledgements
The authors wish to thank all the patients and staff who participated in this study.
Funding
No.
Author information
Authors and Affiliations
Contributions
LW, TZ, and JY: proposed this study; LW, CS, and YW: collected and analyzed the data and drafted the manuscript; C-YN, JY, and SH: offered help; LC: helped revise the manuscript. All authors have approved the final version of the manuscript and attest that it has not been previously published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest relevant to this article.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Ll., Sun, Cj., Wang, Y. et al. Effects of dance therapy on non-motor symptoms in patients with Parkinson’s disease: a systematic review and meta-analysis. Aging Clin Exp Res 34, 1201–1208 (2022). https://doi.org/10.1007/s40520-021-02030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-02030-7