Abstract
Zinc (Zn) is an essential micronutrient of plants and other organisms and is involved in many cellular processes. Zn deficiency is defined as the insufficient Zn available for optimal growth and can lead to a sharp decline in crop yield and quality. About 30% of the world's soils have Zn deficiency. Zn efficiency can be defined as the ratio of grain yield or above-ground dry matter yield to the total Zn uptake under both Zn-deficient and Zn-sufficient conditions. The present review focuses on the potential roles of Zn in the maintenance of plant physiological process, its uptake and translocation, plant response to Zn deficiency with emphasis on wheat, and biofortification strategies to enhance the bioavailability of Zn to wheat grains which might help in addressing significant human nutrition problems related to Zn deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an important micronutrient of plants as it is involved in many cellular processes, including enzyme activation, protein synthesis, and membrane stability (Hansch & Mendel, 2009; Umair Hassan et al., 2020). Zn deficiency is defined as insufficient Zn available for optimal growth, can lead to a sharp decline in crop yield and quality. Zn deficiency has become a serious agricultural problem and is considered to be one of the most common micronutrient problems in crops (Assuncao et al., 2013), with 30% of the world's soils being Zn deficient (Alloway, 2009). Although, Zn is required in a small amount in the event of its insufficiency, plants will suffer physiological stress caused by the dysfunction of various enzyme systems and other metabolic functions in which Zn plays a significant role (Hafeez et al., 2013). By using fertilizers containing trace elements such as Zn, the quality and yield of crops are continuously improved. The lack of this element will lead to a decrease in plant photosynthesis, damage the RNA, and reduce the amount of carbohydrates and proteins which eventually affects the crop yield and quality (Mousavi et al., 2007).
Zn deficiency
Zn deficiency exists all over the world, and almost all crops show a positive response to the application of Zn (Welch, 2002). Normal soil mainly inherits its trace elements, including Zn, from rocks through geochemical and soil weathering processes. In addition to the mineral, the composition of the Zn in the soil also depends on the type, intensity, climate, and many other factors that dominate the formation of the soil (Nael et al., 2009). In calcareous soils, due to the high pH, the solubility of micronutrients is much lower, which reduces the ability of plants to absorb nutrients (Alloway, 2009). Excessive use of phosphate fertilizers in micronutrient deficient soils can also lead to micronutrient deficiencies in plants (Hafeez et al., 2013; Singh et al., 1988). As a result, the concentration of micronutrients in dry matter and crop yield will decrease. The possible reasons that limit the availability of Zn nutrient to plants include the following:
-
Due to the intense leaching of the soil, the total amount of Zn may be very low in the acidic soils (Toribio & Romanya, 2006).
-
The availability of Zn decreases with the increase of soil pH, due to reduced solubility of minerals along with greater absorption of Zn by colloidal soil particles, such as clay minerals, iron, and aluminum oxides, organic matter, and calcium carbonate (Mousavi et al., 2013).
-
Due to the restricted root development, the availability of Zn decreases as temperature and light intensity decrease (Marschner & Cakmak, 1989).
-
High levels of phosphorus in the soil reduce the availability of Zn to plants (Mousavi et al., 2012).
-
High concentrations of bicarbonate ion (HCO3−) prevent plant shoots from absorbing Zn (Gokhan, 2002).
-
The absorption of Zn by plants is inhibited by metal cations of copper and iron, because these elements share the same carriers in plant roots (Lequeux et al., 2010).
Characteristic symptoms of Zn deficiency include chlorosis of young leaves, necrotic patches on the older leaf blades, smaller leaves, dwarf stems wilting and curling of old leaves accompanied by extensive chlorosis and growth retardation (Cakmak, 2008; Lombn & Singh, 2003; Ova et al., 2015). Due to Zn deficiency, the area between the plant veins turns yellow (Vitosh et al., 1994). In dicotyledonous plants, the internode distance and leaf size reduce while in monocotyledonous plants, especially wheat, the whitish-brown necrotic patches are developed on leaf blades, predominantly in the middle and older leaves under Zn deficiency (Gokhan, 2002). Plants having a Zn concentration in their tissues of less than 20 ppm will suffer from Zn insufficiency (Mousavi, 2011). Various plants have different resistance to Zn deficiency. Plants such as wheat, beans, corn, onions, sorghum, rice, citrus fruits and grapes are most sensitive to Zn deficiency (Vitosh et al., 1994).
Zn efficiency
There is significant genetic variation within plant species in their ability to maintain significant growth and performance under Zn-deficient conditions; this is called Zn efficiency (Graham et al., 1993). The durum wheat varieties are more sensitive to Zn deficiency as compared to bread wheat (Cakmak et al., 1996a). Interest to conduct research to elucidate the physiological mechanism that confers Zn efficiency is increasing in the recent years. Many different wheat (Triticum aestivum) genotypes have been examined for their response to low Zn levels in Zn-deficient calcium soils and significant differences in Zn efficiency have been consistently found among certain wheat genotypes (Hacisalihoglu et al., 2001).
Zn toxicity
A higher concentration of Zn in soil is toxic to plants as it may result in various changes in plants including reduced plant growth (Nagajyoti et al., 2010). General symptoms of Zn toxicity in plants include stunted shoot growth, curling and rolling of young leaves, and death of leaf tip (Rout & Das, 2009). Increased concentration of Zn in chloroplast decreases NADPH production in plant. The activity of RUBP carboxylase and photosystem II is also reduced. Zn toxicity reduces ATP synthesis, chloroplast activity and photosynthesis (Reichman, 2002). In addition, it also hinders absorption of phosphorus and iron (Mousavi et al., 2013).
Physiological functions of Zn in plants
Zn is an essential micronutrient for plant and required in various enzymatic reactions, metabolic processes, and oxidation–reduction reactions. Therefore, it is highly important to maintain constant and continuous supply of Zn for proper growth and development of plants. Production of carbohydrate, protein, and chlorophyll content gets significantly reduced in Zn-deficient plants as it is an important component of various enzymes involved in various metabolic reactions. The total average concentration of Zn in cultivated soil is about 65 mgkg−1 (Alloway, 2009). It is mostly absorbed in the form of divalent cations (Zn2+), however, it may also be absorbed in the form of monovalent cations (ZnOH+) (Kochian, 1993). During the long-distance transportation through the xylem, Zn binds to organic acids or appears as free Zn2+, whereas during movement via phloem sap, it may form complex with low molecular weight organic solutes (Kochian, 1993). The metabolic function of Zn is based on its strong tendency to form tetrahedral complexes with N and O, especially with ligands, which play a role in enzymatic reactions (Vallee & Auld, 1990). The role of Zn in protein molecules involved in transcription and gene expression regulation has attracted lot of attention (Broadley et al., 2007). The metabolic changes caused by Zn deficiency are very complicated. However, some changes are typical and can be explained by the function of Zn in specific enzymatic reactions or steps in specific metabolic pathways.
Zn-containing enzymes
In higher plants, Zn is required for the activity of many enzymes (Table 1). It is estimated that more than 1200 types of proteins contain, bind, or transport Zn2+, including a large number of proteins that contain Zn fingers and transcription factors, oxidoreductases, and hydrolases, such as metalloproteases (Kramer & Clemens, 2005). Many Zn containing enzymes are involved in the regulation of different stages of central dogma (Kramer & Clemens, 2005). It is reported that about 4% of total proteins in Arabidopsis possess Zn finger motifs, which are active transcription factors and mediate protein–protein interactions (Kawagashira et al., 2001). The Zn-metalloproteinases in the cell organelles is required to destroy and cleave signal peptide (Stahl et al., 2002). In chloroplasts, the stromal processing peptidase depends on Zn for its activity (Richter & Lamppa, 2003). In addition, the photosystem II repair process in chloroplasts is performed by the light-damaged D1 protein which is degraded by Zn containing proteolytic enzymes (Bailey et al., 2002).
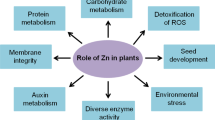
Physiological roles of Zn in plants
Zn is regulator or promoter of various physiological functions in plant viz protein synthesis, carbohydrate metabolisms, auxin synthesis, maintenance of membrane integrity and reproduction (Fig. 1).
Role of Zn in protein synthesis
Zn is a structural component of the ribosome and is essential for its structural integrity. In the absence of Zn, ribosomes dissemble and degrade, but can regenerate once the supply of Zn is restored (Jeganathan et al., 2015). The Zn deficiency in plants leads to a significant reduction in the protein synthesis due to reduced transcription and translation rates, which in turn causes an increase in the accumulation of amino acids (Cakmak et al., 1989). An increase in RNase activity causing RNA degradation in Zn deficient plants is also one of the reasons of reduced protein concentration (Broadley et al., 2012; Pandey et al., 2002).
Role of Zn in carbohydrate metabolism
Many Zn-dependent enzymes are usually involved in carbohydrate metabolism. The Zn deficiency in plants leads to a rapid reduction in the activity of carbonic anhydrase enzyme which significantly affects the carbohydrate metabolism. The activity of fructose 1,6-bisphosphatase involved in glycolysis also drops quite quickly, in the case of mild Zn deficiency (Mousavi et al., 2013). The Zn deficiency in the plant leaves leads to decreased rate of photosynthesis and an increase in sugar and starch accumulation (Mousavi, 2011).
Role of Zn in indole acetic acid synthesis
Tryptophan is the precursor to Indole-3-Acetic Acid (IAA) biosynthesis (Hull et al., 2000) and plants require Zn for the synthesis of tryptophan. In the leaves of Zn-deficient plants, the increase in tryptophan concentration occurs, as similar to that of other amino acids which is probably the result of protein synthesis inhibition. The most obvious symptoms of Zn deficiency may be related to disorders of auxin metabolism, especially IAA (Wang & Yang, 2021). The low concentration of IAA in Zn-deficient plants may be the result of inhibition of IAA synthesis or enhanced degradation (Brown et al., 1993). The biosynthesis of IAA via precursor molecule (tryptophan) does take place but it is degraded by reactive oxygen species (ROS) in the absence or deficiency of Zn (Brown et al., 1993). It is reported that not only IAA but also the level of gibberellin hormone is known to decline during Zn-deficiency (Sekimoto et al., 1997).
Role of Zn on membrane integrity and lipid peroxidation
Zn is necessary to maintain the integrity of biomembranes. It combines with the phospholipids and sulfhydryl groups of the membrane components or forms a tetrahedral complex with the cysteine residues of the polypeptide chain, thereby protecting membrane lipids and proteins from oxidative damage (Mogielnicka‐Brzozowska et al., 2015). It is reported that Zn- deficiency can cause peroxidative damage to the membranes due to the ROS. This explains the well-known phenomenon of increased leakage of organic and inorganic solutes from root cells (Brown et al., 1993). Therefore, the main role of Zn in membranes is to protect membrane lipids and proteins from peroxidation.
Role of Zn in plant reproduction
It is well known that Zn deficiency can severely inhibit flowering and seed production (Pandey et al., 2006). The lower seed yield during Zn deficiency can be attributed to (1) the formation of more abscisic acid in plants, leading to premature shedding of leaves and flower buds; (2) changes in the development and physiology of anthers and pollen grains. Pandey et al (2009) reported decreased dry matter especially seed yield in black gram (Vigna mungo) cv. IPU 94 grown in sand culture with poor Zn nutrition. These plants also showed reduction in the size of anthers and stigma heads, pollen production capacity of anthers, and stigma exudates. Zn deficiency hinders the germination of pollen grains and the growth of pollen tubes. Decrease in the number of pods, seeds per pod and seed quality, changes the topography of the seed coat, and reduced rate of seed germination was also observed. Chen and Ludewig (2018) reported that the main regulator of flowering FLOWERING LOCUS T (FT) expression was suppressed in Arabidopsis thaliana grown under Zn deficiency conditions. FT inhibition increases rosette diameter by delaying the transition to flowering, resulting in longer leaf growth, and a greater number of leaves.
Adaptive response of wheat to Zn deficiency
Apparently, the crop plants evolve several adaptive mechanisms to survive the abiotic stresses caused by various soil factors, such as insufficient or excessive amounts of minerals or metal ions. Zn deficiency is one of the most common limiting factors in crop production, and many plant species, such as beans, corn, wheat, rice, and tomatoes, are considered to be less tolerant to Zn deficiency (Hacisalihoglu & Kochian, 2003). Several plant species exhibit significant intraspecific variability in Zn efficiency, which appears to be under genetic control (Graham & Rengel, 1993). Zn deficiency in wheat (Triticum aestivum L.) occurs in various parts of the world, and the growth capacity of wheat genotypes in Zn-deficient soils shows great diversity (Hacisalihoglu et al., 2001). Several mechanisms have been proposed to explain the efficiency of Zn in crop plants, including increased Zn absorption, increased bioavailability of Zn in the rhizosphere due to the release of root exudates, and more internal utilization (Hacisalihoglu et al., 2001; Singh et al., 2005). Different adaptive strategies for overcoming Zn deficiency have been reported in wheat (Table 2).
Modification in root processes increases the bioavailability of soil Zn
Root structure may vary slightly among the cultivars of wheat, and affects plant Zn availability and Zn efficiency (Kutman et al., 2012). Due to complete exploration of the soil, finer roots with a larger root surface can increase the availability of Zn and other nutrients (Singh et al., 2005). Root-mediated chemical changes in the rhizosphere of wheat change the pH of the rhizosphere (towards acidic) due to the release of organic components from the roots or the activity of soil microbiome that can chelate Zn and increase its availability. It was also observed that with the increase in soil pH, the availability of Zn to plants decreases (Khoshgoftarmanesh et al., 2018). Therefore, root-mediated processes reduce the pH of the rhizosphere which increases the availability of plant Zn by dissolving Zn from the solid phase of organic and inorganic soils (Fig. 2).
The Zn-deficiency in soil stimulates secretion of phytosiderophores from the roots, which together with the secretions from soil microbiome, change the pH of the rhizosphere towards acidic, thereby increasing the bioavailability of Zn2+ to the roots of wheat (conceived from Cakmak et al., 1996b; Khoshgoftarmanesh et al., 2018)
Zn deficiency induces release of phytosiderophores from the roots and this increased amount of phytosiderophores promotes the movement of Zn which enhances the efficiency of Zn in plants. Therefore, it can also improve the fluidity of Zn in plants. The sensitivity of crops to Zn deficiency is negatively correlated with the ability of genotypes to release phytosiderophore from roots under Zn deficiency conditions. Cakmak et al (1996b) observed greater release of phytosiderophore (2′- deoxymugineic acid) from the roots and increased Zn efficiency in the bread wheat genotypes as compared to durum wheat genotypes.
Increased root absorption and the translocation of Zn to above-ground parts
Zn first diffuses into the free space of the root cell wall and then passes through the plasma membrane with the help of ion transporters. Zn is absorbed mainly by plant roots in the form of Zn2+. There are at least four different transporter families that can promote Zn transport in wheat including:
ZIP (Zrt- and Irt-related proteins) transporters
The ZIP transporter proteins have eight potential transmembrane domains, where both the ends (amino and carboxy ends) are present in the outer surface of the plasma membrane (Fig. 3a). ZIP proteins range from 309 to 476 amino acids in length (Guerinot, 2000). Li et al. (2019) identified a total of 58 ZIP genes in wheat (TaZIP) through genome wide association studies (GWAS). Durmaz et al. (2011) reported expression of ZIP protein coding genes in the roots and shoots of various germplasm materials maintained at different concentrations of Zn. They also identified and cloned a full-length ZIP1 carrier protein, named TdZIP1, and further analyzed the structural properties of the corresponding protein sequence. Authors observed that over-expression of ZIP transporter resulted in the excessive accumulation of Zn in the cell thereby creating a toxic environment.
CDF (Cation Diffusion Facilitator) transporters
Plant CDF is commonly referred to as MTP (metal tolerance protein). According to early phylogenetic analysis, the CDF family is divided into three categories based on the specificity of metal ions: (1) CDF that carries Mn2+, (2) CDF that carries Fe2+and Zn2+, and (3) CDF that carries Zn2+ and other metal ions, except Fe2+ or Mn2+ (Montanini et al., 2007). The CDF transporters have six transmembrane domains and both the terminals of the polypeptide chain are present on the cytoplasmic side (Kolaj-Robin et al., 2015). A histidine-rich interconnecting loop (IL2) is present between the 4th and 5th transmembrane domains (Fig. 3b).
Vatansever et al. (2017) identified and characterized genes for a total of 20 MTPs in wheat and named them as a series of TaMTP. All the TaMTPs showed characteristic structure of CDF proteins and most of them contained a Zn transporter dimerization domain. They showed that MTPs were mostly expressed in the aleurone layer of the grain, which is well known as a reservoir for nutrients like Fe and Zn.
P-type ATPase (metal transporting ATPases)
The P-type ATPases may have six to twelve transmembrane domains and three cytoplasmic domains called the N-domain (nucleotide binding), P-domain (phosphorylation) and A-domain (actuator) that confers the ATP hydrolyzing activity (Bublitz et al., 2010) (Fig. 3c). Moradi and Mandoulakani (2020) studied the expression pattern of P-type ATPase genes (HMA1, HMA2 and HMA9) in Zn-efficient and Zn- inefficient bread wheat cultivars under Zn deficient and sufficient conditions and reported many folds higher expression of these genes in Zn-efficient wheat cultivar grown under Zn deficient conditions.
NRAMP (natural resistance-associated macrophage proteins)
The transporter protein has 12 transmembrane domains where N and C-termini are located intracellularly, and a loop between 7th and 8th domains is extracellular (Nevo & Nelson, 2006) (Fig. 3d). Peng et al. (2018) identified a NRAMP transporter (TpNRAMP3) in Polish wheat (Triticum polonicum L.) and reported that the expression of the transporter was high in the leaf blades and roots during the jointing and booting stages.
Approaches to enhance Zn biofortification in wheat
Wheat is an important source of daily food intake for the people, but its Zn bioavailability is low. Agronomical or genetic/molecular breeding methods could help to develop micronutrient-intensive wheat genotypes to increase the Zn concentration in wheat. The biofortification of Zn in wheat can be achieved by the following approaches:
Agronomic approaches for Zn biofortification
Agronomic methods involve fertilizer application strategies and it is a quick solution to reduce Zn deficiency and increase Zn concentration in grains (Cakmak & Kutman, 2018). This approach is most effective when Zn fertilizers are used in combination with nitrogen fertilizers, organic fertilizers and improved varieties (De Valenca et al., 2017). The application of organic / inorganic Zn fertilizers can increase the Zn content of wheat grains (Table 3). However, the biofortification of Zn through agronomic approaches is a non-ecofriendly approach as only about 20% of the supplied Zn is absorbed by the plants and the rest remains adsorbed on the soil particles (Gupta et al., 2021).
Breeding approaches for Zn biofortification
Biofortification of Zn through conventional cross-breeding is a cost-effective and sustainable method for delivering micronutrients in food (Stein et al., 2007). Plant breeders have crossed Zn efficient landraces/ varieties of wheat to generate wheat varieties with enhanced Zn grain content (Table 4). Recently, with the technical support of CIMMYT in Mexico, the Bangladesh Wheat and Maize Research Institute (BWMRI) has launched a promising new Zn biofortified wheat variety "BARI Gom 33", which is the result of a conventional breeding program. It also possesses resistance towards the wheat blast caused by Magnaporthe oryzae. This variety can help millions of people in South Asia including India (Das et al., 2019). The biofortified wheat varieties with high Zn content which have been recently released in India are summarized in Table 5.
Development and dissemination of agronomical qualified wheat varieties with high grain zinc content is a major objective under wheat biofortification breeding program of HarvestPlus and its South Asian program partners. In India, through public–private partnerships (PPPS), seed sector is playing important role in expediting the distribution and commercialization of biofortified wheat varieties so that it can reach and benefit millions of farmers. For example, in Bihar, state government is promoting production and consumption of biofortified wheat varieties like Rajendra-Gehu-3, developed by the Rajendra Prasad Central Agricultural University, and BHU31 and BHU-25 launched by seed companies (https://www.pwc.in/assets/pdfs/consulting/mc/as/integrating-nutrient-rich-crops-into-foodsystems-a-tool-of-change.pdf). Rajendra-Gehu-3 contains 38 ppm of zinc which is the target level of zinc content for biofortified zinc wheat varieties with nutrition and health benefit when consumed regularly. In India, zinc wheat is currently grown by 4,42,078 farming households overall, with ~ 2.1 million household members benefiting. It is expected that in the next few years zinc wheat shall reach more than one million farming households in Bihar (https://www.harvestplus.org/knowledge-market/in-the-news/zinc-wheat-events-bihar-attracthundreds-farmers) and https://www.harvestplus.org/knowledge-market/in-the-news/bihar-stateindia-promote-biofortified-crops-address-nutrition-security. HarvestPlus in collaboration with Banaras Hindu University, Varanasi distributed seed of zinc biofortified wheat varieties to thousands of farmers. During the year 2015–2016, more than 50,000 farmers adopted zinc-biofortified wheat varieties through PPPs. Varieties like “Zinc Shakthi” showed promising performance and benefitted thousands of farmers (https://www.cimmyt.org/news/farmers-in-india-embrace-highzinc-wheat-for-its-nutritional-benefits/).
Transgenic approaches for Zn biofortification
The availability of enormous sequence data in wheat and model cereal crop species like rice and barley provides an opportunity to identify and utilize potential homologs and candidate genes (with putative Fe and Zn transporter function) for improving Zn and Fe biofortification in wheat. In rice, over-expression of genes involved in pathway for Fe and Zn transport showed increased bioavailability of Fe without affecting yield. Lee et al., (2009) showed that over-expression of NA synthase (NAS) led to threefold increases in Fe and Zn content in paddy grown grain. Moreover, when anaemic mice were served, with these grains, hemoglobin and haematocrit were recovered to normal levels within two weeks. Zn content in barley has been improved by overexpressing Arobdopsis Zn transporters (Ramesh et al., 2004). In order to increase the bioavailability of iron and Zn, phytase activity was increased in barley seeds by expression of phytase gene i.e. HvPAPhy_a (Holme et al., 2012). These studies strengthen the view that grain Fe and Zn can be modified in wheat through transgenic approaches. However, in wheat transformation efficiency is still a major bottleneck due to which transformation to any elite wheat cultivar is still in its infancy thus limiting proper utilization of elite lines in breeding programmes.
Role of phytic acid hindering the bioavailability of Zn
Most of the phosphorus present in the soil is in organic form, and the main component of soil organic phosphorus is phytic acid (PA), which is mainly composed of inositol penta and hexaphosphate (Dalai, 1977). Due to its negative charge, PA can chelate various mineral cations (Zn2+, Fe2+, Mg2+, Ca2+) present in the soil and make it unavailable for absorption by the plants. Most plant species cannot use soil PA as a source of phosphorus because they lack extracellular phytase, an enzyme that releases inorganic phosphate from PA (Fig. 4). Phytase is produced by many microorganisms, including bacteria, yeasts, and fungi (Eida et al., 2012). The microbial degradation of PA is presumed to occur in the soil because the microorganisms that produce phytase are widely distributed in various soils (Hayatsu, 2013; Richardson, 2001).
In cereal crops, 85% of total phosphorus is stored in seeds and other plant organs in the form of phytic acid (Cominelli et al., 2020; Perera et al., 2018). The remaining 15% of phosphorus in living cells is stored in the form of soluble inorganic phosphate, used for multiple functions, including regulation of different cells signals and process plants (Sparvoli & Cominelli, 2015). PA readily chelate Zn2+ which generate Zn phytate and hinders with its bioavailability (Perera et al., 2018). Hence, even after biofortification of Zn in wheat grains, it may remain unavailable to humans (Gupta et al., 2021). However, PA has some beneficial health aspects as it may act as anti-oxidant, anti-inflammatory, and anti-cancer molecules (Doria et al., 2009; Jenab & Thompson, 1998). Hence, the biofortification strategies need to focus on developing Zn biofortified wheat varieties with balanced PA, to avoid compromising its beneficial effects. The United States Department of Agriculture—Agricultural Research Service (USDA-ARS) in collaboration with the University of Nebraska, has released eight wheat varieties (N16MD9012, N16MD9140, N16MD9275, N16MD9268, N16MD9046, N16MD9204, N16MD9074 and N16MD9153) with low PA and high micronutrient content. Some of these varieties also possess resistance to leaf, stem and stripe rust diseases (Venegas et al., 2018).
Conclusion
The research on Zn deficiency stress in wheat has a long history. Some recent findings provided some information about the physiological basis of Zn efficiency. The possible mechanisms have been studied, including root absorption and translocation of Zn, chelation in leaves, and biochemical utilization of Zn; however, there are still many questions about the Zn efficiency mechanism which needs to be answered in near future. Root uptake of Zn is generally considered to be one of the key components of Zn efficiency. However, it is not adequately evident by the literature and we still do not fully understand this association. More information is needed on the exact role of biochemical Zn using different components. With in-depth research, our understanding and cognition of the basis of the Zn efficiency mechanism will definitely need to be revised.
The Zn deficiency in wheat is generally marked by enhanced accumulation of ROS, dehydroascorbate, glutathione-sulfhydryl, and glutathione-disulphide, and some other phytochemicals. The physiological responses to Zn deficiency include enhanced production of ROS scavenging enzymes, release of excessive phytosiderophores from roots, elevated production and expressions of Zn transporters, and higher accumulation of other macro and micronutrients. The problem of Zn bioavailability in wheat can be addressed by using novel biofortification approaches. A great deal of knowledge on biofortification and bioavailability of Zn has been generated till date. Some wheat varieties have also been successfully developed to increase the Zn content of grains. However, the available information is not fully utilized in breeding programs. Since the sequences of several genes involved in wheat biofortification are now known (Gupta et al., 2021), these genes can be used to develop new transgenic wheat varieties with enhanced Zn efficiency.
References
Abreu, I. A., & Cabelli, D. E. (2010). Superoxide dismutases—a review of the metal-associated mechanistic variations. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1804(2), 263–274.
Ahmad, R., Kim, Y. H., Kim, M. D., Kwon, S. Y., Cho, K., Lee, H. S., & Kwak, S. S. (2010). Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses. Physiologia Plantarum, 138(4), 520–533.
Alloway, B. J. (2009). Soil factors associated with Zn deficiency in crops and humans. Environmental Geochemistry and Health, 31(5), 537–548.
Assuncao, A. G., Persson, D. P., Husted, S., Schjørring, J. K., Alexander, R. D., & Aarts, M. G. (2013). Model of how plants sense Zn deficiency. Metallomics, 5(9), 1110–1116.
Auld, D. S., & Bergman, T. (2008). Medium-and short-chain dehydrogenase/reductase gene and protein families: The role of Zn for alcohol dehydrogenase structure and function. Cellular and Molecular Life Sciences: CMLS, 65(24), 3961–3970.
Badger, M. R., & Price, G. D. (1994). The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Biology, 45(1), 369–392.
Bailey, S., Thompson, E., Nixon, P. J., Horton, P., Mullineaux, C. W., Robinson, C., & Mann, N. H. (2002). A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo. Journal of Biological Chemistry, 277(3), 2006–2011.
Bassi, D., Kaur, K., Singh, T. P., & Kaur, J. (2021). Quality attributes and Chapatti making property of biofortified wheat as influenced by particle size. Journal of Food Science and Technology, 58(3), 1156–1164.
Bhat, F. A., Ganai, B. A., & Uqab, B. (2017). Carbonic anhydrase: Mechanism, structure and importance in higher plants. Asian J Plant Sci Res, 7(3), 17–23.
Broadley, M., Brown, P., Cakmak, I., Rengel, Z., & Zhao, F. (2012). Function of nutrients: micronutrients. In P. Marschner (Ed.), Marschner’s mineral nutrition of higher plants (3rd ed., pp. 191–248). Academic Press.
Broadley, M. R., White, P. J., Hammond, J. P., Zelko, I., & Lux, A. (2007). Zn in Plants. New Phytologist, 173(4), 677–702.
Brown, P. H., Cakmak, I., & Zhang, Q. (1993). Form and function of zinc plants. In A. D. Robson (Ed.), Zinc in soils and plants. Developments in plant and soil sciences (pp. 93–106). Springer.
Bublitz, M., Poulsen, H., Morth, J. P., & Nissen, P. (2010). In and out of the cation pumps: P-type ATPase structure revisited. Current Opinion in Structural Biology, 20(4), 431–439.
Burnell, J. N., & Hatch, M. D. (1988). Low bundle sheath carbonic anhydrase is apparently essential for effective C4 pathway operation. Plant Physiology, 86(4), 1252–1256.
Cakmak, I., (2008). Zn deficiency in wheat in Turkey. In Micronutrient deficiencies in global crop production (pp. 181–200). Springer.
Cakmak, I. (2000). Tansley Review No. 111: possible roles of Zn in protecting plant cells from damage by reactive oxygen species. New Phytologist, 146(2), 185–205.
Cakmak, I., & Kutman, U. Á. (2018). Agronomic biofortification of cereals with Zn: A review. European Journal of Soil Science, 69(1), 172–180.
Cakmak, I., Marschner, H., & Bangerth, F. (1989). Effect of Zn nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.). Journal of Experimental Botany, 40(3), 405–412.
Cakmak, I., Sari, N., Marschner, H., Ekiz, H., Kalayci, M., Yilmaz, A., & Braun, H. J. (1996b). Phytosiderophore release in bread and durum wheat genotypes differing in Zn efficiency. Plant and Soil, 180(2), 183–189.
Cakmak, I., Yilmaz, A., Kalayci, M., Ekiz, H., Torun, B., Ereno, B., & Braun, H. J. (1996a). Zn deficiency as a critical problem in wheat production in Central Anatolia. Plant and Soil, 180(2), 165–172.
Candan, N., Cakmak, I., & Ozturk, L. (2018). Zn-biofortified seeds improved seedling growth under Zn deficiency and drought stress in durum wheat. Journal of Plant Nutrition and Soil Science, 181(3), 388–395.
Che, Y., Zhang, N., Zhu, X., Li, S., Wang, S., & Si, H. (2020). Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Scientia Horticulturae, 261, 108949.
Chen, X., & Ludewig, U. (2018). Biomass increase under Zn deficiency caused by delay of early flowering in Arabidopsis. Journal of Experimental Botany, 69(5), 1269–1279.
Cominelli, E., Pilu, R., & Sparvoli, F. (2020). Phytic acid and transporters: What can we learn from low phytic acid mutants? Plants, 9(1), 69.
Crespo-Herrera, L. A., Govindan, V., Stangoulis, J., Hao, Y., & Singh, R. P. (2017). QTL mapping of grain Zn and Fe concentrations in two hexaploid wheat RIL populations with ample transgressive segregation. Frontiers in Plant Science, 8, 1800.
Dalai, R. C. (1977). Soil Organic Phosphorus. Advances in Agronomy, 29, 83–117.
Das, S., Chaki, A. K., & Hossain, A. (2019). Breeding and agronomic approaches for the biofortification of Zn in wheat (Triticum aestivum L.) to combat Zn deficiency in millions of a population: a Bangladesh perspective. Acta Agrobotanica, 72(2), 1–13.
De Valenca, A. W., Bake, A., Brouwer, I. D., & Giller, K. E. (2017). Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Global Food Security, 12, 8–14.
Doria, E., Galleschi, L., Calucci, L., Pinzino, C., Pilu, R., Cassani, E., & Nielsen, E. (2009). Phytic acid prevents oxidative stress in seeds: evidence from a maize (Zea mays L.) low phytic acid mutant. Journal of Experimental Botany, 60(3), 967–978.
Durmaz, E., Coruh, C., Dinler, G., Grusak, M. A., Peleg, Z., Saranga, Y., Fahima, T., Yazici, A., Ozturk, L., Cakmak, I., & Budak, H. (2011). Expression and cellular localization of ZIP1 transporter under Zn deficiency in wild emmer wheat. Plant Molecular Biology Reporter, 29(3), 582–596.
Eida, M. F., Nagaoka, T., Wasaki, J., & Kouno, K. (2012). Phytate degradation by fungi and bacteria that inhabit sawdust and coffee residue composts. Microbes and Environments, p.ME12083.
Genc, Y., Verbyla, A. P., Torun, A. A., Cakmak, I., Willsmore, K., Wallwork, H., & McDonald, G. K. (2009). Quantitative trait loci analysis of Zn efficiency and grain Zn concentration in wheat using whole genome average interval mapping. Plant and Soil, 314(1), 49–66.
Gokhan, H. (2002). Physiological and biochemical mechanisms underlying Zn efficiency in monocot and dicot plants. Plant Physiology, 131, 595–602.
Graham, R. D., Ascher, J. S., & Hynes, S. C., 1993. Selecting Zn-efficient cereal genotypes for soils of low Zn status. In Genetic aspects of plant mineral nutrition (pp. 349–358). Springer, Dordrecht.
Graham, R.D. & Rengel, Z. (1993). Genotypic variation in Zn uptake and utilization by plants. In Zn in soils and plants (pp. 107–118). Springer, Dordrecht.
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochimica et Biophysica Acta (BBA)- Biomembranes, 1465(1–2), 190–198.
Gupta, P. K., Balyan, H. S., Sharma, S., & Kumar, R. (2021). Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theoretical and Applied Genetics, 134(1), 1–35.
Hacisalihoglu, G., Hart, J. J., & Kochian, L. V. (2001). High-and low-affinity Zn transport systems and their possible role in Zn efficiency in bread wheat. Plant Physiology, 125(1), 456–463.
Hacisalihoglu, G., & Kochian, L. V. (2003). How do some plants tolerate low levels of soil Zn? Mechanisms of Zn efficiency in crop plants. New Phytologist, 159(2), 341–350.
Hafeez, B. M. K. Y., Khanif, Y. M., & Saleem, M. (2013). Role of Zn in plant nutrition-a review. Journal of Experimental Agriculture International, 3, 374–391.
Hansch, R., & Mendel, R. R. (2009). Physiological functions of mineral micronutrients (cu, Zn, Mn, Fe, Ni, Mo, B, cl). Current Opinion in Plant Biology, 12(3), 259–266.
Hao, Y., Velu, G., Peña, R. J., Singh, S., & Singh, R. P. (2014). Genetic loci associated with high grain Zn concentration and pleiotropic effect on kernel weight in wheat (Triticum aestivum L.). Molecular breeding, 34(4), 1893–1902.
Hayatsu, M. (2013). Utilization of phytic acid by cooperative interaction in rhizosphere. Microbes and Environments, 28(1), 1–2.
Holme, I. B., Dionisio, G., Brinch-Pedersen, H., Wendt, T., Madsen, C. K., Vincze, E., & Holm, P. B. (2012). Cisgenic barley with improved phytase activity. Plant Biotechnology Journal., 10(2), 237–47.
Hull, A. K., Vij, R., & Celenza, J. L. (2000). Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proceedings of the National Academy of Sciences, 97(5), 2379–2384.
Imtiaz, M., Alloway, B. J., Khan, P., Memon, M. Y., Siddiqui, S. U. H., Aslam, M., & Shah, S. K. H. (2006). Zn deficiency in selected cultivars of wheat and barley as tested in solution culture. Communications in Soil Science and Plant Analysis, 37(11–12), 1703–1721.
Jeganathan, A., Razi, A., Thurlow, B., & Ortega, J. (2015). The C-terminal helix in the YjeQZn- finger domain catalyzes the release of RbfA during 30S ribosome subunit assembly. RNA, 21(6), 1203–1216.
Jenab, M., & Thompson, L. U. (1998). The influence of phytic acid in wheat bran on early biomarkers of colon carcinogenesis. Carcinogenesis, 19(6), 1087–1092.
Jin, Y., Richards, N. G., Waltho, J. P., & Blackburn, G. M. (2017). Metal fluorides as analogues for studies on phosphoryl transfer enzymes. AngewandteChemie International Edition, 56(15), 4110–4128.
Kawagashira, N., Ohtomo, Y., Murakami, K., Matsubara, K., Kawai, J., Carninci, P., Hayashizaki, Y., Kikuchi, S., & Higo, K. (2001). Multiple Zn finger motifs with comparison of plant and insect. Genome Informatics, 12, 368–369.
Khande, R., Sharma, S. K., Ramesh, A., & Sharma, M. P. (2017). Zn solubilizing Bacillus strains that modulate growth, yield and Zn biofortification of soybean and wheat. Rhizosphere, 4, 126–138.
Khoshgoftarmanesh, A. H., Afyuni, M., Norouzi, M., Ghiasi, S., & Schulin, R. (2018). Fractionation and bioavailability of Zn (Zn) in the rhizosphere of two wheat cultivars with different Zn deficiency tolerance. Geoderma, 309, 1–6.
Khoshgoftarmanesh, A. H., Shariatmadari, H., Karimian, N., Kalbasi, M., & Khajehpour, M. R. (2005). Zn efficiency of wheat cultivars grown on a saline calcareous soil. Journal of Plant Nutrition, 27(11), 1953–1962.
Kochian, L. V. (1993). Zn absorption from hydroponic solutions by plant roots. In Zn in soils and plants (pp. 45–57). Springer.
Kolaj-Robin, O., Russell, D., Hayes, K. A., Pembroke, J. T., & Soulimane, T. (2015). Cation diffusion facilitator family: Structure and function. FEBS Letters, 589(12), 1283–1295.
Kramer, U. & Clemens, S. (2005). Functions and homeostasis of Zn, copper, and nickel in plants. In Molecular biology of metal homeostasis and detoxification (pp. 215–271). Springer.
Krishnappa, G., Rathan, N. D., Sehgal, D., Ahlawat, A. K., Singh, S. K., Singh, S. K., Shukla, R. B., Jaiswal, J. P., Solanki, I. S., Singh, G. P., & Singh, A. M. (2021). Identification of novel genomic regions for biofortification traits using an SNP marker-enriched linkage map in wheat (Triticum aestivum L.). Frontiers in Nutrition, 8.
Krishnappa, G., Singh, A. M., Chaudhary, S., Ahlawat, A. K., Singh, S. K., Shukla, R. B., Jaiswal, J. P., Singh, G. P., & Solanki, I. S. (2017). Molecular mapping of the grain iron and Zn concentration, protein content and thousand kernel weight in wheat (Triticum aestivum L). PLoS One, 12(4), k.
Kutman, U. B., Kutman, B. Y., Ceylan, Y., Ova, E. A., & Cakmak, I. (2012). Contributions of root uptake and remobilization to grain Zn accumulation in wheat depending on post-anthesis Zn availability and nitrogen nutrition. Plant and Soil, 361(1), 177–187.
Lee, S., Jeon, U. S., Lee, S. J., Kim, Y. K., Persson, D. P., Husted, S., Schjørring, J. K., Kakei, Y., Masuda, H., Nishizawa, N. K., & An, G. (2009). Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proceedings of the National Academy of Sciences, 106(51), 22014–22019.
Lequeux, H., Hermans, C., Lutts, S., & Verbruggen, N. (2010). Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiology and Biochemistry, 48(8), 673–682.
Li, S., Liu, Z., Guo, L., Li, H., Nie, X., Chai, S., & Zheng, W. (2019). Genome-wide identification of ZIP gene family members in wheat. Research Square, pp. 1–23.
Liu, J., Wu, B., Singh, R. P., & Velu, G. (2019). QTL mapping for micronutrients concentration and yield component traits in a hexaploid wheat mapping population. Journal of Cereal Science, 88, 57–64.
Lombn, S. P., & Singh, B. R. (2003). Varietal tolerance to Zn deficiency in wheat and barley grown in chelatorbuffered nutrient solution and its effect on uptake of Cu, Fe, and Mn. Journal of Plant Nutrition and Soil Science, 166(1), 76–83.
Marschner, H., & Cakmak, I. (1989). High light intensity enhances chlorosis and necrosis in leaves of Zn, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. Journal of Plant Physiology, 134(3), 308–315.
McCall, K. A., Huang, C. C., & Fierke, C. A. (2000). Function and mechanism of zinc metalloenzymes. The Journal of Nutrition, 130(5), 1437S-1446S.
Mogielnicka-Brzozowska, M., Strzeżek, R., Wasilewska, K., & Kordan, W. (2015). Prostasomes of canine seminal plasma–Zn-binding ability and effects on motility characteristics and plasma membrane integrity of spermatozoa. Reproduction in Domestic Animals, 50(3), 484–491.
Montanini, B., Blaudez, D., Jeandroz, S., Sanders, D., & Chalot, M. (2007). Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genomics, 8(1), 1–16.
Moradi, K., & Abdollahi Mandoulakani, B. (2020). Expression pattern of HMA1, HMA2 and HMA9 genes under Zn deficiency conditions in bread wheat cultivars with different Zn uptake efficiency. Cereal Research, 9(4), 347–357.
Mousavi, S. R. (2011). Zn in crop production and interaction with phosphorus. Australian Journal of Basic and Applied Sciences, 5(9), 1503–1509.
Mousavi, S. R., Galavi, M., & Ahmadvand, G. (2007). Effect of Zn and manganese foliar application on yield, quality and enrichment on potato (Solanum tuberosum L). Asian Journal of Plant Sciences, 6(8), 1256–1260.
Mousavi, S. R., Galavi, M., & Rezaei, M. (2012). The interaction of Zn with other elements in plants: A review. International Journal of Agriculture and Crop Sciences, 4(24), 1881–1884.
Mousavi, S. R., Galavi, M., & Rezaei, M. (2013). Zn (Zn) importance for crop production—a review. International Journal of Agronomy and Plant Production, 4(1), 64–68.
Nael, M., Khademi, H., Jalalian, A., Schulin, R., Kalbasi, M., & Sotohian, F. (2009). Effect of geo- pedological conditions on the distribution and chemical speciation of selected trace elements in forest soils of western Alborz. Iran. Geoderma, 152(1–2), 157–170.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8(3), 199–216.
Nevo, Y., & Nelson, N. (2006). The NRAMP family of metal-ion transporters. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1763(7), 609–620.
Ova, E. A., Kutman, U. B., Ozturk, L., & Cakmak, I. (2015). High phosphorus supply reduced Zn concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant and Soil, 393(1), 147–162.
Pandey, N., Pathak, G. C., & Sharma, C. P. (2006). Zn is critically required for pollen function and fertilisation in lentil. Journal of Trace Elements in Medicine and Biology, 20(2), 89–96.
Pandey, N., Pathak, G. C., & Sharma, C. P. (2009). Impairment in reproductive development is a major factor limiting yield of black gram under Zn deficiency. Biologia Plantarum, 53(4), 723.
Pandey, N., Pathak, G. C., Singh, A. K., & Sharma, C. P. (2002). Enzymic changes in response to Zn nutrition. Journal of Plant Physiology, 159(10), 1151–1153.
Peng, F., Wang, C., Cheng, Y., Kang, H., Fan, X., Sha, L., Zhang, H., Zeng, J., Zhou, Y., & Wang, Y. (2018). Cloning and characterization of TpNRAMP3, a metal transporter from polish wheat (Triticum polonicum L.). Frontiers in Plant Science, 9, 1354.
Perera, I., Seneweera, S., & Hirotsu, N. (2018). Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice, 11(1), 1–13.
Ram, H., Rashid, A., Zhang, W., Duarte, A. Á., Phattarakul, N., Simunji, S., Kalayci, M., Freitas, R., Rerkasem, B., Bal, R. S., & Mahmood, K. (2016). Biofortification of wheat, rice and common bean by applying foliar Zn fertilizer along with pesticides in seven countries. Plant and Soil, 403(1), 389–401.
Ramesh, S. A., Choimes, S., & Schachtman, D. P. (2004). Over-Expression of an Arabidopsis Zinc Transporter in Hordeum Vulgare Increases Short-Term Zinc Uptake after Zinc Deprivation and Seed Zinc Content. Plant Molecular Biology, 54, 373–385.
Rana, A., Joshi, M., Prasanna, R., Shivay, Y. S., & Nain, L. (2012). Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. European Journal of Soil Biology, 50, 118–126.
Rathan, N. D., Sehgal, D., Thiyagarajan, K., Singh, R. P., Singh, A. M., & Velu, G. (2021). Identification of genetic loci and candidate genes related to grain Zn and iron concentration using a Zn-enriched wheat ‘Zn-Shakti’.
Reichman, S. M. (2002). The responses of plants to metal toxicity: A review forusing on copper, manganese & Zn (pp. 22–26). Australian Minerals & Energy Environment Foundation.
Richardson, A. E. (2001). Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Functional Plant Biology, 28(9), 897–906.
Richter, S., & Lamppa, G. K. (2003). Structural properties of the chloroplast stromal processing peptidase required for its function in transit peptide removal. Journal of Biological Chemistry, 278(41), 39497–39502.
Rout, G. R. & Das, P. (2009). Effect of metal toxicity on plant growth and metabolism: I. Zn. Sustainable agriculture, pp.873–884.
Sekimoto, H., Hoshi, M., Yokota, T., & Nomura, T. (1997). Zn efficiency of dwarf Zea mays treated with a gibberellin-biosynthesis inhibitor. In Plant nutrition for sustainable food production and environment (pp. 263–264). Springer.
Sharma, P. N., Kumar, P., & Tewari, R. K. (2004). Early signs of oxidative stress in wheat plants subjected to Zn deficiency. Journal of Plant Nutrition, 27(3), 451–463.
Singh, B., Natesan, S. K. A., Singh, B. K., & Usha, K., (2005). Improving Zn efficiency of cereals under Zn deficiency. Current Science, pp.36–44.
Singh, R., Govindan, V., & Andersson, M. S. (2017). Zn-biofortified wheat: harnessing genetic diversity for improved nutritional quality (No. 2187–2019–666).
Singh, J. P., Karamanos, R. E., & Stewart, J. W. B. (1988). The mechanism of phosphorus-induced Zn deficiency in bean (Phaseolus vulgaris L.). Canadian Journal of Soil Science, 68(2), 345–358.
Sparvoli, F., & Cominelli, E. (2015). Seed biofortification and phytic acid reduction: A conflict of interest for the plant? Plants, 4(4), 728–755.
Stahl, A., Moberg, P., Ytterberg, J., Panfilov, O., Von Lowenhielm, H. B., Nilsson, F., & Glaser, E. (2002). Isolation and identification of a novel mitochondrial metalloprotease (PreP) that degrades targeting presequences in plants. Journal of Biological Chemistry, 277(44), 41931–41939.
Stein, A. J., Nestel, P., Meenakshi, J. V., Qaim, M., Sachdev, H. P. S., & Bhutta, Z. A. (2007). Plant breeding to control Zn deficiency in India: How cost-effective is biofortification? Public Health Nutrition, 10(5), 492–501.
Takáč, T., Novák, D., & Šamaj, J. (2019). Recent advances in the cellular and developmental biology of phospholipases in plants. Frontiers in Plant Science, 10, 362.
Tiwari, C., Wallwork, H., Arun, B., Mishra, V. K., Velu, G., Stangoulis, J., Kumar, U., & Joshi, A. K. (2016). Molecular mapping of quantitative trait loci for Zn, iron and protein content in the grains of hexaploid wheat. Euphytica, 207(3), 563–570.
Tiwari, V. K., Rawat, N., Chhuneja, P., Neelam, K., Aggarwal, R., Randhawa, G. S., Dhaliwal, H. S., Keller, B., & Singh, K. (2009). Mapping of quantitative trait loci for grain iron and Zn concentration in diploid A genome wheat. Journal of Heredity, 100(6), 771–776.
Toribio, M., & Romanya, J. (2006). Leaching of heavy metals (Cu, Ni and Zn) and organic matter after sewage sludge application to Mediterranean forest soils. Science of the Total Environment, 363(1–3), 11–21.
Umair Hassan, M., Aamer, M., Umer Chattha, M., Haiying, T., Shahzad, B., Barbanti, L., Nawaz, M., Rasheed, A., Afzal, A., Liu, Y., & Guoqin, H. (2020). The critical role of Zn in plants facing the drought stress. Agriculture, 10(9), 396.
Vallee, B. L., & Auld, D. S. (1990). Active-site Zn ligands and activated H2O of Zn enzymes. Proceedings of the National Academy of Sciences, 87(1), 220–224.
Vatansever, R., Filiz, E., & Eroglu, S. (2017). Genome-wide exploration of metal tolerance protein (MTP) genes in common wheat (Triticum aestivum): Insights into metal homeostasis and biofortification. BioMetals, 30(2), 217–235.
Velu, G., Crespo-Herrera, L., Guzman, C., Huerta, J., Payne, T., & Singh, R. P. (2019). Assessing genetic diversity to breed competitive biofortified wheat with enhanced grain Zn and Fe concentrations. Frontiers in Plant Science, 9, 1971.
Velu, G., Ortiz-Monasterio, I., Cakmak, I., Hao, Y., & Singh, R. Á. (2014). Biofortification strategies to increase grain Zn and iron concentrations in wheat. Journal of Cereal Science, 59(3), 365–372.
Velu, G., Tutus, Y., Gomez-Becerra, H. F., Hao, Y., Demir, L., Kara, R., Crespo-Herrera, L. A., Orhan, S., Yazici, A., Singh, R. P., & Cakmak, I. (2017). QTL mapping for grain Zn and iron concentrations and Zn efficiency in a tetraploid and hexaploid wheat mapping populations. Plant and Soil, 411(1–2), 81–99.
Venegas, J. P., Graybosch, R. A., Baenziger, P. S., Bai, G., & St. Amand, P. (2018). Registration of great plains–adapted reduced phytate winter wheat germplasm. Journal of Plant Registrations, 12(3), 405–410.
Verma, A., & Singh, G. P. (2020). Simultaneous application of AMMI measures and yield for stability analysis of wheat genotypes evaluated under irrigated late sown conditions of Central Zone of India. Journal of Applied and Natural Science, 12(4), 541–549.
Vitosh, M. L., Warncke, D. D., &and Lucas, R. E. (1994). Zn determine of crop and soil. Michigan State University Extension.
Wang, J., & Yang, S. (2021). Dose-dependent responses of Arabidopsis thaliana to Zn are mediated by auxin homeostasis and transport. Environmental and Experimental Botany, 189, 104554.
Welch, R. M. (2002). The impact of mineral nutrients in food crops on global human health. Plant and Soil, 247(1), 83–90.
Xia, H., Xue, Y., Liu, D., Kong, W., Xue, Y., Tang, Y., Li, J., Li, D., & Mei, P. (2018). Rational application of fertilizer nitrogen to soil in combination with foliar Zn spraying improved Zn nutritional quality of wheat grains. Frontiers in Plant Science, 9, 677.
Xu, Y., An, D., Liu, D., Zhang, A., Xu, H., & Li, B. (2012). Molecular mapping of QTLs for grain Zn, iron and protein concentration of wheat across two environments. Field Crops Research, 138, 57–62.
Yadava, D. K., Hossain, F., & Mohapatra, T. (2018). Nutritional security through crop biofortification in India: Status & future prospects. The Indian Journal of Medical Research, 148(5), 621.
Zhang, Y. Q., Sun, Y. X., Ye, Y. L., Karim, M. R., Xue, Y. F., Yan, P., Meng, Q. F., Cui, Z. L., Cakmak, I., Zhang, F. S., & Zou, C. Q. (2012). Zn biofortification of wheat through fertilizer applications in different locations of China. Field Crops Research, 125, 1–7.
Zou, C., Du, Y., Rashid, A., Ram, H., Savasli, E., Pieterse, P. J., Ortiz-Monasterio, I., Yazici, A., Kaur, C., Mahmood, K., & Singh, S. (2019). Simultaneous biofortification of wheat with Zn, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. Journal of Agricultural and Food Chemistry, 67(29), 8096–8106.
Zou, C. Q., Zhang, Y. Q., Rashid, A., Ram, H., Savasli, E., Arisoy, R. Z., Ortiz-Monasterio, I., Simunji, S., Wang, Z. H., Sohu, V., & Hassan, M. (2012). Biofortification of wheat with Zn through Zn fertilization in seven countries. Plant and Soil, 361(1), 119–130.
Acknowledgements
Thanks are due to the Department of Biotechnology (DBT), Govt of India for providing funds in the form of research Projects awarded to Shailendra Sharma (SS). The authors are also thankful to Ch. Charan Singh University, Meerut for providing various research facilities. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shukla, G., Sharma, S., Gaurav, A. et al. Physiological role and biofortification of zinc in wheat (Triticum aestivum L.). Plant Physiol. Rep. 27, 665–679 (2022). https://doi.org/10.1007/s40502-022-00677-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-022-00677-6