Abstract
To describe the impact of population mobility between different cities on the spread of infectious disease, a new infectious disease complex dynamical model is proposed. Moreover, we obtain the basic regeneration number of the model based on applied spectral analysis. And the disease-free equilibrium points and local equilibrium points of the model are discussed, and it is found that two kind equilibrium points are globally asymptotically stable. In addition, the final scale of the presented model is analyzed and an expression for the final scale is obtained. Furthermore, we analyze the impact of population mobility on the spread of infectious diseases via numerical simulations. Our results reveal that the increase of population mobility between two cities leads to more intense disease transmission. Finally, the influence of media effects on the spread of infectious diseases is investigated. It is shown that the spread of diseases is suppressed because of the increase of individual's self-isolation rate. Therefore, controlling the population mobility is an effective initiative to curb outbreaks of infectious diseases throughout the network. These results can provide a theoretical basis for preventing and controlling the spreading of infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Infectious diseases are transmitted by pathogenic (micro) organisms. The spread of infectious diseases is both covert and sudden. Infectious diseases have always caused great harm to human health and caused great losses to social and economic development. The global outbreak of influenza in 1918 [1, 2], with a death toll of 20 million, the outbreak of SARS virus [3, 4] in 2003, and the global outbreak of COVID-19 [5, 6] in 2019 all posed a great threat to human health. In the last few decades, the study of complex networks has gradually become a hot issue in the field of complexity disciplines. Scholars have made significant contributions to the study in these areas such as transportation, social [7,8,9], financial [10,11,12] and biological [13, 14] networks. As research into complex networks have continued, the spreading of computer viruses in computer networks, contagious diseases in social networks can have an effect on the development of human society [15]. Therefore, the behavior of transmission dynamics on complex networks has become one of the research directions of great interest.
The dynamics propagation on complex network has achieved some very important studies in recent decades [16,17,18,19,20,21,22,23]. Due to the complex topology of the network, the relationships between individuals can be accurately depicted. Study on the transmission of infectious diseases on complex networks mostly focuses on static networks [24,25,26]. Static networks are effective tools for studying short-term infectious diseases [27], but for some infectious diseases with a long duration, population mobility factors are likely to change the topological structure of the network [28,29,30]. Hence, studying the spread of infectious diseases on complex dynamic networks is of great significance. Jin et al. designed an infectious disease model that incorporates population statistics into complex network theory to study the impact of population statistics on population distribution [31]. Pan et al. established the community structure of the SIS epidemic model and discussed the impact of population statistics on disease transmission [32]. Also, authors proposed an infectious diseases model that considers the demographic characteristics on a complex network. They study the spread of the dynamic of two different infectious diseases [33]. Therefore, it is very meaningful to study the impact of population mobility on complex network.
In recent years, researchers have increasingly considered the impact of population mobility on the spread of diseases. For example, Ref. [34]. proposed a spatiotemporal model that estimates the causal effect of changes in community mobility (intervention) on infection rates. Researchers used mobile phone data to describe the connectivity among areas and study the impact of this connectivity on the spread of COVID-19 during the second wave of the pandemic in 2020 [35]. Authors mutually compared time series of covid-19-related data and mobility data across Belgium’s 43 arrondissements. They concluded that there is a strong correlation between physical movement of people and viral spread in the early stage of the sars-cov-2 epidemic in Belgium, though its strength weakens as the virus spreads [36]. Ref. [37] investigated the bi-directional relationship between human mobility and COVID-19 spread across U.S. counties during the early phase of the pandemic when infection rates were stabilizing and activity-travel behavior reflected a fairly steady return to normal following the drastic changes observed during the pandemic’s initial shock. Authors studied the interaction between disease transmission and disease related awareness transmission, and then proposed a new coupled disease transmission model on a two-layer multiple network [38, 39]. Subrata Ghosh proposed a deterministic compartmental model of infectious disease that considers the test kits as an important ingredient for the suppression and mitigation of epidemics. Furthermore, they consider heterogeneous networks to study the impact of long and short-distance human migration among the patches [40].
However, these existing works are inadequate in fully describing the realistic roles of the population mobility on complex network. In real life, the population mobility plays crucial role in the spreading of infectious disease. Most importantly, individuals typically move not only on single-layer networks, but also on more complex multi-layer networks. According to the above considerations, this paper focuses on studying the impact of population mobility on disease transmission on a two-layer network.
The paper is organized as follows. In Sect. 2, an infectious disease model is presented that takes into account population movements between two cities. Section 3 investigates the basic regeneration number and the global stability of the model using the next-generation matrix scheme. Section 4 verifies the theoretical results through numerical simulation. Finally, Sect. 5 summarizes the study.
2 Model formulation
As is well known, the spread of infectious diseases between two cities due to floating populations become more complex. In the following, let \(N_{i,k} \left( t \right)\), \(S_{i,k} \left( t \right),S_{{q_{i,k} }} \left( t \right),E_{i,k} \left( t \right),I_{i,k} \left( t \right),Q_{i,k} \left( t \right),R_{i,k} \left( t \right)\) denote the number of individuals whose states are \(N\), \(S,S_{q} ,E,I,Q,R\) of degree \(k\) in city \(i\) at time \(t\).
And the total human population size is denoted as \(\left( N \right)\), and the total population \(N\) is divided into six subclasses: people who are easily infected are called susceptible people \(\left( S \right)\), susceptible individuals have a sense of self-isolation, and those who adopt self-isolation measures are referred to as self-quarantine susceptible \(\left( {S_{q} } \right)\), infected individuals in the incubation period are called exposed \(\left( E \right)\), people who are infected with the virus and have symptoms are called infected individuals \(\left( I \right)\), exposed and infected individuals separated from uninfected individuals are referred to as quarantined \(\left( Q \right)\), the group of patients including exposed, infected and quarantined have undergone treatment and rehabilitation are called recovered \(\left( R \right)\).
For the total human population, we suppose that \(N_{i,k} \left( t \right) = S_{i,k} \left( t \right) + S_{{q_{i,k} }} \left( t \right) + E_{i,k} \left( t \right) + I_{i,k} \left( t \right) + Q_{i,k} \left( t \right) + R_{i,k} \left( t \right)\).
First, some assumptions and symbols are given by
-
(1)
It is assumed that there are no independent individuals. That is to say, an individual must contact with people. And an individual can be associated with \(m\) individuals at most. That is \(k \in \left\{ {1, \cdots m} \right\},i \in \left\{ {1,2} \right\}.\) An individual only contact with a maximum of m individuals.
-
(2)
We assume the following equation holds:
$$\begin{aligned} \sum\limits_{k = 1}^{m} {S_{i,k} \left( t \right)} &= S_{i} \left( t \right),\sum\limits_{k = 1}^{m} {S_{{q_{i,k} }} \left( t \right) = S_{{q_{i} }} \left( t \right),\sum\limits_{k = 1}^{m} {E_{i,k} \left( t \right)} }\\ & = E_{i} \left( t \right),\sum\limits_{k = 1}^{m} {I_{i,k} \left( t \right) = I_{i} \left( t \right),}\end{aligned} $$$$ \begin{aligned}&\sum\limits_{k = 1}^{m} {Q_{i,k} \left( t \right) = Q_{i} \left( t \right)} ,\\ &\sum\limits_{k = 1}^{m} {R_{i,k} \left( t \right)} = R_{i} \left( t \right),\sum\limits_{k = 1}^{m} {N_{i,k} \left( t \right) = N_{i} } \left( t \right). \end{aligned}$$These equations represent the sum of individuals with different degrees as all individuals in city \(i\).
-
(3)
Suppose that all newborns are susceptible. And newborns enter the city \(i\) with a probability of \(\delta_{i,k}\).The number of newborn individual in city \(i\) is \(A_{i}\) at each step time.
-
(4)
The network’s nodes do not have multiple edges and self-loop. This implies that we consider the contact number between different individuals is single. In addition, any individual cannot be infected unless interconnecting other individuals.
-
(5)
The probability of a susceptible individual will be infected by exposed in city \(i\) is \(\lambda_{i}\). The probability of a susceptible individual will be infected by infectious in city \(i\) is \(\beta_{i}\). And the natural mortality rate of \(d_{1}\), the mortality rate due to disease is \(d_{2}\). We assume that \(q\) stands for self-isolation rate,\(q_{1}\) represents the probability that the self-quarantined individuals will be released from isolation. We define \(\xi_{i}\) as the rate of population movement from city \(i\) to another one. Let \(\gamma_{i}^{1}\) represents the recovery rate of quarantined individuals, let \(\gamma_{i}^{2}\) represents the recovery rate of infectious. And,\(\alpha_{i}^{1}\) and \(\alpha_{i}^{2}\) represent the isolation rate of exposed individuals and the isolation rate of infectious, respectively. Furthermore, the conversion rate from the exposed to infectious is \(\varepsilon_{i}\) in city \(i\). The number of individuals contacted in city \(i\) due to population mobility is \(\omega_{i}\).
-
(6)
The proportion of individuals whose degree is \(k\) in city \(i\) can be defined as \(p_{i,k} = N_{i,k} /N_{i}\).The probability of randomly selecting an edge connecting to an infective neighbor is given by
$$ \theta_{i} \left( t \right) = \left( {\sum\limits_{k = 1}^{m} {kp_{i,k} I_{i,k} \left( t \right)/N_{i,k} \left( t \right)} } \right)/\left\langle {k_{i} } \right\rangle = \left( {\sum\limits_{k = 1}^{m} {kI_{i,k} \left( t \right)} } \right)/\left( {\sum\limits_{k = 1}^{m} {kN_{i,k} \left( t \right)} } \right) $$(1)And \(\left\langle {k_{i} } \right\rangle = \sum\limits_{k = 1}^{m} {kp_{i,k} ,i = 1,2.}\)
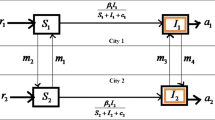
Figure 1 shows the propagation mechanism of the epidemic. The blue and green boxes represent city 1 and city 2, respectively. City 1 and city 2 contain different individuals that are connected through human contacts. The blue and green lines represent the individual’s states that change in different cities due to population mobility. The parameters are marked next to the line bar, what are the paths they represent for a group to transition from one state to another (Table 1).
Based on the above assumptions and mechanism of transmission, the infectious disease model taking into account the population mobility is established as follows
In the model (2),\(\delta_{i,k} A_{i}\) shows the number of newborn individuals in city \(i\) that have a degree of \(k\) as they are connected to \(k\) existing individuals.\(q_{1} S_{{q_{i,k} }}\) denotes that self-isolated individuals become susceptible person.\(\left( {q + d_{1} } \right)S_{i,k}\) means natural death or self-isolation of susceptible individuals.\(\left( {\beta_{i} + \lambda_{i} } \right)k\left( {1 - \xi_{i} } \right)\theta_{i} \left( t \right)S_{i,k}\) describes the susceptible individuals does not move across cities, but contacts the latent and sick people in city \(i\).
\(\omega_{i} k\xi_{i} \left( {\lambda_{{i{\prime} }} + \beta_{{i{\prime} }} } \right)\left( {\frac{{I_{{i{\prime} }} + E_{{i{\prime} }} }}{{N_{{i{\prime} }} }}} \right)S_{i,k}\) indicates that susceptible individuals are infected as latent people because of population movements, contact with lurkers and infected people in another city.
3 Dynamical analysis
In this section, we carry out a theoretical analysis on the basic regeneration number and the stability of the equilibrium point of the proposed model (2).
3.1 The basic regeneration number
From hypothesis, we have \(N^{\prime}_{i,k} = \delta_{i,k} A_{i} - d_{1} N_{i,k} - d_{2} \left( {I_{i,k} + Q_{i,k} } \right).\)
Theorem 3.1
The model (2) has solutions in the invariant region.
Proof
We have
then
Now, we let \(N_{i,k} \left( 0 \right) \le \frac{{\delta_{i,k} A_{i} }}{{d_{1} }}.\)
In the end, the following equation can be obtained
Thus, the region \(\Omega\) is positively invariant and attracting for the Eq. (2).
According to the model (2), the disease-free equilibrium point of the model is
Next, we discuss the basic regeneration number of the model.
\(R_{0}\) is defined as the average number of secondary cases caused by an infected individual during his infectivity period when he is introduced to a population of susceptible individuals without intervention.
In general, the next generation matrix method is used to calculate \(R_{0}\). The input matrix \({\varvec{F}}_{{\varvec{1}}}\) and the output matrix \({\varvec{V}}_{{\varvec{1}}}\) are given below, respectively.
The partial derivative of the input matrix and output matrix at the disease-free equilibrium point are as follows:
where \(m_{1l} = \left( {\beta_{2} + \lambda_{2} } \right)k\xi_{1} \frac{{\omega_{1} }}{{N_{2} }}S_{1,l}^{0} ,m_{2l} = \left( {\beta_{1} + \lambda_{1} } \right)k\xi_{2} \frac{{\omega_{2} }}{{N_{1} }}S_{2,l}^{0} ,\quad l = 1 \cdots m.\)
where \(\eta_{1} = d_{1} + \varepsilon_{1} + \alpha_{1}^{1} ,\eta_{2} = d_{1} + \varepsilon_{2} + \alpha_{2}^{1}\),\(\sigma_{1} = \gamma_{1}^{1} + d_{1} + d_{2} ,\sigma_{2} = \gamma_{2}^{1} + d_{1} + d_{2} .\)
Based on the above calculation, it can be concluded that.
Let the right term of model (2) be 0, and we get the local equilibrium point.
wherein,
where \(\varphi_{1} = \gamma_{i}^{2} \left( {d_{1} + d_{2} + \gamma_{i}^{1} } \right)\),\(\varphi = \left[ {\left( {d_{1} + q_{1} } \right)\left( {\delta_{i,k} A_{i} - d_{1} } \right) - d_{1} q} \right]\).
where \(\theta_{i}^{*} = \left( {\sum\limits_{k = 1}^{m} {kI_{i,k}^{*} } } \right)/\left( {\sum\limits_{k = 1}^{m} {kN_{i,k}^{*} } } \right)\).
3.2 The stability of equilibrium
In this section, we analyze the stability analysis on the two equilibrium points of the model and obtained the following two conclusions. According to Theorem 2 in reference [41], we can obtain the following lemma,
Lemma 3.1
For model (2), the disease-free equilibrium \(E_{0}\) is locally asymptomatically stable in \(\Omega\) when \(R_{0} < 1\), but unstable if \(R_{0} > 1\), where \(R_{0}\) is defined by next generation matrix method.
Firstly, the stability of the disease-free equilibrium point at \(R_{0} < 1\) has been demonstrated and we obtained the result of Theorem 3.2.
Theorem 3.2
For system (2), if \(R_{0} < 1\), the disease-free equilibrium \(E_{0}\) is globally asymptomatically stable in \(\Omega\).
Then, we derive a proof of the stability of local equilibrium points when \(R_{0} > 1\) and we obtained the result of Theorem 3.3.
Theorem 3.3
If \(R_{0} > 1\),the endemic equilibrium \(E^{*}\) is globally asymptomatically stable in \(\Omega\) for model (2).
Please, refer to “Appendix A” for details on the stability analysis.
3.3 Final sizes
This subsection is devoted to the calculation of final size of model. It is well known that the final size can be used to describe the degree of transmission of infectious diseases. Usually, we analyze the final number of susceptible individuals because it is determined how many people will have contracted the disease at the end of the epidemic. Here, we neglect birth and death, and then model (2) can be described as
Then, add the first and second equations in model (8) to obtain the density of all susceptible individuals \(\left( {\frac{{dS_{a} }}{dt}} \right)\).
Wherein \(S_{a} = S_{i,k} + S_{{q_{i,k} }}\).
Without loss of generality, let \(\overline{\omega } = \int\limits_{0}^{\infty } {\omega \left( t \right)dt}\).
For model (8), diseases will eventually die out. So we have \(I_{i,k} \left( \infty \right) = 0,E_{i,k} \left( \infty \right) = 0,Q_{i,k} \left( \infty \right) = 0\).
Add the first and third equations in model (8)
Integrate from 0 to infinity on both sides of the Eq. (9)
According to Eqs. (8) and (9), Eq. (10) can be rewritten as
Similarly,
Integrate from 0 to infinity on both sides of the Eq. (12)
Similarly, Add the fourth and fifth equations in model (8)
Integrate both sides of the Eq. (15) from 0 to \(\infty\),
Substitute Eqs. (11) and (14) into (16):
Integrate the first equation in model (8) from 0 to \(t\).
When \(t \to \infty\), we can obtain the relationship formula for the final scale.
4 Numerical simulations
In this section, we investigate the influence of basic reproduction number on the spread of infectious diseases and the influence of parameters related to population flow on the spread of infectious diseases.
Figure 2 shows the variation of all individual density of \(S,S_{q} ,E,I,Q,R\) at \(R_{0} < 1\) over time, the black dashed line represents the changing trends of different subcategories in city 1, while the red line represents the changing trends of different subcategories in city 2. It can be seen from Fig. 2, when \(R_{0} < 1\), the density of individuals such as \(E,I\) and \(Q\) gradually stabilizes towards 0 over time, at this point, the disease gradually disappears after the outbreak. Figure 3 shows the variation of all individual density of \(S,S_{q} ,E,I,Q,R\) at \(R_{0} < 1\) over time. Also, Fig. 3 shows when \(R_{0} > 1\), the density of infected persons tends to a certain stable value. At this point, infectious diseases become endemic and always exist.
Figure 4 indicates the relationship between \(I_{1} \left( t \right)\) and population mobility rate. In Fig. 4, different lines represent different population mobility rates, which mainly reveal the relationship between population mobility rates \(\xi\) and infected individuals \(I_{1} \left( t \right)\) in city 1. It is found that when the population mobility rate increased, the speed of disease outbreak accelerated, and the number of patients increased significantly. This behavior exacerbates the outbreak of diseases and also increases the number of infected individuals.
In Fig. 5, different lines represent different values of the number of contacts. From Fig. 5, we can see that the lines with larger values of contacts which have larger peak values and the earliest time to reach the peak value. And the vertical axis represents the infected individuals in city 1. From the perspective of disease transmission, when a disease spreads between two cities due to population mobility, the more people encounter during individual mobility, the greater risk of encountering infected individuals and contracting the virus.
Figure 6 shows the relationship between self-isolation rate \(\left( q \right)\) and infected individual \(I_{1} \left( t \right)\) in city 1, different lines represent different values of self-isolation rate. From Fig. 6, we can observe that the higher of the self-isolation rate \(\left( q \right)\) is, the slower of the speed of disease outbreak is, and the number of patients is significantly reduced. In fact, when diseases spread, people will develop a sense of self isolation and take some self- isolation measures, such as working from home, wearing masks, reducing going out, and so on. The result of this approach is a decrease in population mobility, which can reduce the chance of exposure to the virus and control the spread of diseases. In particular, we notice that the media effect plays positive role on curbing the spreading of infectious diseases. That is, the media calls on people to take self-protection measures or reflect on the trajectories of nearby infected individuals, thereby increasing individual self-isolation rates. This measure can greatly avoid the probability of individual infection.
To better characterize the model behavior with respect to its main parameters, we have also discussed the relationship between the basic regeneration number and the population movement rate in two cities.
The relationship between \(R_{0}\) and two population mobility rates is depicted in Fig. 7a. While Fig. 7b can be obtained from a top-down three-dimensional graph. The color bar in the figure indicates that as the color changes from blue to red, the corresponding \(R_{0}\) value also increases. We observe an increase of population mobility in both cities as the basic regeneration number \(R_{0}\) increases. In fact, if the flow of population between cities is reduced, the basic number of regenerations can be controlled and the spread of diseases can be effectively suppressed.
The relationship between \(R_{0}\) and the number of contact \(\omega\) in two cities is shown in Fig. 8. Figure 8a presents the change of \(R_{0}\) with \(\omega_{1}\) and \(\omega_{2}\) in the form of a three-dimensional graph, Fig. 8b is observed from the perspective of a top-down 3D view. It is found that as the number of effective contacts increases in both cities, the basic regeneration number \(R_{0}\) gradually increases. This is of great significance for the spread of diseases in real life. If the number of effective contacts between cities is reduced, the basic number of regenerations can be controlled and the spread of diseases can be effectively suppressed.
The blue line in Fig. 9 represents \(I_{1} \left( t \right)\) with different degrees, while the green line represents \(I_{2} \left( t \right)\) with different degrees. Figure 9 depicts the changes of \(I_{1} (t)\)(blue curve) and \(I_{2} (t)\)(green curve) as the degree of the initial node increases. We can observe that as the degree increases,\(I(t)\) also increases. The higher the degree of the initial node, the more neighbors it has. When infectious diseases spread, the transmission rate of infected nodes also increases. In other words, an increase in the number of neighboring nodes of a node results in an increase in the density of infected individuals.
In what follows, we intend to analyze the relationship between the infection balance states of nodes and the degree of nodes. Besides, the NW small world network and BA scale-free network were used in numerical simulation, and Fig. 10 gives the trend of the infection equilibrium state curve and the trend of degree curve.
As shown in Fig. 10a, the node with large degree has large infected equilibrium state. However, Fig. 10b indicates that the relevance is a little weaker for the BA scale-free network. As a consequence, the node with higher degree is easier to be infected.
These numerical results imply that population mobility between cities has a significant impact on disease transmission. If we want to quickly curb the spread of the disease, we need to control population mobility, such as policies such as school closures, to isolate the spread of the disease from the outside world.
5 Conclusions
This paper proposed an improved infectious disease model, taking into account the factors of population mobility and self-isolation in two cities. Furthermore, the dynamic analysis of the model, including the basic regeneration number and the stability of the equilibrium point, have been theoretically derived. In addition, we analyzed the final scale of the presented model and obtained the according expression. Furthermore, the impact of population mobility on the spread of infectious diseases is investigated via numerical simulations. Our results show that people are more susceptible to infection when the rate of population movement within cities increases. When the rate of population movement between the two cities increases, the rate of infection of susceptible individuals accelerates, and the rate of spread of infectious diseases increases rapidly. Meanwhile, if the media effect is greater, it means that the number of self-isolated individuals will increase, which can slow down the spread of infectious diseases.
Availability of data and materials
We do not analyse or generate any datasets, because our work proceeds within a theoretical and mathematical approach.
Code availability
Code will be made available on request.
References
Koziol JA, Schnitzer JE (2022) Déjà vu all over again: racial, ethnic and age disparities in mortality from influenza 1918–19 and COVID-19 in the United States. Heliyon 8(4):e09299
Chowell G, Viboud C, Simonsen L et al (2011) The 1918–1920 influenza pandemic in Peru. Vaccine 29:B21–B26
Ghosh JK, Ghosh U (2023) Three dimensional epidemic model with non-monotonic incidence and saturated treatment: a case study of SARS infection of Hong Kong 2003 Scenario. Results Control Optim 11:100239
Rodriguez T, Dobrovolny HM (2021) Quantifying the effect of trypsin and elastase on in vitro SARS-COV infections. Virus Res 299:198423
Basyouni KS, Khan AQ (2022) Discrete-time COVID-19 epidemic model with chaos, stability and bifurcation. Results Phys 43:106038
Song H, Wang R, Liu S et al (2022) Global stability and optimal control for a COVID-19 model with vaccination and isolation delays. Results Phys 42:106011
Akhtar MU, Liu J, Liu X et al (2023) NRAND: An efficient and robust dismantling approach for infectious disease network. Inf Process Manag 60(2):103221
Nunner H, Buskens V, Teslya A et al (2022) Health behavior homophily can mitigate the spread of infectious diseases in small-world networks. Soc Sci Med 312:115350
Voinson M, Smadi C, Billiard S (2022) How does the host community structure affect the epidemiological dynamics of emerging infectious diseases? Ecol Model 472:110092
Schimit PHT, Monteiro LHA (2010) Who should wear mask against airborne infections? Altering the contact network for controlling the spread of contagious diseases. Ecol Model 221(9):1329–1332
Stensen DB, Cañadas RA, Småbrekke L et al (2022) Social network analysis of Staphylococcus aureus carriage in a general youth population. Int J Infect Dis 123:200–209
Lymperopoulos IN (2021) Stay home to contain Covid-19: neuro-SIR-neuro dynamical epidemic modeling of infection patterns in social networks. Expert Syst Appl 165:113970
Bouveret G, Mandel A (2021) Social interactions and the prophylaxis of SI epidemics on networks. J Math Econ 93:102486
Yao Y, Guo Z, Huang X et al (2023) Gauging urban resilience in the United States during the COVID-19 pandemic via social network analysis. Cities 138:104361
Marqués SP, Martínez FMC, Leirós R et al (2023) Leadership and contagion by COVID-19 among residence hall students: a social network analysis approach. Soc Netw 73:80–88
Zhao D, Gao B, Wang Y et al (2018) Optimal dismantling of interdependent networks based on inverse explosive percolation. IEEE Trans Circuits Syst II Express Briefs 65(7):953–957
Zhao D, Wang L, Wang Z et al (2018) Virus propagation and patch distribution in multiplex networks: modeling, analysis, and optimal allocation. IEEE Trans Inf Forensics Secur 14(7):1755–1767
Wang X, Wang Z (2022) Bifurcation and propagation dynamics of a discrete pair SIS epidemic model on networks with correlation coefficient. Appl Math Comput 435:127477
Zhu L, Liu W, Zhang Z (2020) Delay differential equations modeling of rumor propagation in both homogeneous and heterogeneous networks with a forced silence function. Appl Math Comput 370:124925
Li M, Ling Y, Ma J (2023) The dynamics of the risk perception on a social network and its effect on disease dynamics. Infect Dis Model 8(3):632–344
Ghosh S, Kundu P et al (2023) Dimension reduction in higher-order contagious phenomena. Chaos 33:053117
Das P, Upadhyay RK et al (2021) Mathematical model of COVID-19 with comorbidity and controlling using non-pharmaceutical interventions and vaccination. Nonlinear Dyn 106(2):1213–1227
Ghosh S, Senapati A et al (2021) Reservoir computing on epidemic spreading: a case study on COVID-19 cases. Phys Rev E 104:014308
Wu Q, Hadzibeganovic T (2018) Pair quenched mean-field approach to epidemic spreading in multiplex networks. Appl Math Model 60:244–254
Wang X, Wang Z, Shen H (2019) Dynamical analysis of a discrete-time SIS epidemic model on complex networks. Appl Math Lett 94:292–299
Xia C, Wang Z, Zheng C et al (2019) A new coupled disease-awareness spreading model with mass media on multiplex networks. Inf Sci 471:185–200
Zhang H, Shu P, Wang Z et al (2017) Preferential imitation can invalidate targeted subsidy policies on seasonal-influenza diseases. Appl Math Comput 294:332–342
Huang S, Chen F, Chen L (2017) Global dynamics of a network-based SIQRS epidemic model with demographics and vaccination. Commun Nonlinear Sci Numer Simul 43:296–310
Gong Y, Small M (2018) Epidemic spreading on meta population networks including migration and demographics. Chaos Interdiscip J Nonlinear Sci 28(8):083102
Jing W, Jin Z, Peng XL (2018) Adaptive SIS epidemic models on heterogeneous networks with demographics and risk perception. J Biol Syst 26(02):247–273
Jin Z, Sun G, Zhu H (2014) Epidemic models for complex networks with demographics. Math Biosci Eng 11(6):1295–1317
Pan W, Sun GQ, Jin Z (2015) How demography-driven evolving networks impact epidemic transmission between communities. J Theor Biol 382:309–319
Yao Y, Zhang J (2016) A two-strain epidemic model on complex networks with demographics. J Biol Syst 24(04):577–609
Giffin A, Gong W, Majumder S et al (2022) Estimating intervention effects on infectious disease control: the effect of community mobility reduction on Coronavirus spread. Spatial Stat 52:100711
Ensoy MC, Nguyen MH, Hens N et al (2023) Spatio-temporal model to investigate COVID-19 spread accounting for the mobility among municipalities. Spatial Spatio Temporal Epidemiol 45:100568
Rollier M, Miranda GHB, Vergeynst J et al (2023) Mobility and the spatial spread of sars-cov-2 in Belgium. Math Biosci 360:108957
Rafiq R, Ahmed T, Uddin MYS (2022) Structural modeling of COVID-19 spread in relation to human mobility. Transp Res Interdiscip Perspect 13:100528
Xia C, Wang Z, Zheng C (2019) A new coupled disease-awareness spreading model with mass media on multiplex networks. Inf Sci Int J 471:185–200
Wang S (2019) The impact of awareness diffusion on SIR-like epidemics in multiplex networks. Appl Math Comput 349:134–147
Ghosh S, Senapati A et al (2021) Optimal test-kit-based intervention strategy of epidemic spreading in heterogeneous complex networks. Chaos 31:071101
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Acknowledgements
First of all, I would like to give my heartfelt thanks to all the people who have ever helped me in this paper. My sincere and hearty thanks and appreciations go firstly to my supervisor, Mrs Yang Lixin, whose suggestions and encouragement have given me much insight into these translation studies. It has been a great privilege and joy to study under his guidance and supervision. Furthermore, it is my honor to benefit from his personality and diligence, which I will treasure my whole life. My gratitude to him knows no bounds. I am also extremely grateful to all my friends and classmates who have kindly provided me assistance and companionship in the course of preparing this paper. In addition, I would like to thank the National Natural Science Foundation of China for its support. This research was supported by The National Natural Science Foundation of China under Grant No.11702195.
Funding
This research was supported by The National Natural Science Foundation of China (11702195).
Author information
Authors and Affiliations
Contributions
YQ: Writing-Original Draft, Validation, Formal analysis, Visualization, Methodology. LY: Methodology, Writing-Review&Editing, Funding acquisition, Supervision, Resources. ZG: Resources, Visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financialor proprietary interests in any material discussed in this article. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Appendix A: Specific derivation of stability analysis
Appendix A: Specific derivation of stability analysis
Stability plays a crucial role in the dynamic analysis of infectious disease models. The following content is the theoretical derivation of stability analysis. Let's first derive the global stability of the disease-free equilibrium point at \(R_{0} < 1\).
Firstly, the disease-free equilibrium \(E_{0}\) of system (2) is \(\left( {S_{i,k}^{0} ,S_{{q_{i,k} }}^{0} ,0,0,0} \right)\).
Where
System (2) is rewritten in the form.
Where \(X = \left( {S_{i,k} \left( t \right),S_{{q_{i,k} }} \left( t \right)} \right)^{\prime }\)\(Z = \left( {E_{i,k} \left( t \right),I_{i,k} \left( t \right),Q_{i,k} \left( t \right)} \right)^{\prime }\).
\(U_{0} = \left( {X_{0} ,0} \right) = \left( {S_{i,k}^{0} ,S_{{q_{i,k} }}^{0} ,0,0,0} \right)\) denotes the disease-free equilibrium of system.
Then, we have the following two conditions:
\(\left( {H_{1} } \right)\) For \(\frac{dX}{{dt}} = F\left( {X,0} \right) = \left( \begin{gathered} \delta_{i,k} A_{i} + q_{1} S_{{q_{i,k} }} - \left( {q + d_{1} } \right)S_{i,k} \hfill \\ qS_{i,k} - d_{1} S_{{q_{i,k} }} - q_{1} S_{{q_{i,k} }} \hfill \\ \end{gathered} \right)\),\(x_{0} = \left( {S_{i,k}^{0} ,S_{{q_{i,k} }}^{0} ,0,0,0} \right)\) is a globally asymptomatically stable equilibrium of \(\frac{dx}{{dt}} = F\left( {x,0} \right)\). Hence,\(U_{0}\) is globally asymptomatically stable.
And \({\varvec{A}}_{21} \user2{ = - V}_{21}\), \({\mathbf{A}}_{22} {\mathbf{ = }} - {\mathbf{V}}_{22}\), \({\varvec{A}}_{31} \user2{ = } - {\varvec{V}}_{31}\), \({\varvec{A}}_{32} \user2{ = } - {\varvec{V}}_{32}\), \({\varvec{A}}_{33} \user2{ = } - {\varvec{V}}_{33}\).
Where \(\rho_{1l} = \left( {\beta_{2} + \lambda_{2} } \right)k\xi_{2} \omega_{2} \frac{{S_{1,l}^{0} }}{{N_{2} }},\rho_{2l} = \left( {\beta_{1} + \lambda_{1} } \right)k\xi_{1} \omega_{1} \frac{{S_{2,l}^{0} }}{{N_{1} }},l = 1 \cdots m.\)
Note that \({\varvec{A}}\) is an Metzler matrix,\(\user2{ - A}\) is an M-matrix, and all eigenvalues with respect to \({\varvec{A}}\) have negative real parts when \(R_{0} < 1\).We derive that \(Z\left( t \right)\) is globally asymptomatically stable, which means.
Then, the disease-free equilibrium \(E_{0}\) is globally asymptomatically stable when \(R_{0} < 1\).
Next, we discuss the global stability of the local equilibrium point at \(R_{0} { > }1\).
Proof
Consider the following Lyapunov function candidate
where \(h_{1} ,h_{2} ,h_{3} ,h_{4}\) and \(h_{5}\) are positive constants to be determined later. Differentiating the function with respect to time yields.
Substituting system (A5) into the above formula and sorted out:
where \(x_{1} = \frac{{S_{i,k} }}{{S_{i,k}^{*} }},x_{2} = \frac{{S_{{q_{i,k} }} }}{{S_{{q_{i,k} }}^{*} }},x_{3} = \frac{{E_{i,k} }}{{E_{i,k}^{*} }},x_{4} = \frac{{I_{i,k} }}{{I_{i,k}^{*} }},x_{5} = \frac{{Q_{i,k} }}{{Q_{i,k}^{*} }}\).
The above formula can be simplified
Considering \(h_{3} = 1\), by setting the coefficients of \(x_{2} ,x_{3} ,x_{4} ,x_{5}\) to 0 and solving for \(h_{1} ,h_{2} ,h_{4}\) yields.
\(h_{1} = {{\left[ {\left( {q + d_{1} } \right) + a} \right]} \mathord{\left/ {\vphantom {{\left[ {\left( {q + d_{1} } \right) + a} \right]} q}} \right. \kern-0pt} q}.\)\(h_{2} = {{\left[ {\alpha_{i}^{2} \varepsilon_{i} + \alpha_{i}^{1} \left( {d_{1} + d_{2} + \alpha_{i}^{2} + \gamma_{i}^{2} } \right)} \right]} \mathord{\left/ {\vphantom {{\left[ {\alpha_{i}^{2} \varepsilon_{i} + \alpha_{i}^{1} \left( {d_{1} + d_{2} + \alpha_{i}^{2} + \gamma_{i}^{2} } \right)} \right]} {\left[ {\alpha_{i}^{2} \left( {d_{1} + \varepsilon_{i} + \alpha_{i}^{1} } \right)} \right]}}} \right. \kern-0pt} {\left[ {\alpha_{i}^{2} \left( {d_{1} + \varepsilon_{i} + \alpha_{i}^{1} } \right)} \right]}}\).\(h_{4} = {{\left[ {\left( {d_{1} + d_{2} + \alpha_{i}^{2} + \gamma_{i}^{2} } \right)} \right]} \mathord{\left/ {\vphantom {{\left[ {\left( {d_{1} + d_{2} + \alpha_{i}^{2} + \gamma_{i}^{2} } \right)} \right]} {\alpha_{i}^{2} }}} \right. \kern-0pt} {\alpha_{i}^{2} }}.\)\(a = \left( {\beta_{i} + \lambda_{i} } \right)k\left( {1 - \xi_{i} } \right)\theta_{i} + \left( {\beta_{{i^{\prime}}} + \lambda_{{i^{\prime}}} } \right)\left( {\frac{{I_{{i^{\prime}}} + E_{{i^{\prime}}} }}{{N_{{i^{\prime}}} }}} \right)k\xi_{i} \omega_{i}\).
Using the arithmetic–geometric means inequality, it is found that \(L\) is less or equal to zero with equality only if \(x_{1} = 1,x_{2} = 1,x_{3} = 1,x_{4} = 1,x_{5} = 1\). By LaSalle’s invariance principle, we know the largest invariant set in \(\Omega\) is reduced to the endemic equilibrium \(E^{*}\), and the largest invariant set contained in
Then, we conclude that the endemic equilibrium is globally asymptotically stable in \(\Omega\).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, Y., Yang, L. & Gu, Z. Dynamics behavior of a novel infectious disease model considering population mobility on complex network. Int. J. Dynam. Control 12, 2295–2309 (2024). https://doi.org/10.1007/s40435-023-01371-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40435-023-01371-7