Abstract
The snowmelt timing is a major factor controlling plant phenology in alpine regions. Presently, the warming is pushing forward snowmelt timing which may influence species’ seasonal cycle throughout the alpine landscape of Himalaya. Nevertheless, very few studies have studied the response of species’ phenology to advanced snowmelt in the Himalayas. The present study investigated different alpine species’ responses to early snowmelt present in different alpine communities. Five communities were identified and two sites were selected in each community (early snowmelt: ES, late snowmelt: LS) with a 50 × 50 m plot marked permanently for species monitoring. The observations for phenophase initiation and durations of all species were recorded fortnightly. Kruskal–Wallis test was performed to examine species-specific differences in phenophase durations. Pairwise differences were tested with Dunn’s post hoc test. The present study hypothesized early snowmelt advances and lengthens phenophases timing and duration in all alpine species. The results showed that phenophases initiations and duration were earlier and longer in ES sites for the majority of species but no significant relationship was found between snowmelt timing and species’ phenophase duration. Many species showed two distinctive phenophases (reproductive and fruiting). The divergence was higher in the reproductive phenophase than in other phenological stages. Hence, it seems that the early snowmelt is an important driver influencing the early spring phenology of herb species, the species-specific effects of already happening phenological adjustment for higher reproductive success in the current warming of alpine meadows points towards other limiting factors too that remain to be better understood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Early seasonal warming and changes in precipitation regimes, leading to earlier snowmelt, have significant implications for alpine systems (Ernakovich et al. 2014). Warming trends in alpine regions show elevation and geographic-dependent effects on snow regimes, with regional trends deviating from global trends (Theurillat and Guisan 2001; Rangwala and Miller 2012; Zhang et al. 2007; Stocker et al. 2013) emphasizing local topographic influence on the direction and magnitude of changes, especially concerning snow persistence, melt pattern, growing season, plant production, etc. These changes expose alpine ecosystems to faster climate change, affecting species redistribution, richness, growth, and phenology (Korner and Hiltbrunner 2021; Palaj and Kollar 2021; Steinbauer et al. 2018; Adhikari and Kumar 2020; Fazlioglu and Wan 2021) on or near mountain summits, resulting in group extinction of range-restricted species (Sonntag et al. 2022).
Snow plays a crucial role in controlling micro-climate and plant growth in alpine regions (Berauer et al. 2019). Its duration determines the beginning of the growth season, influencing composition, richness, and phenology of alpine communities globally (Aalto et al. 2018; Fazlioglu and Wan 2021), while its impact on temperature sums determines when germination takes place. Alpine plants have evolved cold resistance and phenology regulation as adaptations (Korner et al. 2016), making them highly sensitive to environmental changes, including alterations in plant phenology (Pervey et al. 2017; Kudo 2020).
Long-term monitoring studies have shown that warming events can advance plant reproductive phenology, but early snowmelt without temperature increase may not consistently advance phenology and can lead to false growth season initiation and negative ecological impacts (Wolkovich et al. 2012; Gehrmann et al. 2021; Reyes-Fox et al. 2014) including false growth season initiation, increasing frost damage because of early dehardening, and may alter the functional attributes of alpine communities by increasing productivity at the cost of depleting nutrient reserves in alpine soils. Responses to warming events vary among species with some species neither advancing nor delaying the timing of spring events (Reyes-Fox et al. 2014), while other experiments show advance and delay in species phenology of species in the same habitat under changes in snowmelt regime (Adhikari et al. 2018, 2020), causing phenological mismatches and affecting survival (Jerome et al. 2021), reproductive synchrony, and pollinator interactions (Memmott et al. 2007; Liu et al. 2011). These changes can also limit suitable habitats for endemic and cryophilic species (Dirnbock et al. 2011).
The Indian Himalayan Region (IHR) is one of the most threatened non-polar regions, with projected significant temperature rise and precipitation increases (Singh and Negi 2018). Studies on alpine plant phenology in the Western Himalayan alpine landscape are limited (Sundriyal et al. 1987; Ram et al. 1988; Negi et al. 1992; Nautiyal et al. 2001; Vashistha et al. 2009, 2011; Bijalwan et al. 2013; Bisht et al. 2014), with few focusing on species’ seasonal cycle and phenological responses to climate change or advanced snowmelt (Adhikari et al. 2018; Adhikari and Kumar 2020). This knowledge gap constrains our understanding of alpine ecosystem dynamics in the rapidly changing environment of the IHR.
Given the unique eco-climatic conditions and spatial variations in the Himalayan alpine landscape, species may exhibit broader phenological responses, distinct from manipulation experiments. Therefore, this study focuses on the response of various alpine species to changes in natural snowmelt timing, particularly their phenology behaviour and adaptation strategies being adopted to tackle warming in the Himalaya. The present study aimed to determine alpine species’ response to early snowmelt in alpine meadows, which may act as baseline information for future studies as the phenology is strongly dependent on the snowmelt timing and temperature.
2 Material and methods
Study area
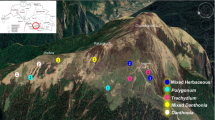
– The study was conducted at and around the Tungnath region (30° 29′ 12.26″ N Lat. and 79° 13′ 16.13″ E Long.) lies in upper catchment of Alaknanda and Mandakini rivers in Uttarakhand, West Himalaya, forms a part of Kedarnath Wildlife Sanctuary (Fig. 1). Four distinct seasons are discernible in the study area viz., short summer (May–June), monsoon (July to mid-September), autumn (mid-September–October), and long winter (November–April). The period with total snow cover is usually between 4–5 months and melts during April–May marking the arrival of favourable conditions for plant growth. The mean annual temperature (MAT) ranged between -8.9 (January) and + 25.6 °C (May) with an average of 6.7 ± 0.7 °C at timberline ecotone (3300 m) in 2008–2010 (Adhikari et al. 2012). The mean temperature of the warmest month was 12.6 ± 1.2 °C in July. The annual precipitation was 2410.5 ± 432.2 mm, of which 90% was recorded during rainy season (June–September). An increase in relative humidity, dew/frost point, maximum and minimum temperature, and annual rainfall over a period of over 30 years (1981–2017) were observed by Adhikari et al. (2018).
Selected communities
– The phytosociological study was conducted in the alpine meadow at and around Tungnath region and five major herbaceous communities, viz. Trachydium, Mixed Herbaceous, Mixed Danthonia, Polygonum, and Danthonia were identified (Kumar and Adhikari 2023). Due to the physiognomy of the region, alpine meadows are interspersed with krummholz and form a natural boundary between some communities in alpine meadows.
All the species present in each community were selected for the phenological study. The recorded species were divided into different growth forms following Pérez-Harguindeguy et al. (2013), namely semi-basal herbs, short basal herbs, erect leafy herbs and tussock-forming grasses, dwarf shrubs and climbers; as the growth form of a species is largely associated with the eco-physiological adaptations, such as maximizing photosynthetic production, sheltering from severe climatic conditions, and resistance to grazing by optimizing the height and positioning of the leaves and rosettes and prostrate growth forms are associated with heavy grazing, thus playing a significant role in species phenology behaviour.
Study design
– The area of alpine meadows is finite resulting in small communities. To determine the effects of natural snowmelt on species phenology, two sites were selected within each community on basis of snow cover as recorded on 28 March 2017, when the sites were for the first time accessed after a winter snowfall. Based on orography, the thickness of snow cover varies in each community ranging from 0 m (no snow) to 0.3 m in low snow cover regions (hereafter referred to as early snowmelt site or ES) and up to 1 m in high snow cover region (hereafter referred as late snowmelt site or LS), based on measurements at 10 random locations within each community, which is good enough to indicate two sites differed in snow cover. The snow cover duration in different communities in ES sites was 57–74 days and for LS sites 67–88 days.
Due to the habitat specificity of plant species, communities were interspersed and based on snow cover duration, ES and LS sites were randomly selected for each community. Subsequently, a difference of 7 to 14 days’ snowmelt time was observed between ES and LS sites in individual communities. A 50 × 50 m plot was permanently marked in the ES and LS sites of each community. Twenty-five (1 × 1 m) random quadrats were laid in each site, fortnightly, to record the abundance and phenophase of the species. The focus of the study was to record all major phenophases with corresponding principal stages for all species within quadrats for the growing season (May–October 2017).
Phenology observations
– The phenological stage of each species was recorded through a modified Biologische Bundesanstalt Bundessortenamt und CHemische Industrie (BBCH) following Adhikari et al. (2018). BBCH scale is a detailed observation key providing uniform coding primarily to phenological criteria instead of differentiating analogous stages. It depends on the frequency distribution of population phenophase so the presence is not required at the beginning; however, in alpine herb species, it is often difficult to pinpoint the dominant secondary phenophase stages, and sometimes even primary stages as per the BBCH scale. Since the growing period in the alpine is short (6–7 months), there are many herbaceous species with a short life cycle (< 2 months to 2–4 months). These species may skip some primary stages depending on the micro-climate. Oftentimes, it was observed that the species individuals/population growth was fast enough between recorded observations that some primary stages were not recorded leading to the illusion of herb species skipping primary stages depending on micro-climate. Therefore, for ease of data recording, the ten primary phenophase stages (code 0–9) of the BBCH scale were modified (Adhikari et al. 2018) and enumerated into four principal stages viz. vegetative stage (0–4), reproductive stage (5–6), fruiting stage (7–8), and senescence stage (9).
The abundance of individual species and phenophase in a quadrat was recorded fortnightly. Since species population showed different primary stages at the same temporal point (sampling day), therefore, to avoid phenophase overlapping and to decrease noise, the existence of a particular stage was considered if, in a quadrat, more than 5% of the individuals showed that primary stage; if more than 20% quadrats showed the particular stage in a plot; and if more than 30% plots showed the particular stage in a site. The principal phenophase initiation for a species was considered on the day when its corresponding lowest primary phase was recorded in at least one plot for both ES and LS sites. Its end was the day when the next principal phenophase was recorded or when species individuals in that principal phenophase were recorded less than 5% after its initiation. The principal phenophase duration was calculated from the day of phenophase initiation to the day of phenophase end.
Statistical analysis
– The Kruskal–Wallis test, a nonparametric equivalent of analysis of variance (ANOVA), was used to compare species phenophase duration between ES and LS sites and between different growth forms. Pairwise differences from the Kruskal–Wallis test were tested with the Dun post hoc test to determine the significant differences. The average duration of principal phenophase for different species and growth forms in different sites (ES and LS) was calculated by averaging the phenophase duration of species growth forms. Data were first tested for parametric assumptions such as normality and homogeneity of variance prior to using nonparametric analysis. Analysis was completed using PAST (PAleontological Statistics) software.
3 Results
The growth period in alpine meadow lasted for 6 months from mid-April to mid-October. Overall, eighty species were recorded during the study period, of which 36 species were common to both ES and LS sites (Fig. 2, 3, 4 and 5). In the present study, 22 species were common to all communities, 8 species each were common to 4 and 3 communities, 13 were common to two communities, and 29 were unique to a single community, respectively (See Kumar and Adhikari 2023). Twenty species of these common species, namely Anaphalis nepalensis (Spreng.) Hand.-Mazz., Bistorta affinis (D.Don) Greene, Bistorta amplexicaulis (D.Don) Greene, Carex setosa Boott, Danthonia cachemyriana Jaub. & Spach, Gaultheria trichophylla Royle, Gentiana argentea (Royle ex. D.Don), Gerbera gossypina (Royle) Beauverd, Geranium wallichianum D.Don ex Sweet, Geum elatum Wall. ex G.Don, Kobresia duthiei C.B.Clarke, Oxygraphis polypetala Hook.f. & Thomson, Pedicularis hoffmeisteri Klotzsch., Plantago ovata Forssk., Ranunculus hirtellus Royle, Rumex nepalensis Spreng., Saussurea taraxacifolia (Lindl. ex Royle) Wall. ex DC., Sibbaldia cuneata Edgew., Sibbaldia parviflora Willd., Taraxacum officinale F.H.Wigg., and Trachydium roylei Lindl., contributed most to the abundance in both ES and LS sites.
Plant communities composition
– Kumar and Adhikari (2023) have studied the phytosociological characteristics of plant communities at Tungnath in detail and the gist is presented. Forbs were the major contributor (90%) in all the communities. The pattern of growth forms was in following order: short basal (31) > semi-basal (26) > erect leafy (20) > dwarf shrub, climber, and tussock (1 each). The species mostly exhibited an intermediate growth cycle and primarily characterized by hemicryptophytes (70%). The species count was similar between the ES and LS sites for Trachydium, Polygonum, and Danthonia communities; however, the Mixed Herbaceous and Mixed Danthonia communities exhibited greater variation in species count between ES and LS sites.
The species richness for the communities ranged between 4.5 and 21.8 species m−2 throughout the growing period, maximum with Mixed Herbaceous community (8.9–21.8 species m−2) and minimum with Polygonum community (4.5–13.3 species m−2). The diversity was generally higher in LS sites (1.46 to 3.21) than in ES sites (1.02 to 2.83) in all communities, except for specific months and communities where ES sites showed higher diversity. Based on density, Trachydium roylei was the dominant species in the Trachydium, Mixed Herbaceous and Mixed Danthonia communities, while Ranunculus hirtellus and Danthonia cachemyriana dominated the Polygonum and Danthonia communities, respectively.
Phenophase response to snowmelt
− Among common species, the onset of three principal phenophases viz. vegetative, reproductive and fruiting in a majority of species responded strongly to early snowmelt. The vegetative and reproductive phenophases were observed in the early growth period (May and June), while senescence was observed from September onwards. In both ES and LS sites, the vegetative phase dominated in June, the reproductive phase in August, and the senescence phase in late September and October. As per the BBCH scale, the maximum species were in different primary stages of the vegetative phase during May and June (76.3 and 60.0%, respectively), while the majority of species flowered between June (33.3%) and September (30.8%) with a peak in August (76.8%).
Growth form and species response to early snowmelt
– Overall, the average vegetative phase duration for ES sites was less than LS sites for erect leafy species and short basal species, while it was longer for semi-basal species. The reproductive phase duration was much more in ES sites than LS sites in all growth forms, while the fruiting phase duration was marginally higher in ES sites for erect leafy and semi-basal species, but was longer in LS sites for short basal species. The duration of senescence was shorter in ES sites for erect leafy and short basal species, while it was much longer in ES sites for semi-basal species (Table 1).
To see the influence of snowmelt timing on phenophase duration, the Kruskal–Wallis test was conducted for different phenophases and growth forms. Between sites (ES and LS), no significant differences were observed for phenophase duration between individual stages of vegetative, flowering, fruiting and seeding stages. Similarly, the Kruskal–Wallis test was also conducted between growth forms, where no significant differences were observed for phenophase duration between individual stages of vegetative, fruiting and senescence stages but a significant difference was observed for the reproductive stage (p < 0.04). Dun post hoc test showed reproductive phase duration differed significantly between erect leafy species and short basal species (p < 0.01).
Erect leafy growth form
– Of the total 20 erect leafy species, 10 species were common to both ES and LS sites, while 5 species each were unique to ES sites and LS sites (Fig. 2). Among common species, the vegetative phase started in early May for 7 species (Bistorta affinis, B. amplexicaulis (D.Don) Greene, Gaultheria trichophylla Royle, Ranunculus diffuses DC., R. hirtellus, Rumex nepalensis Spreng., and Tanacetum dolichophyllum (Kitam.)) in both ES and LS sites, while remaining species (Bupleurum candollei Wall. ex DC., Euphrasia himalayica Wettst., and Polygonum polystachyum Wall. ex Meisn.) started in June.
Among common species, germination and vegetative phase was first recorded in ES sites for all species, except Bupleurum candollei and Polygonum polystachyum where the germination was initially in LS sites (Fig. 2). The reproductive phase was initiated first in ES sites, except in Bistorta affinis, Polygonum polystachyum and Rumex nepalensis, which were recorded first in LS sites. Ranunculus hirtellus and R. diffuses showed two distinct reproductive phases, first in May–June and second during August. The fruiting phase was recorded in only 6 common species. It initiated early in ES sites than LS sites in Gaultheria trichophylla and at the same time in ES and LS sites in Rumex nepalensis. It was recorded only in 3 species (Bupleurum candollei, Euphrasia himalayica Wettst., and Ranunculus hirtellus Royle) in ES sites and only in one species (Bistorta affinis (D.Don) Greene) in LS sites. The senescence phase was initiated early in LS sites (Fig. 2).
Among 10 species unique to ES and LS sites, the early-growing species (germination before June) only showed vegetative phase, except Veronica cana Wall. ex Benth., which completed its life cycle. The reproductive phase was recorded in all unique species except Pimpinella diversifolia DC. and Aconitum heterophyllum Wall. ex Royle. The fruiting phase was only recorded in Veronica cana in ES sites and Phlomis bracteosa Royle ex Benth. and Prunella vulgaris L. in LS sites. Among late-growing unique species (germination after May), the senescence was not recorded for Bistorta macrophylla (D.Don) Sojak, while it was present in remaining species (Fig. 2).
Semi-basal growth form
– Eleven, out of 26 semi-basal species were common in both ES and LS sites, while 5 species were present only in ES and 10 species in LS sites (Fig. 3). Among common species, 6 species (Anaphalis nepalensis (Spreng.) Hand.-Mazz., Carex setosa Franch. & Sav., Dactylorhiza hatagirea (D.Don) Soo, Kobresia duthiei S.R.Zhang, Pedicularis hoffmeister Klotzsch, and Saussurea taraxacifolia (Lindl. ex Royle) Wall. ex DC.) germinated in May, 2 species (Geranium wallichianum D.Don ex Sweet and Poa annua L.) in June, and 3 species (Geranium collinum Stephan ex Willd., Goodyera repens (L.) R.Br., and Selinum vaginatum (Edgew.) C.B.Clarke) in July. The germination and vegetative phase happened simultaneously in both ES and LS sites for both early and late-growing species, except Geranium wallichianum and Goodyera repens (Edgew.) C.B.Clarke, which germinated first in ES sites (Fig. 3). The reproductive phase was initiated first in Anaphalis nepalensis, Carex setosa and Geranium wallichianum in ES sites and in Pedicularis hoffmeisteri, Saussurea taraxacifolia, and Selinum vaginatum (Edgew.) C.B.Clarke in LS sites. The reproductive phase was initiated at same time in both ES and LS sites in Kobresia duthiei and Geranium collinum, while it was not recorded for Goodyera repens. The fruiting phase was recorded early in Carex setosa and Pedicularis hoffmeisteri in ES sites and in Kobresia duthiei at the same time in both ES and LS sites. In Saussurea taraxacifolia, the fruiting was recorded first in the LS site, while in Anaphalis nepalensis, it was recorded only in the LS site, respectively. The senescence phase was initiated in both ES and LS sites at the same time or early in LS sites for all common species (Fig. 3).
Among 15 species unique to ES and LS sites, the 6 early-growing unique species only showed either vegetative phase or reproductive phase or both with only one species (Geum elatum Wall. ex G.Don) in the ES site completing its’ life cycle. The majority of late-growing unique spices in ES and LS sites completed their life cycle, except Elsholtzia strobilifera (Benth.) Benth. and Geranium pratense L. Among 9 late-growing species, the reproductive phase was recorded in all except Geranium pratense, while the fruiting phase was recorded only in 2 ES unique species and 3 LS unique species. Among species unique to ES or LS sites, the senescence was recorded for only eight species (Fig. 3).
Short basal growth form
– Of the total 31 short basal species, 14 species (Cyananthus lobatus Wall. ex. Benth., Fragaria nubicola (Lindl. ex Hook.f.), Gentiana argentea (Royle ex D.Don) Royle ex D.Don, Gerbera gossypina (Royle) Beauverd, Jurinea dolomiaea Boiss., Oxygraphis polypetala Hook.f. & Thomson, Plantago ovata Forssk., Polygonum vaccinifolium Wall. ex Meisn., Potentilla lineata Trevir., Sibbaldia cuneata Edgew., S. parviflora, Taraxacum officinale F.H.Wigg., Trachydium roylei Lindl. and Viola biflora L.) were common to both ES and LS sites. By comparison, 10 and 7 species were present only in ES or LS sites, respectively (Fig. 4).
Among common species, 8 species were early-growing species, of which 3 germinated before May and 5 in May (Fragaria nubicola (Lindl. ex Hook.f.) Lacaita, Plantago ovata, Sibbaldia cuneata, Taraxacum officinale and Trachydium roylei), while 6 species were late-growing with 3 species (Cyananthus lobatus, Polygonum vaccinifolium and Potentilla lineata Trevir.) germinating in June and 3 species (Gerbera gossypina, Jurinea dolomiaea and Viola biflora L.) in July (Fig. 4). The species germinated first in ES sites; therefore, the vegetative phase started first in ES sites in all species. Gentiana argentea, Oxygraphis polypetala and Sibbaldia cuneata started germination immediately after snowmelt before the start of the growing season (May), therefore their vegetative phase in the early-growing season was not recorded. The reproductive phase was first initiated in either ES sites or simultaneously in ES and LS sites in all common species, except Fragaria nubicola as it disappeared in ES sites by end of May, and only showed vegetative phase. Fragaria nubicola and Oxygraphis polypetala showed two distinctive reproductive phases in both ES and LS sites, first in May–June and second during August–September, while Sibbaldia parviflora showed two distinct reproductive phases only in ES sites, first in May and second during June-July. Of 14 common species, the fruiting phase was recorded for only 9 species, of which 5 species initiated early in ES sites, simultaneously in 2 species in both ES and LS sites and only in ES sites for Trachydium roylei and in LS sites for Fragaria nubicola, respectively. For most of common species, the senescence phase was first recorded in ES sites except in Jurinea dolomiaea and Taraxacum officinale, which senesced first in LS sites (Fig. 4).
Among 17 unique species, 4 were early-growing species and 13 were late-growing species. The early-growing species only showed vegetative phase and then disappeared by May end except Potentilla anserina L. which reappeared early July completing its life cycle, but did not showed fruiting phase. The majority of late-growing unique spices in ES and LS sites entered the reproductive phase. Two distinctive reproductive phases were recorded in Polygonum delicatulum Meisn., a unique species to ES sites, first in July and second in August–September. The fruiting phase was recorded for 4 and 3 unique species for ES and LS sites, respectively. Among species unique to ES or LS sites, the senescence was recorded for only 13 species (Fig. 4).
Other growth forms
– Danthonia cachemyriana Jaub. & Spach (a tussock) was present in both ES and LS sites. The germination took place in early and mid-July in ES and LS sites, respectively. The reproductive phase started in early August in both ES and LS sites. The fruiting phase was not observed and senescence started in mid-September. Cassiope fastigiata (Wall.) D.Don (dwarf shrub) was present only in ES sites for a month (August) and showed only the vegetative phase. Codonopsis rotundifolia Benth. (climber) germinated in early July, flowering lasted from mid-July to mid-August and senescence by late August (Fig. 5).
Changes in herbaceous phenology over a period
– To understand changes in the herbaceous phenology of wide-ranging species with respect to snowmelt timing in the study area, the phenophase of common species in the present study is contrast with the study conducted by Adhikari and Kumar (2020) for treeline herbaceous species. The timberline region became snow-free by about two weeks before the alpine region. Fifty-two species were common in both studies. Among these species, 20 species were common to both ES and LS sites at treeline and alpine zones, while 10 species were common in treeline but ES sites only in alpine, 13 species were common in treeline but LS only in alpine, 4 species were in ES only in treeline but common in alpine, 2 species were in ES only in both treeline and alpine and 3 species were in LS only in both treeline and alpine zones (Table 2).
In contrast to treeline, the average initiation and duration of phenophase of 52 common species in alpine communities showed a delay and shortening of 21.3 and 21.2 days for the vegetative phase, 8.7 and 13.1 days for the reproductive phase, advances in initiation of fruiting and seeding and senescence phases (11.4 and 3.6 days) and shortening of duration by 2.8 and 14 days for fruiting and seeding and senescence phases, respectively. However, based on the majority of species contributing to the abundance in communities throughout the growing season, the phenophase initiation advanced for all phenophases in alpine communities, while the duration was shortened for vegetative, fruiting and seeding and senescence phases and lengthened only for the flowering phase (Table 2).
4 Discussion
Snowmelt water is a precursor for initial plant growth in alpine regions during the early-growing season, which was received by the area in the form of snowfall (4 days during November–December in 2016 and 35 days during January to April in 2017). In the present study, the snowmelt in ES sites started by end of March 2017 and the sites became snow-free by the second week of April, while snowmelt in LS sites started in early April and all sites became snow-free by the last week of April. As per BBCH scale, the majority of species present directly transitioned to stage 3 from stage 0–1, as they showed rapid stem elongation/shoot development. The starting of vegetative phase in the present study was observed, but the end could not be discernible as per the BBCH scale. Similar findings were reported by Adhikari et al. (2020) for treeline herbaceous communities in Tungnath. Therefore, whenever species individuals reached up to 5% of the next stage, the termination of the previous stage was considered. The senescence showed an abrupt increase in ES and LS sites in September, especially in LS sites.
The timing of different phenophases, especially reproductive, varied between different communities, i.e. less variability was observed between communities like Trachydium and Mixed Herbaceous, Mixed Danthonia and Danthonia occupying similar habitats and more variability between communities occupying different habitats like Polygonum and Mixed Danthonia. This is expected as many authors (Körner 1999; Wipf et al. 2009; Bjorkman et al. 2015; Adhikari and Kumar 2020; Kudo 2020) also reported a strong correlation between flowering and snowmelt timing determined by local community assemblage, i.e. species organize themselves as per snow-depth (snowmelt timing) gradient across communities. Furthermore, the differences between phenophase initiation between communities can be further accredited to different micro-climatic conditions including orography, as was observed by Nautiyal et al. (2001) in an alpine meadow.
Early snowmelt advanced phenology
– The species in alpine communities showed a positive response to phenophase initiation in response to early snowmelt in the present study. The phenophases among a majority of species initiated in ES sites across the communities initially show the dominant effect of early snowmelt timing on vegetative phenology and reproduction. This result is consistent with many other active snow manipulation experiments (Dunne et al. 2003; Sherry et al. 2007; Wipf et al. 2009), which suggests snowmelt timing has a large influence on phenology, especially vegetative (Semenchuk et al. 2016) and reproductive (Wipf et al. 2009) phenophases. This is especially true in relation to the advancement of the first flowering day or flowering in general of early-growing species (Abeli et al. 2012; Bjorkman et al. 2015). Although the majority of species’ phenophase initiation showed a positive response with snowmelt timing, the phenophase duration of species in present study did not show a significant relationship with early snowmelt. This suggests that the alpine species have high phenotypic plasticity in phenophase initiation with respect to snowmelt timing and at the same time they show conservation in duration, which is consistent with many other warming experiments (Price and Waser 1998; Semenchuk et al. 2016; Jabis et al. 2020). However, some long-term experiments done in alpine regions in the Tibetan plateau (Yu et al. 2010) and ITEX experiment (Oberbauer et al. 2013) showed changes (shortening and lengthening) in flowering duration (duration between the first and last flowering day) with respect to experimental warming, similar to temperate grasslands where experimental warming resulted in longer phenophase duration (Reyes-Fox et al. 2016).
The germination in multiple species (Aconitum sp., Bistorta sp., Caltha palustris L., Gentiana argentea, Kobresia duthiei, Lysimachia prolifera Klatt, Oxygraphis polypetala, Picrorhiza kurroa Royle ex Benth., Ranunculus sp., Trachydium roylei) was initiated as soon as the area was snow-free by mid-April. The growth of these early-growing species was fast and some of them immediately entered into the reproductive phase in early May, e.g. Carex setosa, Plantago ovata, Sibbaldia parviflora and Trachydium roylei were recorded in the bud development stage, while Caltha palustris L., Gentiana argentea, Oxygraphis polypetala, Picrorhiza kurroa, Trachydium roylei and Gaultheria trichophylla Royle in the flowering stage. This is probably due to an increase in soil moisture and microhabitat temperature, however, the role of soil moisture in advance or cessation of flowering is still debatable as some studies found that it has a minimum role in flowering (Dunne et al. 2003; Sherry et al. 2007). However, Dorji et al. (2013) reported its immediate negative influence on reproductive initiation in lieu of soil dry down due to warming. Gehrmann et al. (2021) reported the early snowmelt without an increase in temperature. Most of the early-growing species with short growth cycle (less than 3 months) flowered early for reproductive success, thereafter building reserve for next season (Kimball et al. 2014). Early flowering in early-growing species may also decrease competition with long growth cycle species (more than 4 months) for reproductive success as they avoid early spring initiation to avoid damages from freezing events (Gezon et al. 2016).
Oftentimes, frost damage was also recorded simultaneously with germination in ES sites forcing the individuals to senescence resulting in false growth start before May. It was interesting to note that although these early-growing species started flowering before May in both ES and LS sites, none of the above species showed fruiting before May and did not enter fruiting phenophase at all. This was because although early snowmelt initiates early growth, it also exposes plants to extreme temperatures resulting in frost damages with drastic consequences for early-growing alpine plants including reduced leaf growth, survival and individual flowering (Baptist et al. 2010; Kawai and Kudo 2018) and at same time affecting individual reproductive success (Inouye 2008; Tonin et al. 2019). Therefore, early-growing species may take advantage of early snowmelt due to warming resulting in early flowering, they are at a risk of reduced reproductive potential/success (Wipf et al. 2009; Cooper et al. 2011; Gezon et al. 2016) than late-growing species as is evident from no to minimum fruiting recorded in these species.
Altitudinal difference in species phenology
– With a change in altitude, the wide-ranging species search for apt habitats to take roots in adjusting their phenological events suited with the environment. To understand these changes, the species phenology of alpine species was compared with some common species of treeline (Adhikari and Kumar 2020). A majority of common species showed advancement in the phenophase initiation, as well as shrinkage in phenophase durations in the present study. This is probably due to temperature exerting main control on the pre-flowering and flowering period with snowmelt timing and soil moisture also playing a secondary role as the trees and krummholz at timberline may provide a blanket effect for underlying herb species. In last few decades, the temperature of treelines is increasing across the globe (Tian et al. 2022), which can induce moisture stress and have negative influence on plant growth (Wu et al. 2019) as is observed in arctic tundra (Buntgen et al. 2015). However, in treeline, melt water from upslope region (snowmelt in alpine) may have curbed the above-mentioned negative influence making favourable conditions (warm and moist) for vegetative growth and simultaneously delaying reproductive phenophase. This is consistent with multiple manipulation experiments (Smith et al. 2012; Dorje et al. 2013; Jabis et al. 2020), which correlated high soil moisture with delay in flowering onset and termination.
The flowering period of plant species is controlled by snow cover (Krenová et al. 2022) only in early-growing species, while in late flowering plants flowering period is controlled by photoperiod (Schuchardt et al. 2021), thus determining reproductive success (Prieto-Benítez et al. 2021). This is evident from multiple early flowering species which showed only vegetative phenophase in the alpine, while they completed or showed at least two phases in treeline (Adhikari and Kumar 2020). Similarly, Anaphalis royleana Hook.f., Corydalis cornuta Royle, Polygonum filicaule Wall. ex Meisn, Nepeta govaniana (Wall. ex Benth.) Benth. and Ranunculus hirtellus showed fruiting and seeding phase in alpine, while it was absent in the treeline. Contrary to this it was absent in Epilobium royleanum Hausskn., Parnassia nubicola Wall. ex Royle, Swertia ciliata (D.Don) B.L.Burtt in alpine, but present in treeline (Adhikari and Kumar 2020). This may be due to the absence of overhead canopy cover which might result in not enough heat retention in alpine communities, thus affecting the growth of these plants in their respective alpine habitats.
Overtime, the alpine communities have developed complementary phenological strategies by spreading individual species’ peak across the growing season (Sherry et al. 2007), which resulted in better resource utilization. However, in the study area, the temperature is getting warmer and soil moisture is constantly high. Over the period, an obvious change in environmental parameters (relative humidity, frost point, rainfall, temperature, decrease in diurnal range) has been reported (Adhikari et al. 2020). These rapid changes coupled with individual species sensitivity and adaptation strategies may give advantage to some species and disadvantage to others. Considering early snowmelt (by March) and less temperature variation (minima and maxima) during the peak growth period in the past few years’, multiple species have started their growth in early April (Adhikari et al. 2018; Adhikari and Kumar 2020). At the species level, phenological modifications in multiple species was also recorded, especially with respect to phenophase’ onset and duration. This can be seen from the comparative shrinkage and delay of vegetative and reproductive phase duration, respectively, and the advancing of the senescence phase in alpine communities. Many workers have also suggested positive growth responses and advances in phenophase initiation with advances in snowmelt and temperature (Gehrmann et al. 2021; Schuchardt et al. 2021). The flowering and fruiting phenophases has also seen an advance/extension for initiation/duration in treeline compared to the alpine (Adhikari et al. 2018; Adhikari and Kumar 2020) and past studies (Sundriyal et al. 1987; Nautiyal et al. 2001; Vashistha et al. 2009, 2011). But multiple species have also shown delay/shortening in phenophases initiation/duration in timberline and ES sites, probably due to need of higher temperature requirement coupled with early snowmelt (Gehrmann et al. 2021) or these species may be showing a lagging effect to rise in temperature as reported by Mulder et al. (2017) and need detailed further study.
Two distinctive reproductive periods were recorded in 11 species either in ES or LS or both sites in alpine zone, of which Fragaria nubicola, Oxygraphis polypetala, Prunella vulgaris, Ranunculus hirtellus and Trachydium roylei also showed two reproductive periods in treeline zone (Adhikari and Kumar 2020), but were absent in past (Sundriyal et al. 1987; Nautiyal et al. 2001). The breaking of reproductive phase may indicate species resort to fruiting under stress (dominantly temperature), while favourable conditions (warm and moist) promote vegetative growth at the expense of reproductive growth (Adhikari and Kumar 2020). This may end up influencing individual species fitness and population dynamics within communities, causing migration, and changing interspecific interactions leading to changes in community structure as is reported by multiple studies (Parmesan 2006; Elzinga et al. 2007). The above phenological responses, if persistent for longer period, may alter reproductive success of species by either, disrupting plant-pollinator synchrony or by shortening of reproductive duration synchrony (Memmott et al. 2007; Liu et al. 2011), or increased synchrony between co-flowering species (Forrest et al. 2010). This may result in development of new assemblage patterns of plant communities over time.
The present study is an attempt to highlight the phenological behaviour of alpine herbaceous species in the west Indian Himalayan Region (IHR). The present study suggests that the early snowmelt (without any manipulation) greatly advances phenophase initiation in early growth species (between timberline and alpine and as well as ES and LS sites) while late growth species (germinating after June) are not much influenced by snowmelt timing as they probably depend on temperature sums for triggering specific phenophase events. The study also finds phenophase duration to be conserved especially in late growth species, indicating less capitalisation on warmer seasons. These findings are consistent with most of the manipulation experiments conducted in the temperate or boreal forests with inconsistencies in experimental settings, as these experiments are mostly in-situ. The growing season duration in the alpine Himalaya is much longer (~ 6 months) than in other regions across the globe (3–4 months), therefore, the species responses may not comply with the findings of studies conducted in temperate or boreal alpines. The species modification of their phenophase across alpine communities already suggests a complex community response to climate change, with many species already splitting and modifying their reproductive phenophase to either avoid peak warming or to facilitate neighbour recruitment to maintain synchrony and reproductive success. These changes (advance flowering) if persistent, could affect reproductive success by altering plant-pollinator relationships in alpine communities, especially if pollinator phenology responds in a different manner to the changing environment. This may result in a different response across lifeforms, especially anthesis of herb species across different functional groups, which will result in completion and final adjustment between species for the resource (pollinators). This will ultimately affect community composition resulting in changes in assemblage patterns across sites. Furthermore, considering the wide elevation treeline range in IHR (~ 2000 m) and increasing temperatures across the globe. Therefore, there is a need to study the phenological sensitivity of herbaceous species in alpines across the IHR as species populations may show different responses under similar conditions due to orography as well as in context to snowmelt influence on community phenology especially introducing spatial variation across IHR, as the temporal relationship of plants (overlapping of anthesis, etc.) will evolve.
Data availability
Not applicable.
Code availability
Not applicable.
References
Aalto J, Scherrer D, Lenoir J et al (2018) Biogeophysical controls on soil-atmosphere thermal differences: implications on warming Arctic ecosystems. Environ Res Lett 13:074003. https://doi.org/10.1088/1748-9326/aac83e
Abeli T, Rossi G, Gentili R et al (2012) Response of alpine plant flower production to temperature and snow cover fluctuation at the species range boundary. Plant Ecol 213:1–13
Adhikari BS, Kumar R (2020) Effect of snowmelt regime on phenology of herbaceous species at and around treeline in Western Himalaya, India. Not Sci Biol 12:901–919. https://doi.org/10.15835/nsb12410716
Adhikari BS, Kumar R, Singh SP (2018) Early snowmelt impact on herb species composition diversity and phenology in a western Himalayan treeline ecotone. Trop Ecol 59:365–382
Baptist F, Yoccoz NG, Choler P (2010) Direct and indirect control by snow cover over decomposition in alpine tundra along a snowmelt gradient. Plant Soil 328:397–410. https://doi.org/10.1007/s11104-009-0119-6
Berauer BJ, Wilfahrt PA, Arfin-Khan MA et al (2019) Low resistance of montane and alpine grasslands to abrupt changes in temperature and precipitation regimes. Arct Antarct Alp Res 51:215–231. https://doi.org/10.1080/1523043020191618116
Bijalwan R, Vats M, Joshi SP (2013) Plant phenological response to microclimatic variations in an alpine zone of Garhwal Himalaya. J App Nat Sci 5:47–52
Bisht VK, Kuniyal CP, Bhandari AK et al (2014) Phenology of plants in relation to ambient environment in a subalpine forest of Uttarakhand western Himalaya. Physiol Mol Biol Plants 20:399–403. https://doi.org/10.1007/s12298-014-0238-2
Bjorkman AD, Elmendorf SC, Beamish AL et al (2015) Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Glob Change Biol 21:4651–4661. https://doi.org/10.1111/gcb.13051
Buntgen U, Hellmann L, Tegel W et al (2015) Temperature-induced recruitment pulses of Arctic dwarf shrub communities. J Ecol 103:489–501. https://doi.org/10.1111/1365-2745.12361
Cooper EJ, Dullingerb S, Semenchuk P (2011) Late snowmelt delays plant development and results in lower reproductive success in the high arctic. Plant Sci 180:157–167. https://doi.org/10.1016/j.plantsci.2010.09.005
Dirnböck T, Essl F, Rabitsch W (2011) Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob Change Biol 17:990–996. https://doi.org/10.1111/j1365-2486201002266x
Dorji T, Totland O, Moe SR et al (2013) Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob Change Biol 19:459–472. https://doi.org/10.1111/gcb.12059
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73:69–86
Elzinga JA, Atlan A, Biere A et al (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22(8):432–439
Ernakovich JG, Hopping KA, Berdanier AB et al (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20:3256–3269. https://doi.org/10.1111/gcb12568
Fazlioglu F, Wan JS (2021) Warming matters: alpine plant responses to experimental warming. Clim Change 164:1–17. https://doi.org/10.1007/s10584-021-02996-3
Forrest J, Miller-Rushing AJ (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution. Phil Trans R Soc B 3653101–31122010.https://doi.org/10.1098/rstb.2010.0145
Gehrmann F, Ziegler C, Cooper EJ (2021) Onset of autumn senescence in High Arctic plants shows similar patterns in natural and experimental snow depth gradients. Arct Sci. https://doi.org/10.1139/as-2020-004
Gezon ZJ, Inouye DW, Irwin RE (2016) Phenological change in a spring ephemeral: implications for pollination and plant reproduction. Glob Change Biol 22:1779–1793. https://doi.org/10.1111/gcb.13209
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362
Jabis MD, Winkler DE, Kuppers LM (2020) Warming acts through earlier snowmelt to advance but not extend alpine community flowering. Ecology 101:e03108. https://doi.org/10.1002/ECY.3108
Jerome DK, Petry WK, Mooney KA, Iler AM (2021) Snow melt timing acts independently and in conjunction with temperature accumulation to drive subalpine plant phenology. Glob Change Biol 27:5054–5069. https://doi.org/10.1111/gcb15803
Kawai Y, Kudo G (2018) Variations in ramet performance and the dynamics of an alpine evergreen herb, Gentiana nipponica, in different snowmelt conditions. Am J Bot 105:1813–1823. https://doi.org/10.1002/ajb2.1186
Kimball KD, Davis ML, Weihrauch DM et al (2014) Limited alpine climatic warming and modeled phenology advancement for three alpine species in the northeast United States. Am J Bot 101:1437–1446. https://doi.org/10.3732/ajb.1400214
Körner C (1999) Alpine plants: stressed or adapted? In: Press M, Scholes J, Barker M Physiological Plant Ecology (eds). 39th Symposium of the British Ecological Society, Cambridge University Press 39:297–311
Körner C, Hiltbrunner E (2021) Why Is the Alpine Flora Comparatively Robust against Climatic Warming? Diversity 13:383. https://doi.org/10.3390/d13080383
Körner C, Basler D, Hoch G et al (2016) Where why and how? Explaining the low-temperature range limits of temperate tree species. J Ecol 104:1076–1088. https://doi.org/10.1111/1365-274512574
Krenová Z, Kindlmann P, Shelly JS et al (2022) Are temperate alpine plants with distinct phenology more vulnerable to extraordinary climate events than their continuously flowering relatives in tropical mountains? Front Ecol Evol. https://doi.org/10.3389/fevo2021804102
Kudo G (2020) Dynamics of flowering phenology of alpine plant communities in response to temperature and snowmelt time: Analysis of a nine-year phenological record collected by citizen volunteers. Environ Exp Bot 170:103843. https://doi.org/10.1016/jenvexpbot2019103843
Kumar R, Adhikari B S (2023) Natural snowmelt timing influences community structre and phenological patterns in alpine meadows, West Himalaya: a case study. Proc Natl Acad Sci India Sect B Biol Sci. https://doi.org/10.1007/s40011-023-01509-9
Liu Y, Reich PB, Li G, Sun S (2011) Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 92:1201–1207. https://doi.org/10.1890/10-20601
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecol Lett 10:710–717. https://doi.org/10.1111/j1461-0248200701061x
Mulder CPH, Iles DT, Rockwell RF (2017) Increased variance in temperature and lag effects alter phenological responses to rapid warming in a subarctic plant community. Glob Change Biol 23:801–814. https://doi.org/10.1111/gcb.13386
Nautiyal MC, Nautiyal BP, Prakash V (2001) Phenology and growth form distribution in an alpine pasture at Tungnath, Garhwal Himalaya. Mt Res Dev 21:168–174
Negi GCS, Rikhari HC, Singh SP (1992) Phenological features in relation to growth forms and biomass accumulation in an alpine meadow of the Central Himalaya. Vegetatio 101:161–170. https://doi.org/10.1007/BF00033199
Oberbauer SF, Elmendorf SC, Troxler TG et al (2013) Phenological response of tundra plants to background climate variation tested using the international tundra experiment. Philos Trans R Soc B 368:20120481. https://doi.org/10.1098/rstb.2012.0481
Palaj A, Kollár J (2021) Expansion of phanerophytes above the timberline in the Western Carpathians. Biologia 76:1991–2003. https://doi.org/10.1007/s11756-021-00782-1
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annual Rev Ecol Evol Syst 37:637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Pérez-Harguindeguy N, Díaz S, Garnier E et al (2013) New handbook for standardized measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Prevéy J, Vellend M, Rüger N et al (2017) Greater temperature sensitivity of plant phenology at colder sites: implications for convergence across northern latitudes. Glob Change Biol 23:2660–2671. https://doi.org/10.1111/gcb13619
Price MV, Waser NM (1998) Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79:1261–1271
Prieto-Benítez S, Morente-López J, Rubio Teso ML et al (2021) Evaluating assisted gene flow in marginal populations of a high mountain species. Front Ecol Evol. https://doi.org/10.3389/fevo.2021.638837
Ram J, Singh SP, Singh JS (1988) Community level phenology of grassland above treeline in central Himalaya India. Arct Antarct Alp Res 20:325–332. https://doi.org/10.1080/00040851198812002680
Rangwala I, Miller JR (2012) Climate change in mountains: a review of elevation-dependent warming and its possible causes. Clim Change 114:527–547. https://doi.org/10.1007/s10584-012-0419-3
Reyes-Fox M, Steltzer H, Trlica MJ et al (2014) Elevated CO2 further lengthens growing season under warming conditions. Nature 510:259–262. https://doi.org/10.1038/nature13207
Reyes-Fox M, Steltzer H, LeCain DR, McMaster GS (2016) Five years of phenology observations from a mixed-grass prairie exposed to warming and elevated CO2. Sci Data 3:1–8. https://doi.org/10.1038/sdata.2016.88
Schuchardt MA, Berauer BJ, von Heßberg A et al (2021) Drought effects on montane grasslands nullify benefits of advanced flowering phenology due to warming. Ecosphere. https://doi.org/10.1002/ecs23661
Semenchuk PR, Gillespie MAK, Rumpf SB et al (2016) High Arctic plant phenology is determined by snowmelt patterns but duration of phenological periods is fixed: an example of periodicity. Environ Res Lett. https://doi.org/10.1088/1748-9326/11/12/125006
Sherry RA, Zhou X, Gu S et al (2007) Divergence of reproductive phenology under climate warming. PNAS 104:198–202
Singh P, Negi GCS (2018) Treeline species phenology: shoot growth leaf characteristics and nutrient dynamics. Trop Ecol 59:297–311
Smith JG, Sconiers W, Spasojevic MJ et al (2012) Phenological changes in alpine plants in response to increased snowpack, temperature, and nitrogen. Arct Ant Alp Res 44(1):135–142. https://doi.org/10.1657/1938-4246-44.1.135
Sonntag S, Fourcade Y (2022) Where will species on the move go? Insights from climate connectivity modelling across European terrestrial habitats. J Nat Conserv 66:126139. https://doi.org/10.1016/jjnc2022126139
Steinbauer MJ, Grytnes JA, Jurasinski G et al (2018) Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556:231. https://doi.org/10.1038/s41586-018-0005-6
Stocker TF, Qin D, Plattner GK et al (2013) Technical summary in climate change 2013: the physical science basis contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. pp 33–115 Cambridge University Press
Sundriyal RC, Joshi AP, Dhasmana R (1987) Phenology of high altitude plants at Tungnath in the Garhwal Himalaya. Trop Ecol 28:289–299
Theurillat JP, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109. https://doi.org/10.1023/A:1010632015572
Tian L, Fu W, Tao Y et al (2022) Dynamics of the alpine timberline and its response to climate change in the Hengduan mountains over the period 1985–2015. Ecol Indic 135:108589. https://doi.org/10.1016/j.ecolind.2022.108589
Tonin R, Gerdol R, Tomaselli M et al (2019) Intraspecific functional trait response to advanced snowmelt suggests increase of growth potential but decrease of seed production in snowbed plant species. Front Plant Sci 10:1–12. https://doi.org/10.3389/fpls.2019.00289
Vashistha RK, Rawat N, Chaturvedi AK et al (2009) An exploration on the phenology of different growth forms of an alpine expanse of North-West Himalaya, India. N Y Sci J 2:29–41. https://doi.org/10.1007/s11676-011-0194-4
Vashistha RK, Rawat N, Chaturvedi AK et al (2011) Characteristics of life-form and growth-form of plant species in an alpine ecosystem of North-West Himalaya. J For Res 22:501. https://doi.org/10.1007/s11676-011-0194-4
Wipf S, Stoeckli V, Bebi P (2009) Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Clim Change 94:105–121. https://doi.org/10.1007/s10584-009-9546-x
Wolkovich EM, Cook BI, Allen JM et al (2012) Warming experiments underpredict plant phenological responses to climate change. Nature. 485:494–497. https://doi.org/10.1038/nature11014
Wu X, Guo W, Liu H et al (2019) Exposures to temperature beyond threshold disproportionately reduce vegetation growth in the northern hemisphere. Natl Sci Rev 6:786–795. https://doi.org/10.1093/nsr/nwy158
Yu H, Luedeling E, Xua J (2010) Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. PNAS 107:22151–22156. https://doi.org/10.1073/pnas.1012490107
Zhang X, Zwiers FW, Hegerl GC et al (2007) Detection of human influence on twentieth-century precipitation trends. Nature 448:461–465. https://doi.org/10.1038/nature06025
Acknowledgements
The authors would like to thank Director and Dean, Wildlife Institute of India (WII) for providing necessary facilities and Uttarakhand Forest Department for granting permission to conduct field work in Kedarnath Wildlife Sanctuary. We also thank Prof. S. P. Singh for his suggestions. We would also like to thank Sachin MH and Manish Bisht for their help during the field work.
Funding
This study was funded by the National Mission on Himalayan Studies, MoEF & CC, New Delhi.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by RK and BSA. Data curation, formal analysis, and writing were done by RK. Methodology was done by BSA and RK. Supervision and review were done by BSA.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Adhikari, B.S. Snowmelt influence on phenological events of herbaceous plants in alpine region of West Himalaya. Braz. J. Bot 46, 1041–1054 (2023). https://doi.org/10.1007/s40415-023-00939-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-023-00939-z