Abstract
This study aims to find out the impact of early natural snowmelt on diversity, composition and phenology of herbaceous vegetation of treeline ecotone (3200–3300 m asl) at Tungnath, Uttarakhand Himalaya. Based on orography, four sites were selected across the treeline ecotone and within each site, two microsites based on snowmelt timing were identified. Across the sites, 86 species were recorded at and around treeline ecotone, 90% were perennial forbs, 84% were hemicryptophytes and 70% were native. Both herb species richness and species diversity were higher in exposed microsites. The total plant density ranged between 82 and 626 individuals m−2 in exposed and 69–288 individuals m−2 in unexposed microsites showing that a warming climate might favour an increase in richness and diversity. The early phenophase was noticed in the majority of species in exposed than at unexposed microsites. The timing of different phenophases varied among the sites. Comparing this study with previous studies either in the same or similar study areas based on 13 common species indicates that timing and duration of vegetative and flowering phenophase have advanced and lengthened, while fruiting and seed maturation have shortened presumably because of the increase in mean minimum temperature. This indicates that species might adopt different adaptations to a warming climate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
The high mountain ecosystems are under tremendous pressure at present because of habitat destruction, landscape modification and loss in biodiversity (Korner 2003; Bruun et al. 2006) and are under the threat of climate change (Beniston 2003; Pauli et al. 2012). Some of the major consequences of climate warming are early snowmelt, increase in soluble N deposition in high mountain habitats, limitation of plant growth and diversity in terrestrial ecosystems (Hättenschwiler and Körner 1997; Tørseth and Semb 1997) may restrict plant growth in alpine species (Hiltbrunner et al. 2005; Gong et al. 2015). ‘Alpine’ is commonly used in a broad sense for the treeless areas above a low-temperature determined treeline in the high reaches of mountains, characterized by low temperature, precipitation in form of scanty rainfall, blizzard and snowstorms, high wind velocity and high intensity of ultraviolet (UV) radiation (Nautiyal et al. 2004). A treeline ecotone is a transition zone between a closed canopy upright forest (timberline) and low growing primarily herbaceous alpine. The treeline zone, elevational limit of a tree, is often controlled by temperature. This limit is directly affected by drastic drop in allocation of energy gained via photosynthesis (Körner 1998) and is modulated by several factors such as seasonal snowpack duration, winds, precipitation, soil and high intensity of UV radiation (Nautiyal et al. 2004). Due to the extreme climate, plants adapt to these conditions and in general are dwarfed, stunted, woolly or spiny, and develop a mosaic patch of different life forms (Walker et al. 1994). The community structure and composition show that seasonal fluctuations are strongly influenced by the extent to which periodic phenomena in the individuals are adjusted to each other (Kershaw 1973). The resulting hypothesis is that as the climate warms, trees will move up the slope towards higher reaches. Several studies have shown such geographic responses (Lloyd and Fastie 2002).
Snow is an important environmental factor controlling micro-climate and plant growth (Wipf 2010) and acts as an insulator during the cold season, protecting the plants and soil underneath (Sturm et al. 1997). The increase in soil moisture at the initial stage of the vegetative period due to early snowmelt changes the community composition, species richness and the occurrence patterns of individual species (Korner 2003; le Roux et al. 2013). Snow duration and extent determine the beginning and length of the growing period, and meltwater provides water and nutrients that are crucial for plant growth (Jones et al. 2001). To upland ecosystems, snowmelt timing, depth and duration of the snow cover are of special importance as they define the start and duration of the potential growing season (Inouye and Wielgolaski 2003), the temperatures under the snow (Pomeroy and Brun 2001) and characteristics of alpine plant communities (Korner 2003).
In alpine and arctic systems, plant communities’ occurrence is closely associated with spatial snow distribution patterns (Odland and Munkejord 2008), showing the importance of snow depth and snowmelt with season length in structuring communities as changes in the snow cover can have a wide range of species-specific effects on alpine tundra plants (Wipf et al. 2009). Several workers (Campbell et al. 2005; Sturm et al. 2005) suggested that the snowmelt controls the biochemical, microbial and plant processes during winters, which persisted well into the growing season (Weih and Karlsson 2002). An early snowmelt results in the longer growing season and higher growing degree days, but it also increases frost damage (subzero temperature), earlier dehardening resulting in higher frost sensitivity (Inouye 2000; Gorsuch and Oberbauer 2002).
The changing climate has been affecting high altitude plant communities (Wipf and Rixen 2010) and plant phenology (Smith et al. 2012), which can potentially impact ecosystem services they provide (Winkler et al. 2018) and the range-restricted species (Parmesan 2006), particularly in polar and higher elevation (alpine) regions. The other drivers of change, such as extreme events, drought and forest fires, and anthropogenic disturbance have further intensified impact on the structural and functional attributes of alpine vegetation communities that harbours large number of high-value medicinal, aromatic and threatened plants (Xu et al. 2009; Singh et al. 2011). Therefore, alpine plants are sensitive to climate change and are considered as good indicators for understanding its impacts (Pauli et al. 2007) as compared to other ecosystems (Malik and Nautiyal 2016).
In the Himalayan region, the studies related to assessing the ecological impacts of winter precipitation (snowmelt) on alpine plant communities are very limited (Adhikari et al. 2018). Therefore, considering the gap, in this study we analyse the impact of snowmelt water and snow cover on phenology and structural and functional attributes of herbaceous plant communities at treeline ecotone.
17.2 Study Area
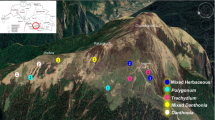
This study was conducted in Tungnath which lies in the upper catchment of Alaknanda (one of the two tributaries of river Ganga) and Mandakini rivers of Uttarakhand, India (Fig. 17.1). Alpine meadow communities consisting of forbs, grasses and sedges occur in Tungnath region along with Rhododendron campanulatum krummholz. A year is divisible into four seasons, viz. short summer (May to June), monsoon (July to mid–September), autumn (mid-September to October) and long winter (November–April). The period with snow cover is of about 4–5 months (December–April) and snowmelts during April–May between 3200 and 3300 m asl. The snowfall is staggered in the study region and area to become inaccessible.
17.3 Methods
17.3.1 Study Design
Treeline ecotone at Tungnath region (Photographs 17.1 and 17.2) is almost 2 km long lying between 3200 and 3300 m asl predominated by Abies spectabilis and Quercus semecarpifolia forests along with Rhododendron krummholz. Four summits namely Ravanshila, Chandrashila, Patankudi and Jhabra were selected based on the orographic lift for the natural snowmelt experiment (Table 17.1). These summits were further divided into two major snow cover regions (high snow cover: hereafter referred to as HSC and snow depth 1.0–1.5 m during January comprised of Patankudi and Jhabra summits and low snow cover: hereafter referred to as LSC and snow depth 0.6–1.0 m during January comprised of Ravanshila and Chandrashila summits). At each site, a plot (50x50m) was selected at the base of summits between 3200 and 3300 m asl. Within the selected plot two different microsites (namely, Exposed microsite: hereafter referred to as EX with canopy cover <20% and Unexposed microsite: hereafter referred to as UX with >20% canopy cover) were identified based on snowmelt timing. The UX microsites had higher snow deposition due to snowdrift and longer snow stay period due to krummholz cover resulting in late snowmelt (delayed 4–14 days). Within each microsite, 3 random quadrats (1x1m each) were permanently laid with a distance of 10–12 m to record plant abundance, richness and diversity.
17.3.2 Lifespan
The recorded plants were categorized based on their lifespan into the following categories: annuals, plants that live for one growing season, perennials; plants that grow and bloom over spring/summer season and die every autumn/winter and grow again from their rootstock; and biennials, plants which complete life cycle in two growing season, germinate, grow and survive through winters and next year grow more, bloom and die.
17.3.3 Growth Form Categories
The direction and extent of growth and branching on the main-shoot axis or axes determine the growth form of a plant. The plants were classified following Pérez-Harguindeguy et al. (2013) under the following growth forms, viz. climber, dwarf shrub, semi-basal, short-basal, erect leafy and tussock to compare with other studies conducted in western Himalaya.
17.3.4 Life Form Categories
Following Raunkier (1934) the plants were assigned to various life-form classes, viz. phanerophyte (surviving buds or shoot apices are borne on shoots into the air), chamaephyte (surviving buds/shoot apices are borne on shoots very close to the ground), hemicryptophyte (surviving buds/shoot apices are situated on the soil surface), geophyte (surviving buds/shoot apices are below soil surface) and therophyte (complete their life cycle from seed to seed and die within a season).
17.3.5 Nativity
The place of origin/first record of the species where it occurs naturally indicates the nativity of the species (Anonymous 1883–1970), while the introduction of the species in an area happened through anthropogenic activities and has not previously occurred in that area are known as ‘Non-Native’. We used Index Kewensis Plantarum Phanerogamarum (Anonymous 1883–1970) to know the nativity of a species.
17.3.6 Species Richness, Diversity and Evenness
Species richness was taken as a count of the total number of species in a particular area. The diversity was calculated after Shannon and Wiener (1949). If Pi is the proportion of individuals (from the sample total) of species i, then diversity (H′) is
where P is the proportion (n/N) of individuals of one particular species found (n) to the total number of individuals found (N), ln is natural log and s is the number of species.
Evenness index was calculated using Pielou (1975).
where H′ is Shannon & Wiener diversity index, H′max = ln S, is the natural log of S.
Sorenson Similarity Index (Is) between different sites was calculated following Sorensen (1948).
where Is the Sorenson Index of Similarity; C, the common species to both comparable sites; A, the total number of species in site A; and B, the total number of species in site B.
17.3.7 Simper
The contribution of each species to the dissimilarity (%) between two groups was calculated from the Bray–Curtis dissimilarity matrix through SIMPER analysis in PAST.
17.3.8 Phenological Observations
The phenological changes were observed visually to monitor how the species respond to climate variations on a monthly interval. Therefore, records of various phenophases such as growth initiation, vegetative phase, flowering, fruiting, seed formation and senescence of each species were taken from May to October in 2017 following the Biologische Bundesanstalt Bundessortenamt und Chemische Industrie (BBCH) scale (Hess et al. 1997). The complete existence of a particular phenophase was considered if 5% of the individuals showed that phenophase within the plot. Based on growth initiation time, the plants were divided into two categories: early growth species (up to mid-May) and late growth species (mid-May to June). The phenology of each species was observed within quadrats under each category in adjacent areas with similar micro-environment within selected plots to minimize the effect of herbivory.
17.4 Results
Plant growth period (the period from the beginning of growth to the time when about 80% of plant shoots senesced) was 6 months from mid-April to mid-October. Soil moisture values (45–48%) observed during the peak growing period (July) showed that it is not a limiting factor for plant growth. The number of snowfall days during winter months (December to April) in 2016–2017 was 39 days.
17.4.1 Lifespan Category, Growth Form, Growth Cycle and Life Form
Across the sites and within EX and UX areas, a total of 86 plant species were recorded, of which 90% were forbs and remaining 10% were climber (1%), shrub (3%) and grasses and sedges (6%), while 90.6% were perennials followed by annuals (8.2%) and biennials (1.2%; Table 17.2). Of the total species, 53% species were early growing (germination before July) while 47% were late growing species (germination after June). Growth forms of the species were highest for semi basal/short basal followed by erect leafy, dwarf shrub and climber/shrub/tussock. The maximum species had a long growth cycle (LGC) followed by an intermediate growth cycle (IGC) and a short growth cycle (SGC). Among various growth forms, tall and short forbs contributed the maximum followed by medium forbs and others (climbers, shrub/undershrub, grasses/sedges; Table 17.2). The majority of the species recorded in this study were native to the Himalayan region (70%) indicating a high conservation value of treeline in the Tungnath region.
17.4.2 Species Richness and Diversity Patterns
The species richness was almost equal in EX and (74) and UX (72) microsites. In general, it was noticed that the richness of the herbaceous species increased across the months from May to September (44–80 species, respectively) and then declined in October (56 species).
The species richness attained a peak between July and September (77–80 species) and 60% of species were common to both EX and UX microsites. Generally, species number in a month was markedly higher in EX microsite than in UX microsite, particularly in Jhabra (EX: 38, UX: 27) in July. Two-way ANOSIM showed significant variations in species richness between sites (r = 0.93) and across months (r = 0.91).
At EX microsites the species richness was highest in July for Chandrashila, Patankudi and Jhabra, while in August for Ravanshila. In UX microsites, species richness was highest in July for Ravanshila and Jhabra and in August for Chandrashila and Patankudi (Fig. 17.2). This could be because these microsites are dominated by krummholz, protecting species against environmental factors prevailing in other microsites. In general, species richness was higher in EX than UX microsites, probably due to the absence of canopy cover, ample sunlight and less competition for resources.
The diversity values from 2.10 to 3.11 in UX microsites and 2.41 to 3.21 in EX microsites and peaked during July for both EX and UX microsites (Fig. 17.3). The change in diversity after July was due to the senescence of early growth cycle species.
The evenness values ranged from 0.46 to 0.77 in UX microsites and 0.40 to 0.68 in EX microsites. The evenness values for Ravanshila, Patankudi and Jhabra sites showed a similar distribution pattern in both EX and UX microsites, while in Chandrashila species were much more evenly distributed in UX microsites. This could be due to the favourable condition for growth and dominance of Ranunculus, Fragaria, Oxygraphis, Trachydium and Prunella over other species.
17.4.3 Site and Microsite Similarity
The similarity among sites was highest between Ravanshila and Patankudi (66%) followed by Ravanshila and Chandrashila (65%), Chandrashila and Patankudi (63%), Ravanshila and Jhabra (62%) and Chandrashila and Jhabra (50%). The reason could be the similar number of species present in Ravanshila (45), Patankudi (46) and Jhabra (42), which is much less than the species present in Chandrashila (66). The micro-climate of Ravanshila and Patankudi is different than that of Chandrashila, which lies in between and have an admixture of micro-climate of both sites, which may lead the species to move in, hence the richness is high. Although the similarity between EX and UXmicrosites was 82% as a whole, the similarity between EX and UXmicrosites within each site was much less (Chandrashila 31%, Patankudi 34%, Ravanshila 36% and Jhabra 38%). Across months, the similarity was highest between microsites during peak growing season (July–September; Table 17.3).
SIMPER (Similarity Percentage) analysis suggested the primarily responsible taxa (species) for observed differences between groups of samples, that is, snow cover region and microsites. The overall dissimilarity between HSC and LSC region was 56.8% and the maximum contribution was generated by following species in decreasing order: Ranunculus > Trachydium > Oxygraphis > Carex > Fragaria > Potentilla polyphylla > Prunella. Comparing average dissimilarity between EX and UX microsites of HSC and LSC region, the average dissimilarity was 63% contributed in EX microsites by 5 species (Ranunculus > Trachydium > Oxygraphis > Carex > Prunella); however, in UX microsite it was 59.2% generated by 11 species (Fragaria > Potentilla polyphylla > Ranunculus > Trachydium > Poa > Galium > Anemone > Polygonum filicaule > Polygonum vccinifolium > Carex > Lysimachia).
Across sites the overall average dissimilarity in UX microsites was 64% between months and 72% between sites; however, in EX microsites it was 59% and 67%, respectively. Within sites, the maximum contribution to dissimilarity (up to 50%) as follows:
EX microsites: Ranunculus > Trachydium > Carex > Oxygraphis > Fragaria > Anaphalis > Prunella > Koenigia > Anemone.
UX microsites: Fragaria > Ranunculus > Polygonum > Carex > Bistorta > Trachydium > Anemone > Poa > Polygonum > Circea > Potentilla.
17.4.4 Habitat Preference of Plant Species
The species like Bistorta amplexicaulis, Fragaria nubicola, Geranium wallichianum, Potentilla atrosanguinea and Viola biflora were present in all microsites. The species specifically present in all UX microsites were Myriactis wallichi and Veronica cana, while Anaphalis nepalensis, Kobresia, Oxygraphis polypetala, Polygonum filicaule, Potentilla lineata, Potentilla polyphylla and Selinum vaginatum occurred in EX microsites. The distribution of certain species was restricted to few sites, such as Bupleurum longicule, Rubus nepalensis, Swertia speciosa, Synotis alata, Arenaria, Euphorbia sp. and Taraxacum officinale in Ravanshila; Aster methodrus, Viburnum glanduliflorum, Swertia auriculata, Arisaema propinquim, Ligularia amplexicaulis, Halenia elliptica, Senecio graciliflorus, Trillium govanianum, Smilacina purpurea and Aster albescens in Chandrashila; Thalictrum foliolosum, Primula edgeworthii and Ligularia sibirica in Patankudi and Rumex nepalensis, Cynoglossum glochidiatum, Primula redii, Morina longifolia, Cerastium cerastoides and Persicaria nepalensis in Jhabra. Aster methodrus, Viburnum glanduliflorum, Arisaema propinquim, Ligularia amplexicaulis, Thalictrum foliolosum, Hemiphragma heterophyllum, Halenia elliptica, Trillium govanianum, Smilacina purpurea, Clematis, Parochetus communis, Galium asperifolium and Caltha palustris were found in UX microsites, and Swertia auriculata, Rumex nepalensis, Aster albescens, Cynoglossum glochidiatum, Primula redii, Ligularia sibirica, Goodyera repens, Persicaria nepalensis, Corydalis cornuta, Caltha palustris, Trachydium roylei, Gaultheria trichophylla, Gentiana argentea, Gentiana tubiflora, Gerbera gossypina and Morina polyphylla in EX microsites.
17.4.5 Community Structure
In all microsites, the herbaceous communities in the initial phase were different in UX microsites. In Ravanshila and Jhabra sites, Fragaria and Ranunculus dominated the community in UX microsites, respectively and showed changes in August onwards in Ravanshila, while Ranunculus dominated the community throughout the growing period in Jhabra. It was interesting to note that in Chandrashila, the community changed after August, while in Patankudi the species dominance changed throughout the growing period in UX microsites. The community in Chandrashila and Jhabra was dominated by Ranunculus throughout the growing period in EX microsites, while Anaphalis dominated in Patankudi throughout the growing period, except May and August. Ranunculus, Fragaria, Oxygraphis and Trachydium dominated the vegetation in EX microsites of Ravanshila (Table 17.4).
Plant density among the sites (Chandrashila, Ravanshila and Patankudi) was more on EX microsites than on UX microsites, except Jhabra where the density values were not affected by exposure (Fig. 17.4). In moist microsites, water was not a limiting factor. In Chandrashila and Ravanshila microsites, plant density at EX microsites was more than twice than that on UX microsites. The snow effect was minimized because of the shadow of higher summit in Patankudi and Jhabra which resulted in faster snowmelt in Ravanshilla and Chandrashilla (45–65 days) as compared to Patankudi and Jhabra (55–80 days) for EX microsites. Plant densities generally were high initially in June–July, then declining from there with some deviations.
17.4.6 Phenological Trends across Microsites
The micro-climatic conditions, which include environmental conditions (aspect, slope, moisture, soil texture, ambient and soil temperature, rainfall etc.) and topographical features (gentle, steep, boundary/rocky) and anthropogenic factors (grazing, trampling, collection of NTFP) influenced the phenophases. The relationship between growth initiation and snowmelt was evident in several micro-sites. Growth in species was initiated immediately after the snowmelt in May. For example, immediately after snowmelt, Anemone rivularis, Fragaria nubicola, Gentiana argentia, Lysimachia prolifera, Oxygraphis polypetala, Plantago major, Rannunculus hirtallus, Selinum vaginatum, Trachydium roylei and Gaultheria trichophylla were observed in flowering, while Arenaria, Caltha palustris, Carex setosa, Nepeta govaniana, Polygonum filicaule, Potentilla polyphylla, Primula denticulata, Trachydium roylei and Plantago himalaica were in bud development phase. The timing of different phenophases varied among microsites and it was quite similar between Ravanshila and Chandrashila and between Patankudi and Jhabra, while more variability in phenophases was observed in extreme sites, that is, Ravanshila and Jhabra.
In the early growing season (May–June) only vegetative and reproductive phenophases were observed in all the microsites, all phenophases were recorded from the mid-growing season (July–September; Fig. 17.5). Goodyera ripens germinated in EX microsites only during August and Epilobiumroylei showed vegetative reproduction in UXmicrosites in September only. Fruiting started in June (Trachydium roylei and Plantago himalaica) in EX microsites and in UX microsites it started in July and last till September.
Oxygraphis polypetala and Ranunculus hirtelus showed dual reproductive phenophase in months of May–June and August–September in both EX and UX microsites, while Fragaria nubicola showed dual reproductive phase in the month of June and August in EX microsite only. Of the total 86 species, only 23 species (26.7%) showed fruit and seed maturation phases. The absence of fruiting and seeding phenophase in most of the species (73.3%) at both EX and UX microsites and the abovementioned species in UX microsites may be due to an increase in mean minimum temperature in Tungnath region, which favours longer vegetative and reproductive phenophase.
17.5 Discussion
The precursor to initial plant growth is snowmelt water, which was received by the microsites in the form of snowfall (39 days; 4 days in December 2016 and 35 days during January to April in 2017), before the onset of monsoon. Due to heavy rainfall in recent years (241.1 ± 43.2 cm) (maximum in September) during 2008–2010 (Adhikari et al. 2012) and 370.7 cm (maximum in July) for 2017 (GBPIHE) in the region helped species to delay their senescence. The other reason could be much variation in temperature (minima and maxima) in the past (7.7 °C in October to 12.2 °C in June in 1984 (Sundriyal et al. 1987) as compared to 2017 and 2.4 °C in July to 9.9 °C in October (GBPIHED). Similarly, the mean monthly temperature was too high (13–23 °C) during the growth period (June to September) in the past (1988–1998) by Nautiyal et al. (2001) as compared 9.6 to 12.6 °C in 2008–2010 (Adhikari et al. 2012).
Comparing this study with that Nautiyal et al. (2001), we found a decrease in Long growth cycle (LGC) species and an increase in short growth cycle (SGC) and intermediate growth cycle (IGC) species for tall forbs. The percentage of LGC species increased and IGC species decreased for medium forbs, while SGC was absent in this study. For short forbs, per cent LGC increased, while IGC and SGC decreased in this study as compared to Nautiyal et al. (2001). We also noticed increase in LGC and IGC species in grasses/sedges, a climber was observed in LGC in this study.
Hemicryptophyte (83.5%) was the predominant life-form which is in conformity with other studies conducted at similar elevations in the alpine region of Garhwal Himalaya (Nautiyal 1996, Nautiyal et al. 2001; Sundriyal et al. 1987; Rawat 2007; Dad and Khan 2010; Vashistha et al. 2011, Kumar et al. 2016; Table 17.5). In general, the life-form spectrum of an area is affected by the grazing intensity as well as anthropogenic factors. The proportion of hemicryptophytes increased in the study area over the last three decades as compared to previous studies (Sundriyal et al. 1987), showing better adaptability of hemicryptophytes to grazing, trampling and other environmental factors, viz. wind, heavy rainfall/erosion, heavy frost than that of other life forms. The proportion of chamaephytes was reported to be high in Rudranath region (31%) by Ram and Arya (1991), in Central Himalaya (47%) by Pangtey et al. (1990) and in Yusmarg (46%) by Dhar and Kachroo (1983), could be due to less rainfall than that of this study site.
The nativity, first record/origin of the species and endemism of any biogeographic province denotes the high conservation value of the area (Anonymous 1883–1970). In Western Himalaya, very few studies (Samant et al. 1998, 2001; Samant and Joshi 2005) on nativity and endemism of the species are available. It indicates that the dominance of native species is quite prevalent in the region, while the introduction of non-natives due to livestock grazing, tourism and fuelwood collection in the region does not have any impact. The alpine meadows struggle with heavy tourism influx, high grazing intensity and exploitation of natural resources, such as high-value medicinal plants, imbalance the natural ecosystems. Samant et al. (1998) opined that the areas with a high percentage of native species deserve more conservation, while due to overexploitation and habitat degradation allow the introduction of non-natives, which leads to less conservation value of the area. The loss of a native and endemic species can be due to direct competition between native and non-native plants, the introduction of non-natives in the area which alters ecosystem properties drastically (Vitousek 1986) and further leads to the community dominated by exotic annuals and through succession process (Stylinski and Allen 1999). The species richness across various sites in western Himalaya is reported to range from 27 to 56 species per site (average 30.5 ± 8.3 species; Rawat 2007), thus species richness of this study site (49.8 ± 5.5 species) is well within this range. The species richness values in this study per square meter (UX and EX microsites between 12.5 and 18.6 and 16.8 and 21.0 species m−2, respectively) are similar to that (15–21 species m−2) reported for Zemu valley in Sikkim by Tambe (2007). It shows that in alpine herbaceous communities the species richness does not differ between Tungnath, Uttarakhand and in Sikkim site, though in lower elevations Sikkim is far more species-rich than Uttarakhand. Across all microsites and months plant density ranged between 69 and 626 individuals m−2 in this study, which are higher than the values reported for the entire western Himalaya (152 ± 90 individuals m−2) by Rawat (2007) and herb density reported for treeline zone (123–239 individuals m−2) by Rai et al. (2012).
The average diversity ranged from 2.77 to 2.83 for this study, which are less than that reported for herb layer of treeline zone (3.06–3.25) by Rai et al. (2012), for south or south-east facing slopes of the Greater Himalaya (3.01–3.30) by Rawat (2007) and treeline gap (3.23) by Kala et al. (1998). The values of this study are higher than the values reported by Rawat (2007) for the entire greater Himalaya (2.1–2.4), Kala et al. (1998) for Valley of Flowers NP (2.47), Singh (1999) in Upper Tirthan Valley (2.39) between 3200 and 3300 m, Adhikari (2004) for Nanda Devi NP (0.587–2.088) and Tambe (2007) for the alpine landscape of Khangchendzonga NP (1.44–2.48), could be due to high grazing pressure and more moisture in the study area. Similar observations were also observed for high arctic tundra by Park et al. (2018).
It is interesting to note that while only 7 species were responsible for more than 50% dissimilarity between HSC and LSC regions, just 5 species contributed for dissimilarity of EX microsites between HSC and LSC against 11 species for UX microsites. Furthermore, all 5 species of EX microsites (Ranunculus, Trachydium, Oxygraphis, Carex and Prunella) contributed to the overall dissimilarity between HSC and LSC, while only 4 species (Fragaria, Potentilla polyphylla, Ranunculus and Trachydium, later 2 species common with EX microsites) out of 11 species contributed to overall dissimilarity. These 5 species search for places with ample sunlight and moisture, so that they can grow gregariously (> 50 individuals m−2, mean average), as they are LGC species. Compared to the above 5 species, Fragaria and Potentilla polyphylla love to grow in moist and shady places with moderate abundance (30–45 individuals m−2, mean average).
It is interesting to note that in EX microsites of Ravanshila, Chandrashila and Jhabra sites, Ranunculus contributed more for dissimilarity, while Anaphalis for Patankudi. This could be due to the fast melting of snow in the first three sites and late melting in Patankudi (Table 17.1). Among sites, Fragaria for Ravanshila and Chandrashila, Bistorta for Patankudi and Ranunculus for Jhabra contributed the most. It is evident that the contribution of these species is directly related to fast snowmelt and less grazing in the treeline ecotone of Ravanshila and Chandrashila, and high grazing pressure and late thawing of snow in Patankudi and Jhabra.
In general, phenophases were in advanced stages in EX than in UX microsites, which is similar to the findings of other experiments conducted in similar alpine environments (Wipf et al. 2009; Wipf 2010). Since different species respond differently to snowmelt effect, it indicates that apart from snowmelt, other factors also play major role (Gehrmann et al. 2018), as some contradictions were seen in different phenophases among different life forms. The absence of vegetative phenophase in some species may be contributed by the chilling effect due to frost and early snowmelt, where newly germinated buds either die or move to the next phenophase for stability.
Nautiyal et al. (2001) reported that immediately after the snowmelt 16 species were in the flowering phase, among them Gentiana argentea and Oxygraphis polypetala emerged early and most dominant in the Tungnath region (mainly alpine). Comparing this study results with Nautiyal et al. (2001) a general shift was observed in the vegetative phase by a month (i.e. June). Over a period of time (from 1984 to 2017) the duration of vegetative phenophase increased from 2 months (Sundriyal et al. 1987), to 4 months (Nautiyal et al. 2001) and 6 months (present study). It was also observed that the phenophase stability and duration was prominent in the studies conducted by Sundriyal et al. (1987) and Nautiyal et al. (2001), but in this study, phenophases overlapped resulting in no clear-cut dominance (stability) of phenophase. As such there was no change in the flowering period, but fruiting and seed maturation ended in September in this study as compared to October during 1988–1998 (Nautiyal et al. 2001). Senescence in this study started in May, which was 2 months prior to that reported by Nautiyal et al. (2001). The longer vegetative phenophase for this study could be due to higher precipitation in recent years (Adhikari et al. 2012; Adhikari et al. 2018) and less difference in minimum and maximum temperature in recent years (Adhikari et al. 2018) as compared to Sundriyal et al. (1987).
Most of the species showed senescence early in UX microsites than that of EX microsites, which may be due to an early drop in temperature in UX microsites than EX microsites. The above discrepancy may be due to a number of reasons like interspecific (early vs late flowering; Petraglia et al. 2014) or intraspecific (collective life form response to snowmelt timing, Khorsand et al. 2015; Livensperger et al. 2016) interactions or maybe due to micro-environmental changes (temperature). Considering early snowmelt (by March) in past few years, it is possible that certain species (Primula edgeworthii, Anemone rivularis, Gentiana spp., Picrorhiza kurroa, Kobresia) have started their growth in early April high water-absorbing ability at low soil temperature, which might be related to a high level of soluble carbohydrate (Mooney and Billings 1960) in early senescence. The species per cent in senescence increased sharply from August to September in this study is in conformity with the study conducted by Nautiyal et al. (2001).
The phenophases of 10 most common species namely Anaphalis royleana, Anemone obtusiloba, Bupleurum longicaule, Danthonia cachemyriana, Geranium walliichianum, Oxygraphis polypetala, Pedicularis pectinata, Selinum vaginatum, Taraxacum officinale and Trachydium roylei in this study were compared with the studies conducted by Sundriyal et al. (1987) for the year 1984, Nautiyal et al. (2001) for the year 1988–1998 (11 years; Fig. 17.6) in Tungnath region and Bijalwan et al. (2013) for the year 2008 in Dyara Bugyal. Anaphalis royleana had a much longer vegetative phase and consequently delayed flowering in 2017 than in 1984. The flowering period lagged by half month compared to 1984. The fruiting period duration was the same, but the timing of fruiting and senescence lagged behind by a month in 2017 as compared to 1984. Anemone obtusiloba had a much longer vegetative phase, and budding and flowering advanced by 1 and 3 weeks, respectively between 2008 and 2017. Fruiting was absent and senescence lagged by a month in 2017 than in 2008 (Bijalwan et al. 2013). In Bupleurum longicaule flowering and fruiting phases advanced by more than a month and 3.5 weeks, respectively for the year 2017 as compared to 2008 (Bijalwan et al. 2013).
Comparison of phenophases for the year 1988–1998 (Nautiyal et al. 2001) and 2017 (present study) in Tungnath region
Danthonia cachemyriana had a much longer vegetative phase and its reproductive phase delayed by 4 weeks in 2017; however, fruiting was restricted to the last week of August in 2017 as compared to mid-August to mid-September in 1984 (Sundriyal et al. 1987). The senescence advanced by 2 weeks in 2017 as compared to 1984 (Sundriyal et al. 1987). The duration of vegetative, budding and flowering phenophases of Geranium wallichianum increased and advanced significantly in 2017 as compared to 2008. In this fruiting was delayed by 2 weeks and senescence advanced by a month in 2017 as compared to 2008 (Bijalwan et al. 2013). Oxygraphis polypetala had a much longer vegetative phase up to August in 2017 as compared to 1984, restricted to the first week of May. Budding, flowering and fruiting phases occurred in two different time periods between May–June and late July–September in 2017 as compared to 1984 (Sundriyal et al. 1987) budding and flowering phases were present in May and fruiting in June to early August. Senescence was delayed by one and a half months in 2017 than in 1984 (Sundriyal et al. 1987). The vegetative phase duration in Pedicularispectinata increased exponentially while the advancement of budding, flowering, fruiting and senescence phases was observed in 2017 than in 1984. The duration of the vegetative phase for Selinum vaginatum increased to early September and the budding and flowering delayed, fruiting phase was absent and there is no change in senescence between 2017 and 2001. The duration for the vegetative phase increased while reproductive and fruiting advanced for Taraxacum officinale during 2008–2017. For Trachydium roylei, the flowering period shortened, and fruiting and senescence phases advanced between 1984 (Sundriyal et al. 1987).
The phenophases for Geum elatum, Parnassia nubicola and Potentilla atrosanguinea were compared at different sites, viz. Dyara in 2008 (Bijalwan et al. 2013; Sundriyal et al. 1987) with this study (Fig. 17.7). In Tungnath, the vegetative phase of Geum elatum extended by 3 weeks, budding lag behind a week, flowering and fruiting advanced by a week and no change in senescence was observed as compared to 1984. However, all the phenophases in Dyara, except the vegetative phase, lag behind 1 to 4 weeks in 2008 (Bijalwan et al. 2013). All phases of Parnesia nubicola were delayed by 1–4 weeks in 2017 as compared to 1984 (Sundriyal et al. 1987), while the reproductive phase was delayed as compared to 2008 (Bijalwan et al. 2013). Potentilla atrosanguinea had an extended vegetative phase, budding and flowering delayed and fruiting and senescence advanced in 2017 as compared to 1984 (Sundriyal et al. 1987).
The less variation in temperature (minima and maxima) during the peak growth period, that is, July–August helped species to remain in different phenophases for a longer period. A shift of phenophase timing and period was observed in general for the species common to past studies in the study site (Nautiyal et al. 2001; Sundriyal et al. 1987). Early onset of growth initiation and extended vegetative phenophase was observed, could be due to early snowmelt/soil water thawing leading to early soil moisture availability and ambient temperature which favoured early growth as suggested by several workers (Holway and Ward 1965; Kudo 1991; Ram et al. 1988; Kudo and Suzuki 1999; Kudo and Hirao 2006). Flowering and fruiting phenophases have also seen a shift (both advance and lag) in phenophase initiation and duration of the period in general and have extended compared to other studies (Sundriyal et al. 1987; Nautiyal et al. 2001). Variation in the phenophase period at spatio-temporal scale due to orography and micro-environmental variables were also observed (Nautiyal et al. 2001). The snowmelt timing with growth initiation and flowering has been observed (Fareed and Caldwell 1975; Bock 1976; Owen 1976), while ambient temperature during snowmelt was strongly favoured the growth initiation in alpine plants (Holway and Ward 1965; Kudo 1991; Kudo and Suzuki 1999; Kudo and Hirao 2006). The patterns of phenological events vary at spatio-temporal scale from species to species due to micro-environmental variables as well as orography of the region, which were also observed by Nautiyal et al. (2001) and the growth initiation depends on soil water availability due to snowmelt and rise in temperature (Ram et al. 1988).
17.6 Conclusion
The treeline ecotone at the Tungnath region is dominated by native hemicryptophytes. The plant density (325.8 ± 67.3 vs 183.9 ± 23.6), species richness (30.7 ± 1.22 vs 25.9 ± 1.10) and diversity (2.83 ± 0.05 vs 2.77 ± 0.05) were generally higher in EX microsites as compared to UX microsites. The herbaceous community changes across the months in all the microsites, except UX of Ravanshila and EX microsites of Chandrashila and Jhabra. Though the early snowmelt promotes species diversity and phonological events as observed in this study, there is a need for further experimentation on their relationships. The results of this study suggest that the herbaceous species in treeline ecotone are undergoing changes in phenological patterns due to the increase in temperature (minima) and precipitation period in the study area (1984 to 2017) in general. Some of the species entered into two distinct pheno-periods for reproductive and fruiting/seed maturation phenophases within the same growing period (Oxygraphis, Fragaria and Ranunculus), while some prefer vegetative propagation for continuity rather than progressing through fruiting and seeding instead. The early snowmelt and ambient temperature strongly favour early growth initiation, vegetative and flowering phases in a few of the species (of the total species (86) 16% in EX and 12% in UX microsites) in April. The availability of snowmelt water followed by monsoon promotes longer vegetative phenophase in most of the species (30% in EX and 31% in UX microsites of the total species). Duration of phenophase in general from the past indicates that they have become longer, while senescence has become truncated. This study provides an insight to the effect of changing climate on alpine vegetation, which requires further investigations to understand the changing dynamics of alpine communities in the light of changing temperature and precipitation regimes. The treeline research in the climate context is increasing and diversifying but important geographical gaps in the context to Himalaya remain. There is a need to shift from traditional monitoring and development and implementation of new tools and techniques to monitor alpine treeline biodiversity. Considering the current state of the literature on higher Himalaya, there is a need for establishing multi-dimensional and multi-institutional long term monitoring projects to understand the impact of changing climate and anthropogenic pressures on peri-glacial vegetation, with a strong emphasis on extreme climatic events in high altitudes ecosystems, viz. timberline, treeline and alpine.
References
Adhikari BS (2004) Ecological attributes of vegetation in and around Nanda Devi National Park. In: Biodiversity Monitoring Expedition Nanda Devi 2003. A report to the Ministry of Environment and Forests, Govt. of India Uttaranchal State Forest Department, Dehradun, pp 15–38
Adhikari BS, Rawat GS, Rai ID, Bhattacharyya S, Bharti RR (2012) Ecological assessment of timberline ecotone in Western Himalaya with special reference to climate change and anthropogenic pressures. Final Report, Wildlife Institute of India, Dehradun
Adhikari BS, Kumar R, Singh SP (2018) Early snowmelt impact on herb species composition, diversity and phenology in a western Himalayan treeline ecotone. Trop Ecol 59(2):365–382
Anonymous. Index Kewensis Plantarum Phanerogamarum. Vol 1–2:1883–1885 & 15 Suppl. 1886–1970. Clarendon Press, Oxford; pp 1883–1970
Beniston M (2003) Climatic change in mountain regions: a review of possible impacts. Clim Chang 59:5–31
Bijalwan R, Vats M, Joshi SP (2013) Plant phenological response to microclimatic variations in an alpine zone of Garhwal Himalaya. J Appl Nat Sci 5(1):47–52
Bock JH (1976) The effects of increased snowpack on the phenology and seed germinability of selected alpine species. In: Steinhoff HW, Ives JD (eds) Ecological impacts of snowpack augmentation in the San Juan Mountains of Colorado. Final report to the Division of Atmospheric Water Resources Management, Bureau of Reclamation, Denver, Colorado, USA
Bruun HH, Moen J, Virtanen R, Grytnes J-A, Oksanen L, Angerbjörn A (2006) Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J Veg Sci 17:37–46
Campbell JL, Mitchell MJ, Groffman PM (2005) Winter in northeastern North America: an often overlooked but critical period for ecological processes. Front Ecol Environ 3:314–322
Dad M, Khan B (2010) Floristic composition of alpine grasslands in Bandipora, Kashmir. Grassl Sci 56:87–94
Dhar U, Kachroo P (1983) Alpine flora of Kashmir Himalaya. Scientific Publishers, Jodhpur
Fareed M, Caldwell MM (1975) Phenological patterns of two alpine tundra plant populations on Niwot Ridge, Colorado. Northw Sci 49:17–23
Gehrmann F, Hänninen H, Liu C, Saarinen T (2018) Phenological responses to small-scale spatial variation in snowmelt timing reveal compensatory and conservative strategies in subarctic-alpine plants. Plant Ecol Divers 10(5–6):453–468
Gong S, Guo R, Zhang T, Guo J (2015) Warming and nitrogen addition increase litter decomposition in a temperate meadow ecosystem. PLoS One 10:e0116013
Gorsuch DM, Oberbauer SF (2002) Effects of mid-season frost and elevated growing season temperature on stomatal conductance and specific xylem conductivity of the arctic shrub, Salix pulchra. Tree Physiol 22:1027–1034
Gupta VC, Kachroo P (1981) Relation between photosynthetic structure and standing biomass of meadowland communities of Yusmarg in Kashmir Himalayas
Hättenschwiler S, Körner C (1997) Annual CO2 budget of spruce model ecosystems in the third year of exposure to elevated CO2. Acta Oecol 18:319–325
Hess M, Barraljs G, Bleiholder H, Buhr L, Eggers T, Hack H, Stauss R (1997) Use of the extended BBCH scale – general for the descriptions of the growth stages of mono- and dicotyledonous weed species. Weed Res 37:433–441
Hiltbrunner E, Schwikowski M, Körner C (2005) Inorganic nitrogen storage in alpine snow pack in the Central Alps, Switzerland. Atmos Environ 39:2249–2259
Holway JG, Ward RT (1965) Phenology of alpine plants in northern Colorado. Ecology 461(2):73–83
Inouye DW (2000) The ecological and evolutionary significance of frost in the context of climate change. Ecol Lett 3:457–463
Inouye DW, Wielgolaski FE (2003) High altitude climates. In: Schwartz MD (ed) Phenology: an integrative environmental science. Kluwer, Dordrecht, pp 195–214
Jones HG, Pomeroy JW, Walker DA, Hoham RW (2001) Snow ecology, 1st edn. Cambridge University Press, Cambridge
Kala CP, Rawat GS, Uniyal VK (1998) Ecology and conservation of the valley of flowers National Park, Garhwal Himalaya. Wildlife Institute of India, Dehra Dun, p 99
Kershaw RA (1973) Quantitative and dynamic plant ecology. Edward Arnold Ltd, London
Khorsand RR, Oberbauer SF, Starr G, La P, Puma I, Pop E, Ahlquist L, Baldwin T (2015) Plant phenological responses to a long-term experimental extension of growing season and soil warming in the tussock tundra of Alaska. Glob Chang Biol 21:4520–4532
Körner C (1998) A re-assessment of high elevation treeline positions and their explanation. Oecologia 115(4):445–459
Korner C (2003) Alpine plant life, 2nd edn. Springer Verlag, Berlin
Kudo G (1991) Effects of snow-free period on the phenology of alpine plants inhabiting snow patches. Arct Antarct Alp Res 23:436–443
Kudo G, Hirao AS (2006) Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul Ecol 48:49–58
Kudo G, Suzuki S (1999) Flowering phenology of alpine plant communities along a gradient of snowmelt timing. Polar Biosci 12:100–113
Kumar A, Mitra M, Adhikari BS, Rawat GS (2016) Flora of Niti Valley: a cold arid region of Nanda Devi biosphere reserve, Western Himalaya, India. Check List 12(1):1824
Le Roux PC, Aalto J, Luoto M (2013) Soil moisture’s underestimated role in climate change impact modelling in low-energy systems. Glob Chang Biol 19:2965–2975
Livensperger C, Steltzer H, Darrouzet-Nardi A, Sullivan PF, Wallenstein MD, Weintraub MN (2016) Earlier snowmelt and warming lead to earlier but not necessarily more plant growth. AOB Plants 8:plw021
Lloyd AH, Fastie CL (2002) Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Clim Chang 52(4):481–509
Malik ZA, Nautiyal MC (2016) Species richness and diversity along the altitudinal gradient inTungnath, the Himalayan benchmark site of HIMADRI. Trop Plant Res 3(2):396–407
Mooney HA, Billings WD (1960) The annual carbohydrate cycle of alpine plants as related to growth. Am J Bot 47(7):594–598
Nautiyal BP (1996) Studies on structure and function in an alpine meadow of Garhwal, central Himalaya. PhD Thesis, HNB Garhwal University, Srinagar, India
Nautiyal MC, Nautiyal BP, Prakash V (2001) Phenology and growth form distribution in an alpine pasture at Tungnath, Garhwal, Himalaya. Mt Res Dev 21(2):168–174
Nautiyal MC, Nautiyal BP, Prakash V (2004) Effect of grazing and climatic changes on alpine vegetation of Tungnath, Garhwal Himalaya, India. Environmentalist 24:125–134
Odland A, Munkejord HK (2008) Plants as indicators of snow layer duration in southern Norwegian mountains. Ecol Indic 81:57–68
Owen HE (1976) Phenological development of herbaceous plants in relation to snowmelt date. In: Steonhoff HW, Ives JD (eds) Ecological impacts of snowpack augmentation in the San Juan Mountains of Colorado. U.S. Department of Interior, Division of Atmospheric Water Resources Management, Bureau of Reclamation, Denver, Colorado, USA, pp 323–341
Pangtey YPS, Rawal RS, Bankoti NS, Samant SS (1990) Phenology of high-altitude plants of Kumaun in central Himalaya, India. Int J Biometeorol 342:122–127
Park JS, Son D, Lee YK, Yun JH, Lee EJ (2018) Multivariate relationship between snow melt and plant distribution in high arctic tundra. J Plant Biol 61(1):32–39
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G (2007) Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob Chang Biol 13:147–156
Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Calzado RF, Ghosn D, Holten JI, Kanka R, Kazakis G, Kollar J, Larsson P, Moiseev P, Moiseev D, Molau U, Mesa JM, Nagy L, Pelino G, Puscas M, Rossi G, Stanisci A, Syverhuset AO, Theurillat J-P, Tomaselli M, Unterluggauer P, Villar L, Vittoz P, Grabherr G (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336:353–355
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, terSteege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, JHC C (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Petraglia A, Tomaselli M, Petit Bon M, Delnevo N, Chiari G, Carbognani M (2014) Responses of flowering phenology of snowbed plants to an experimentally imposed extreme advanced snowmelt. Plant Ecol 215:759–768
Pielou EC (1975) Ecological diversity. Wiley, New York
Pomeroy JW, Brun E (2001) Physical properties of snow. In: Jones HG, Pomeroy JW, Walker DA et al (eds) Snow ecology. Cambridge University Press, Cambridge, pp 45–126
Rai ID, Adhikari BS, Rawat GS, Bargali K (2012) Community structure along timberline ecotone in relation to micro-topography and disturbances in Western Himalaya. Not Sci Biol 4(2):41–52
Ram J, Arya P (1991) Plant forms and vegetation analysis of an alpine meadow of central Himalaya, India. Proc Indian Natl Sci Acad 57(5):311–318
Ram J, Singh SP, Singh JS (1988) Community level phenology of grassland above treeline in central Himalaya, India. Arct Alp Res 20(3):325–332
Rana TS, Datt B, Rao RR (2002) Life forms and biological spectrum of the flora of Tons Valley, Garhwal Himalaya (Uttaranchal), India. Taiwania 47(2):164–169
Raunkier R (1934) The life form of plants and statistical geography. The Clarendon Press, Oxford
Rawat GS (2007) Alpine vegetation of the western Himalaya: species diversity, community structure, dynamics and aspects of conservation. D.Sc. Thesis, Kumaun University, Nainital
Samant SS, Joshi HC (2005) Plant diversity and conservation status of Nanda Devi National Park and comparison with highland National Parks of the Indian Himalayan region. Int J Biodivers Sci Manag 1:65–73
Samant SS, Dhar U, Palni LMS (1998) Medicinal plants of Indian Himalaya: diversity distribution potential values. GyanodayaPrakashan, Nainital
Samant SS, Joshi HC, Pant S, Arya SC (2001) Diversity, nativity and endemism of vascular plants of Valley of Flowers National Park. Himal Biosci Res Bia Bull 3(1–2):1–17
Shannon CE, Wiener W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, IL
Singh S (1999) Assessment of floral and habitat diversity and collection of base line data to monitor vegetation of GHNP conservation area. Indian Institute of Remote Sensing Dehradun, India
Singh SP, Bassignana-Khadka I, Karky BS, Sharma E (2011) Climate change in the Hindu Kush Himalayas. ICIMOD, Kathmandu
Smith JG, Sconiers W, Spasojevic MJ, Ashton IW, Suding KN (2012) Phenological changes in alpine plants in response to increased snowpack, temperature, and nitrogen. Arct Antarct Alp Res 44(1):135–142
Sorensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Videnski Selskab Biol Skr 5:1–34
Sturm M, Schimel J, Michaelson G (2005) Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55:17–26
Sturm W, Willmes K, Orgass B, Hartje W (1997) Do specific attention deficits need specific training? Neuropsychol Rehabil 7:81–103
Stylinski CD, Allen EB (1999) Lack of native species recovery following severe exotic disturbance in southern Californian shrublands. J Appl Ecol 36(4):544–554
Sundriyal RC, Joshi AP, Dhasmana R (1987) Phenology of high altitude plants at Tungnath in the Garhwal Himalaya. Trop Ecol 28:289–299
Tambe S (2007) Ecology and management of alpine landscape in Khangchendzonga National Park, Sikkim Himalaya. Ph.D. Thesis, FRI University, Dehradun, India
Tørseth K, Semb A (1997) Atmospheric deposition of nitrogen, Sulphur and chloride in two watersheds located in southern Norway. Ambio 26:258–265
Vashistha RK, Rawat N, Chaturvedi AK, Nautiyal BP, Prasad P, Nautiyal MC (2011) Characteristics of life-form and growth-form of plant species in an alpine ecosystem of north-west Himalaya. J For Res 22:501
Vitousek PM (1986) Biological invasions and ecosystem properties. Can species make a difference? In: Mooney HA, Drake JA (eds) Ecology of bilogical invasions of North America and Hawaii, Ecological Studies, vol 58. Springer-Verlag, New York, pp 163–178
Walker MD, Webber PJ, Arnold EH, Ebert-May D (1994) Effects of interannual climate variation on aboveground phytomass in alpine vegetation. Ecology 75(2):393–408
Weih M, Karlsson PS (2002) Low winter soil temperature affects summertime nutrient uptake capacity and growth rate of mountain birch seedlings in the subarctic, Swedish Lapland. Arct Antarct Alp Res 34:434–439
Winkler DE, Butz RJ, Germino MJ, Reinhardt K, Kueppers LM (2018) Snowmelt timing regulates community composition, phenology, and physiological performance of alpine plants. Fron Plant Sci 9:1140
Wipf S (2010) Phenology, growth, and fecundity of eight subarctic tundra species in response to snowmelt manipulations. Plant Ecol 207(1):53–66
Wipf S, Rixen C (2010) A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res 291:95–109
Wipf S, Stoeckli V, Bebi P (2009) Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Clim Chang 94:105–121
Xu J, Grumbine ER, Shrestha A, Eriksson M, Yang X, Wang Y, Wilkes A (2009) The melting Himalayas: cascading effects of climate change on water, biodiversity, and livelihoods. Conserv Biol 23(3):520–530
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Adhikari, B.S., Kumar, R. (2023). Herbaceous Vegetation Structure and Phenology at Treeline Ecotone in Relation to Natural Snowmelt. In: Singh, S.P., Reshi, Z.A., Joshi, R. (eds) Ecology of Himalayan Treeline Ecotone. Springer, Singapore. https://doi.org/10.1007/978-981-19-4476-5_17
Download citation
DOI: https://doi.org/10.1007/978-981-19-4476-5_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4475-8
Online ISBN: 978-981-19-4476-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)