Abstract
Climate change has pronounced impacts on plants, particularly in environments vulnerable to warming such as alpine zones. Although plant responses in tundra and alpine environments from high latitudes have been well-studied at the community level, the overall effect of warming on global alpine plant populations and species remains unclear. We collated global data from 46 open-top chamber (OTC) studies conducted on alpine plants from mountain belts worldwide and assessed potential effects of warming on plant performance. In addition, we examined warming responses of plants from the tundra zone (Arctic tundra and alpine tundra) in circumpolar regions. In terms of growth and reproductive output, the overall response of 91 plant species was highly positive. Shrubs grew significantly larger and expressed higher reproduction under warming compared to forbs and graminoids. Arctic tundra plants tended to respond more positively to warming compared to alpine tundra plants. We also found that plant responses were greater with increasing precipitation across circumpolar tundra, but not across alpine zones. Phenotypic plasticity in size- and fitness-related traits were similar for both alpine and tundra zones. Our findings support the notion that global warming can cause significant changes to alpine environments. Due to changes in biotic interactions, alpine tundra plants may be more negatively affected by warming compared to Arctic tundra plants which responded more positively to warming. Similarly, if shrubs are most advantaged under warming, their invasion into higher elevations may threaten the ecological functioning of alpine ecosystems, which is another serious challenge from climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The biota of Arctic tundra and alpine habitat types are among those that are likely to be most affected by global warming. With increasing temperatures, specialist cold-tolerant plant species may not be able to adapt to new climate change-driven changes in abiotic and biotic conditions including the range extension of species from warmer areas (Lesica and McCune 2004; Alexander et al. 2015). Species inhabiting alpine areas may have limited space or elevation to migrate uphill as their lower distribution range contracts (Walther et al. 2005). Alpine species surviving in isolated refugia over millennia may also be negatively impacted by climate change (e.g., through altered ecosystem functioning as a result of higher temperatures, (Birks 2019)), though alpine habitats may have enough heterogeneity to buffer against the effects of warming compared to lowland areas (Scherrer and Körner 2010).
In the alpine belt of mountain ranges around the world, the rate of warming in the twenty-first century is expected to be twice or thrice that observed in the twentieth century (Nogués-Bravo et al. 2007). There has been an increasing number of studies measuring the effects of climate change as it progresses. Globally, the effects of climate change are already quite pronounced where there is an unprecedented increase in the observation of the occurrence of elevated temperatures, variability in precipitation, and drought events across different habitats, including mountain ecosystems (Theurillat and Guisan 2001; IPCC 2013). The consequences of climate change on plants are also apparent across different scales (i.e., from individuals to entire ecosystems), where the growth and phenology of individuals, the genetic and demographic dynamics of populations, and the species composition and functioning of ecosystems are all altered (Grabherr et al. 2010).
Plants can respond to changes in their environment through plastic or evolutionary shifts in phenology, growth, and reproduction (Parmesan and Yohe 2003; Jump and Penuelas 2005; Smith et al. 2012). Plants often respond to warming by initiating flowering earlier in the season (Totland 1999; Gallagher et al. 2009; Mondoni et al. 2012). For example, plants from tundra ecosystems (Prevéy et al. 2019) and subalpine forb species (Dunne et al. 2003) flowered earlier under warming. In Arctic tundra, the height of plants has been found to be positively correlated with warming (Bjorkman et al. 2018). Similarly, plants increased reproductive output under simulated warming (Totland 1999). Many species are also undergoing changes in their distribution range whereby populations contract in areas that have become warmer and/or expand into areas that were previously too cold (Jurasinski and Kreyling 2007; Harsch et al. 2009; Rumpf et al. 2018). For example, species diversity and richness have increased on European mountain summits due to the upward recruitment of species from lower elevations as mountain regions become warmer (Walther et al. 2005; Steinbauer et al. 2018; Scharnagl et al. 2019). Warm-adapted species are predicted to increase, and cold-adapted species may decline where temperature increases are more pronounced (Gottfried et al. 2012).
To date, most of the empirical evidence on the effect of warming on alpine plants consists of community-level studies reported changes in overall abundance, plant cover, and species diversity (Arft et al. 1999; Theurillat and Guisan 2001; Walker et al. 2006; Wilson and Nilsson 2009; Elmendorf et al. 2012a; Alatalo et al. 2015; Peng et al. 2017; Bjorkman et al. 2018; Myers-Smith et al. 2019). While these experiments have provided clear indications of how the plant community may change under experimental warming (e.g., Marion et al. 1997; Klady et al. 2011; Fu et al. 2015; Baruah et al. 2017), there is no consensus on how plant traits (e.g., height and inflorescence number) in individual populations and species from geographically different alpine areas could change in a warming climate. Exploring how individual plant species respond to warming can allow for a better understanding of community-level responses. This understanding is important for the conservation of alpine and tundra environments. Using meta-analysis, we reveal how the plants from alpine and tundra environments respond to warming to gain a better understanding of how plant populations may shift in the heterogeneous tundra biome.
For the current study, plant responses are defined as changes in plant output under warming such as changes in growth rate, height, and reproductive output. Open-top chambers (OTCs) are devices that are reasonably accurate in simulating natural warming (Hollister and Webber 2000), and have been used extensively to assess plant responses under climate change in the ongoing International Tundra Experiment (ITEX), which consists of multiple OTC studies carried out across tundra and alpine habitats in the arctic. OTCs simulate warming in a controlled manner by increasing the ambient temperature by 1–3 °C (Marion et al. 1997), which can be used to assess the effects of warming on soil temperature across different community types (Hollister et al. 2006). A meta-analysis on the ITEX results has found that herbaceous tundra plants demonstrated increased vegetative growth under warming, including plants from some alpine habitats (Arft et al. 1999). While the ITEX studies were extensive and described sites of different categories (i.e., alpine, tundra, and arctic-tundra), these included mostly tundra sites. Whether alpine plants across the world (and not limited to polar alpine areas) also tend to increase their growth with the increasing temperatures is unclear.
Alpine areas within circumpolar tundra environments are relatively small in size but these habitats can serve as refugia for tundra species under a rapidly warming Arctic because the temperature rise is less rapid in these areas (Rühland et al. 2013). However, it is currently unknown whether the plant responses to warming differ among Arctic tundra and alpine tundra environments within circumpolar areas. The previous meta-analyses (e.g., Arft et al. 1999) did not distinguish Arctic tundra versus Arctic alpine habitats within circumpolar areas. Although there are similarities between the two habitats, there are some important differences. Firstly, some alpine habitats have a longer growing season, higher maximum temperatures, lower soil stability on slopes, and greater geographic isolation of populations (Körner 1995) than comparable Arctic tundra habitat types. Arctic tundra plants may respond less to warming than alpine plants owing to the shorter growing seasons and low incidence of beneficial mycorrhizal associations (Gardes and Dahlberg 1996). Secondly, many more plant species inhabit the alpine belt of mountains across the world than are found in the species pool of the Arctic (10,000 for alpine areas vs. 1500 for Arctic areas; Gardes and Dahlberg 1996).
The synthesis of individual studies via meta-analysis offers a standardized statistical framework to identify key patterns (Hedges et al. 1999). Here, we synthesized the data from OTC studies conducted on plants from global alpine habitats in situ to identify patterns in trait responses of plant species in the alpine belt of mountains worldwide. In addition, we assessed the trait responses to warming in a separate dataset consisting of plants from circumpolar regions only, where we compared the responses of plants from Arctic tundra and alpine tundra habitats within the circumpolar regions. We predicted that under warming, (1) alpine plants would increase in biomass depending on life form, where shrubs and graminoids will have the greatest change (e.g., Venn et al. 2014); (2) for circumpolar regions, alpine tundra plants would respond more positively compared to Arctic tundra plants (in terms of growth and survival traits); and (3) size-related traits (such as biomass and height) would change less under experimental warming (i.e., less plastic) than fitness-related traits (such as seed mass, number of flowers; e.g., Totland 1999; Kiełtyk 2018).
2 Materials and methods
We searched ISI Web of Knowledge, Scopus, and Google Scholar in early 2019 using several keywords including “alpine”, “experimental warming”, “simulated warming”, “altitude”, “elevation”, “field”, “shrub*”, and “herb*” as well as combinations of these words to find the most appropriate empirical studies for the meta-analysis. We also searched through the abstract, methods, references, and citation lists of eligible studies to find additional suitable information (e.g., from the reference lists of ITEX studies). For the comparison between Arctic tundra and alpine tundra plants within circumpolar regions, tundra studies were identified from the ITEX studies and by substituting the “alpine” search term with “tundra”. Additional alpine tundra studies were identified from the results of the main literature search.
Only warming studies measuring plant traits at the individual population were included (i.e., experiments with analyses based on the whole community level were excluded). Studies measuring only species abundance, frequency, and cover were excluded. From each experiment, we collected fitness and size trait data from plants both grown under the control (i.e., no warming) and the warming treatment using only OTCs. Studies using different warming devices (e.g., heaters) other than OTCs were not included in this analysis due to possible differences in experimental design and the warming effect on the plants. Experimental warming studies aimed to test the responses of a subset of plant species out of the pool of species present at a given site (mostly 1 to 5 species; Appendix A and C). Therefore species that are at least locally abundant are more likely captured by the sampling.
We obtained data from a single performance trait (i.e., the number of flowers or seeds; or size-related traits such as biomass) from each study. In studies where there were multiple measurements from the same site (i.e., across time), the data point from the latest year was used to avoid oversampling and pseudo-replication (Hedges et al. 1999). Similarly, a single data point was sampled for studies that measured multiple traits. For each plant species, we recorded the mean trait values under control and OTC warming treatment, standard deviations of the means, sample sizes per treatment, experimental duration, the type of trait measured (fitness- or size-related traits), life form (graminoid, forb, or shrub), the elevation, and location of the study. For global alpine zones, the alpine sub-belt (low or high alpine) was recorded. For circumpolar tundra, the habitat type (Arctic tundra or alpine tundra) was recorded. Measurements of leaf number, biomass, and height (i.e., stretched aboveground shoot length) are considered size-related traits, whereas germination, seed or fruit number, and survivorship are considered fitness-related traits.
Due to the characteristics of diverse experimental sites, including only elevation as a factor may not be biologically meaningful. For example, while 4400 m altitude in Eastern Tibet, China is a low alpine zone, 1000 m altitude in Abisko, Sweden is a high alpine zone. Low alpine zones have less frost and a longer growing season than high alpine zones, with a different vegetation composition consisting of different species (Rosbakh et al. 2014). Therefore, we used the technically the correct definition of elevation (McVicar and Körner 2013) and categorized the alpine sub-belt as “low alpine” or “high alpine.” This was determined depending on the site description in each study to analyze warming effects on plants from different zones representing different microclimatic variables (including variation in precipitation, growing season day length, solar radiation, wind speed, and slope aspect).
Latitude, temperature above minimums for growth, and precipitation over the growing season may be important factors because low-alpine plants at high latitudes may respond differently to low-alpine plants close to the equator (among other site environmental factors). Therefore, using QGIS ver. 3.12.2, we extracted two bioclimatic variables in the localities of experimental alpine sites: mean growing degree days (GDD; in Celsius) and growing season precipitation (GSP; in kg/m2). These data were retrieved from raster files available at the CHELSA website (https://chelsa-climate.org) that provides high-resolution climate data (data were available only for the 1979–2013 period) (Karger et al. 2017). Moreover, the historical average annual temperature (°C), precipitation (mm), solar radiation (kJ m −2 day −1) values in each locality were extracted from WorldClim ver. 2.1 that is a database of high spatial resolution global climate data (2.5 min spatial resolution ~ 21 km2; https://www.worldclim.org) using QGIS software. The parameters included in the models are summarized in Table 1.
2.1 Responses of plants with different life-forms
To test our first prediction that alpine plants with different life forms would respond differently to warming and that shrub and graminoid species would have the largest response, we compared the responses of shrub, forb, and graminoid species under OTC treatment for plants from both global alpine zones and circumpolar tundra (Arctic tundra and alpine tundra) zones to assess how species with different life forms respond to rising temperatures, and whether the patterns among life forms differ among these ecosystems.
2.2 Comparison between responses of Arctic and alpine plants
To test our second prediction that Arctic tundra plants would respond more positively to warming than alpine tundra plants, we used a separate dataset consisting of only Arctic tundra and alpine tundra plants within circumpolar regions. The elevation of the study site and descriptions of the local conditions from each study was used to determine whether the site was Arctic tundra or alpine tundra.
2.3 Comparison between size- and fitness-related traits
Measures of reproduction and survival were categorized as fitness-related traits (e.g., number of flowers, inflorescence, seed and fruit number or mass, and survival rate). Vegetative growth traits were categorized as size-related traits (e.g., biomass, size, and height; Appendixes A and C). We used Hedge’s g as a measure of effect size (Hedges and Olkin 1985) to quantify differences between global alpine plants and circumpolar tundra plants under experimental warming treatment and control. A single g value was calculated for each species sampled within each study:
where Mt – Mc is the difference between the means of treatment and control, and SDpooled is calculated using the following equation (Hedges and Olkin 1985):
where SDt and SDc are standard deviation values under treatment and control, respectively.
To test our third prediction, phenotypic plasticity of plant traits was quantified using a well-established phenotypic plasticity index (PIv) (Valladares et al. 2000, 2006):
where Xc is the trait mean under the control; Xt is the trait mean under treatment (simulated warming induced by OTC). PIV represents the overall absolute change in trait mean values and ranges between 0 and 1 (no plasticity and maximum plasticity, respectively).
2.4 Statistical analyses
All analyses were run using restricted maximum likelihood (REML) in random effects mixed models, where experimental duration and study sample size (N) were included as random effects. The site environmental and biological variables were included as single covariates in the mixed models to assess their effects on the response of global alpine plants to OTC warming. In addition, the interactions between life form and plasticity, and trait type and plasticity were tested (Table 1). In separate analyses, we assessed the responses of circumpolar (Arctic tundra and alpine tundra) plants in relation to the same variables (Table 1). The explained heterogeneity Q statistic (QM) was used as the test statistic for significance of the covariates in the analyses. Effect sizes were considered significant where they do not overlap zero (e.g., Peng et al. 2019). Population-level responses were not pooled together before statistical analyses as each can have a unique response ratio as a result of biological and environmental variations (Nakagawa and Santos 2012). All statistical analyses were conducted using R ver. 3.4.4 (R Development Team 2014) using the “metafor” package (ver. 2.0). Graphical presentations were prepared using SigmaPlot ver. 12 (Systat Software, CA, USA) and QGIS Desktop ver. 3.12.2. All confidence interval (CI) values are 95% upper and lower limits.
3 Results
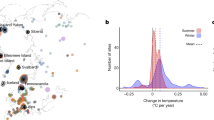
In total, we included 140 data points from 46 OTC studies that examined the effect of simulated warming on alpine plant populations in alpine mountain belts worldwide (Fig. 1). Each data point represents the population-level response of an alpine species from the OTC treatment in relation to the control. The dataset consisted of 91 different species (16 graminoid, 45 forb, and 30 shrub data points) belonging to 65 genera and 27 families (see Appendix A and C for the complete global alpine and circumpolar tundra datasets including the raw data from each study, Appendix B for the full reference list of studies used in this meta-analysis). Of the species, only 7 of 59 were found in both high and low elevation habitats (Appendix C). There were 36 different localities where alpine plant responses were measured across the globe in total (Fig. 1). The elevation of the study sites ranged from 450 m to 4900 m a.s.l. and the latitude ranged from 27.5° S to 78.9° N.
The localities of experimental sites testing the response of plants to warming (using open-top chambers) included in this study. Circles represent studies conducted on alpine zones worldwide, and triangles represent studies on circumpolar tundra sites. The size of the shapes represents the sample size (see inset)
The overall results indicate plants generally have a positive response to experimental warming, where plants grew larger or taller, produced more biomass, or had greater reproductive output (Fig. 2). We analyzed the responses under a suite of site environmental parameters including latitude, average annual temperature, GDD, precipitation, GSP, solar radiation, and elevation (Table 1). The level of response to warming increased with annual precipitation across circumpolar tundra areas (P < 0.05; Fig. 4), but not across global alpine zones (P = 0.38; Table 2). The relationship between the response to warming and the other site parameters (i.e., latitude, average temperature, GDD, GSP, solar radiation, and elevation) were not significant (Tables 2 and 3). For circumpolar tundra plants, alpine tundra plants tended to respond less to warming compared to Arctic tundra (P = 0.05; Table 2). This result is reflected in the elevation data where higher elevations responded less to warming than lower elevations (P = 0.05; Table 2). However, the response to warming did not differ with elevation for plants from global alpine zones (P = 0.98). The other site environmental characteristics (i.e., latitude, average temperature, GSP, GDD, and solar radiation) did not have significant effects on the response of plants to warming, and neither did trait plasticity affect the responses (Tables 2 and 3).
Forest plot indicating the effect size (Hedge’s g) and 95% CI of plants from global alpine zones for different trait types (fitness- and size-related), life forms (graminoid, forb, and shrub), and alpine zones (high and low alpine) at the population-level. Sample size and experimental duration were included as random effects. Effect sizes significantly different to zero are marked with an asterisk
3.1 Responses of plants with different life forms
We analyzed the differences among plants with different life forms (forbs, graminoids, and shrubs) for both alpine plants and tundra (including alpine tundra and Arctic tundra). Plants of different life forms in global alpine zones differed significantly from one another (P < 0.05; Table 2), where shrubs had the greatest response to warming (shrubs: g = 0.75, CI 0.49–1.00; forbs: g = 0.11, CI − 0.24–0.46; and graminoids: g = 0.31, CI − 0.52–1.14; Fig. 2). For tundra plants, the differences in the responses to warming treatment among life forms were not significant (P = 0.59; Table 2). The pattern among life forms of tundra zones are similar to that of global alpine plants, with shrubs demonstrating the greatest response to warming (shrubs: g = 0.59, CI 0.26–0.92; forbs: g = 0.38, CI − 0.06–0.82; and graminoids: g = 0.27, CI = − 0.16–0.71; Fig. 3). The interactions between life form and plasticity, and trait type and plasticity were not significant for both global alpine plants and circumpolar tundra plants (Table 2).
Forest plot indicating the effect size (Hedge’s g) and 95% CI for plants from circumpolar tundra for different trait types (fitness- and size-related), life forms (graminoid, forb, and shrub), and habitat type (Arctic tundra and alpine tundra) data at the population-level for circumpolar tundra areas (Arctic tundra and alpine tundra). Effect sizes significantly different to zero are marked with an asterisk
3.2 Comparison between responses of Arctic tundra versus alpine tundra plants
We analyzed possible differences in the responses of Arctic tundra and alpine tundra plants from the circumpolar regions by extracting 97 data points from 35 publications (20 studies also present in Appendix A and an additional 15 studies) (Appendix C). This dataset includes 59 species, of which 7 occur in both alpine tundra and Arctic tundra habitats. We found that habitat type did not differ significantly among alpine tundra and Arctic tundra plants (P = 0.05; Table 2). However, there were differences among alpine tundra plants (g = 0.35, CI 0.20–0.51) and Arctic tundra plants (g = 0.85, CI 0.08–1.62), where Arctic tundra plants tended to respond more positively in terms of biomass, height, and reproductive output under OTC warming compared to alpine tundra plants (e.g., grew larger and produced more flowers; Appendix C).
3.3 Comparison between size- and fitness-related traits
Fitness-related and size-related traits did not differ from one another. This was the same within alpine plants and circumpolar tundra (i.e., alpine tundra and Arctic tundra) plants (P = 0.60 and P = 0.66, respectively; Table 2). For alpine plants, the responses for size- and fitness-related traits were g = 0.44 (CI 0.15–0.73) and g = 0.33 (CI 0.03–0.63), respectively. For tundra plants, the responses were g = 0.45 (CI 0.13–0.78) and g = 0.35 (CI = 0.02–0.68) for size- and fitness-related traits, respectively.
4 Discussion
We found that experimental warming had an overall positive effect across populations of alpine species (i.e., an increase in biomass and the number of flowers). Our overall finding that plants respond to warming by increasing their size and fitness is consistent with the results of previous studies, which found that experimental warming significantly increased the growth of tundra and alpine plants (Arft et al. 1999; Walker et al. 2006). Interestingly, we found that plants from sites with higher precipitation displayed greater responses to warming for circumpolar tundra environments, but not for alpine zones. A previous experimental study on Antarctic tundra moss species found that precipitation increased their responses to warming (Day et al. 2009). However, a study in southern Norway found that plant responses correlated with temperature rather than precipitation (Guittar et al. 2016). Our global synthesis indicates that precipitation regimes generally can affect the response of plants to warming across various circumpolar areas (Fig. 4).
Relationship between effect size (Hedge’s g) and site annual precipitation (mm/year) for the response of circumpolar tundra plants to open-top chamber (OTC) warming, with the mixed model slope (solid line) and 95% confidence intervals (dashed lines). The pale gray line across g = 0 indicates the threshold where there were no differences between plant responses between OTC treatment and the control. Each circle represents an individual data point, and the size of the circles represents the inverse of variances around g for each data point (studies represented by larger circles have lower variance)
Of different life forms, we found that shrub species had the largest response to warming, whereas forb and graminoid species expressed smaller responses. This result was overall consistent with our first prediction (i.e., warming effects on plant species would depend on life form). Shrub species expressed a highly positive response to warming where they grew faster, taller, and produced more biomass. In some alpine areas, it has been observed that some shrub species increased in abundance with experimental warming (e.g., Vaccinium sp.; Capers and Stone 2011). Moreover, the shrubline was found to be advancing upwards in some areas (e.g., in areas of Alaska, Myers-Smith 2009; Dial et al. 2016); meanwhile shrub recruitment has also increased in response to climate warming (Myers-Smith and Hik 2018). In accordance with observational studies, most of the shrub species in this study (26 of 30) showed increased fitness- and size-related traits under experimental warming. Shrub encroachment can directly decrease species richness in alpine vegetation through replacing herbaceous species in alpine habitats (Elmendorf et al. 2012b; Harte et al. 2015) or competitive exclusion (Wilson and Nilsson 2009; Boscutti et al. 2018). Therefore, there is a strong indication from the literature and our study (i.e., a significant positive response in shrubs under experimental warming) that shrub encroachment is a threat to alpine biomes.
We compared the responses of circumpolar Arctic tundra and alpine tundra plants to experimental warming and predicted that alpine tundra plants would respond more positively to warming than Arctic tundra plants (second prediction). Surprisingly, the response of alpine tundra plants to warming was lower than that of Arctic tundra plants. Thus, our findings indicated alpine tundra species may be more susceptible to environmental changes caused by or associated with warming compared to Arctic tundra species. For global alpine zones, although there was no difference in response between low and high alpine zones, they did both increase in effect size (Hedge’s g) in response to warming. This finding was unexpected because the relatively cold environments at higher elevations were thought to physiologically limit the growth response (Körner 1995, 2003). Alternatively, plants from high alpine zones may be adapted to the harsher local environmental conditions (e.g., cold or wind exposure) that may trade-off with phenotypic plasticity to changing environments (Chevin et al. 2010; Lande 2014).
Contrary to our third prediction, we found that the responses in size- and fitness-related traits did not differ from one another in global alpine and circumpolar tundra environments. In addition, plants from alpine zones and tundra zones displayed similar levels of trait plasticity (Table 3). This finding suggests that maintaining the same level of fitness across diverse environmental conditions (simulated warming in this case) is an adaptive response to variation in the environment (e.g., jack-of-all-trades strategy, Richards et al. 2006; Ghalambor et al. 2007; Matesanz et al. 2010). The trait plasticity captured by the OTC studies may be advantageous across harsh cold ecosystems where plants display a conservative resource strategy; therefore, the result that plants from the different zones did not differ in plasticity is not too surprising (Li et al. 2016).
Overall, our meta-analysis results provide support for the conclusions of previous studies (e.g., warming significantly increased plant growth), such as the meta-analysis performed by Arft et al. (1999) which only included studies from four alpine sites available at the time (while our meta-analysis covers 36 different alpine areas across the world in more recent times). In addition, Walker et al. (2006), Elmendorf et al. (2012a), and Bjorkman et al. (2018) used various methods to measure community or plant responses but did not experimentally assess the effects of warming using OTCs. By focusing on studies employing a standardized experimental method (i.e., OTCs), our comprehensive synthesis study contributes a more detailed understanding of how alpine plants are responding to warming.
The long-term effects of warming on alpine ecosystems are currently difficult to predict (Körner and Hiltbrunner 2018). Several warming experiments indicated that species richness may decrease in alpine and tundra areas, although this decrease may be transient as more species invade from warmer areas (Wilson and Nilsson 2009; Walker et al. 2006; Klady et al. 2011; Elmendorf et al. 2012a). Several long-term observational studies also found increasing species richness on mountain summits (Pauli et al. 2012; Wipf et al. 2013); although, this may also be temporary as high alpine species lose their habitats and become locally extinct (Steinbauer et al. 2018). Conversely, a study on European alpine grasslands found that species abundance remained locally consistent over time with warming (Windmaißer and Reisch 2013). Responses of plants expressed under warming may depend on the prior adaptation experienced by the population, other environmental parameters, and experiment duration. For example, long-term or short-term OTC studies can find contrasting findings (Elmendorf et al. 2012a), albeit the effect of experiment duration was not significant in our study.
Apart from warming, other global change drivers induced by anthropogenic activities such as elevated atmospheric CO2, more sporadic precipitation, land-use change, and nitrogen deposition may disrupt ecosystem services (Tylianakis et al. 2008; IPCC 2013; Flombaum et al. 2017). For example, in the European Alps, nitrogen deposition may cause shifts in alpine plant communities by favoring high-nutrient demanding species (Rumpf et al. 2018). The effects of these global change drivers (including warming) on plants may be additive or synergistic. Results from a large-scale meta-analysis suggest that the effects of multiple drivers on plants can be additive (Yue et al. 2017). Conversely, Darling and Côté (2008) found that global change factors may often have non-additive effects (synergistic or antagonistic effects) on vegetation. Therefore, the response of alpine plants to climate change can be shaped by complex ecological processes which require further study; particularly on mountain summits worldwide to disentangle effects of global change drivers on plants and fragile ecosystem services which might be threatened by warming-driven shrub invasion as suggested by our results.
There are other factors which moderate how alpine and tundra plants respond to warming that could not be accounted for in this study. For example, each mountain range may have unique microclimates including differences in seasonality, geology, soils, wind exposure, water availability, and land-use history that can affect trait changes (Grabherr et al. 2010). Anthropogenic features such as roads and structures could create conditions favorable to plants (Lembrechts et al. 2018); such as those found on screes and warmer slopes (Kulonen et al. 2018). We cannot account for trait shifts that are potentially maladaptive. Although we found that plants grow larger and have higher production under warming (particularly shrub species), plants from both circumpolar tundra areas and alpine areas may face increased mortality from summer freezing events because they may have lower resistance to sub-zero temperatures under warmer conditions (in the Andes mountains: Sierra-Almeida and Cavieres 2010; in Antarctic tundra: Sierra-Almeida et al. 2018). Earlier snowmelt can similarly select for earlier flowering but may make plants more vulnerable to early frost (Inouye 2008; Olson et al. 2018). Conversely, an increase in snow cover can insulate the soil and protect plants from frost (Semenchuk et al. 2013). Taller and denser canopies can also trap heat in the soil in winter, but the extra shading has a cooling effect in summer (Myers-Smith et al. 2019).
We were not able to ascertain whether the species were locally rare or common from the OTC studies, as these studies did not survey local abundance and rare species might not be captured by sampling. Subsequent studies can more explicitly test whether common species have more negative responses to the effects of warming compared to rare species (e.g., within the same site; Pauli et al. 2007), and identify correlations among traits in the plant responses. Currently, there are too few studies to assess whether plants from different geographic locations respond differently to each other. Future studies can explore plant responses in different regions in more detail. By accounting for various abiotic and biological characteristics, our study sheds light on how alpine and circumpolar tundra plants respond to warming.
5 Conclusions
We found that alpine species respond differently to warming depending on growth form. In addition, Arctic tundra plants responded positively to warming while the responses of alpine tundra plants were modest. These results indicate a likely shrub encroachment in alpine areas, as shrub species seem to be most benefitted by warming. Moreover, in circumpolar regions where Arctic tundra exists below the alpine zone, Arctic tundra plants may threaten alpine tundra plants due to their increased growth advantage under climate change. Our results offer valuable insights into the responses of alpine plants to warming for future management and research. Future studies may provide a more detailed assessment of alpine plant responses and identify key principles in management to assist the conservation of these vulnerable alpine ecosystems under precipitation change and warming.
References
Alatalo JM, Little CJ, Jägerbrand AK, Molau U (2015) Vascular plant abundance and diversity in an alpine heath under observed and simulated global change. Sci Rep 5(1):10197. https://doi.org/10.1038/srep10197
Alexander JM, Diez JM, Levine JM (2015) Novel competitors shape species’ responses to climate change. Nature 525:515–518. https://doi.org/10.1038/nature14952
Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, Henry GHR, Jones MH et al (1999) Response patterns of tundra plant species to experimental warming: a meta-analysis of the International Tundra Experiment. Ecol Monogr 69(4):491–511. https://doi.org/10.2307/2657227
Baruah G, Molau U, Bai Y, Alatalo JM (2017) Community and species-specific responses of plant traits to 23 years of experimental warming across subarctic tundra plant communities. Sci Rep 7(1):2571. https://doi.org/10.1038/s41598-017-02595-2
Birks JHB (2019) Contributions of Quaternary botany to modern ecology and biogeography. Plant Ecol Divers 12:189–385. https://doi.org/10.1080/17550874.2019.1646831
Bjorkman AD, Myers-Smith IH, Elmendorf SC, Normand S, Rüger N, Beck PSA, Blach-Overgaard A, Blok D, Cornelissen JHC et al (2018) Plant functional trait change across a warming tundra biome. Nature 562(7725):57–62. https://doi.org/10.1038/s41586-018-0563-7
Boscutti F, Casolo V, Beraldo P, Braidot E, Zancani M, Rixen C (2018) Shrub growth and plant diversity along an elevation gradient: evidence of indirect effects of climate on alpine ecosystems. PLoS One 13:e0196653. https://doi.org/10.1371/journal.pone.0196653
Capers RS, Stone AD (2011) After 33 years, trees more frequent and shrubs more abundant in northeast U.S. Arct Antarct Alp Res 43(4):495–502. https://doi.org/10.1657/1938-4246-43.4.495
Chevin L-M, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8(4):e1000357. https://doi.org/10.1371/journal.pbio.1000357
Darling ES, Côté IM (2008) Quantifying the evidence for ecological synergies. Ecol Lett 11(12):1278–1286. https://doi.org/10.1111/j.1461-0248.2008.01243.x
Day TA, Ruhland CT, Strauss SL, PARK JH, Krieg ML, Krna MA, Bryant DM (2009) Response of plants and the dominant microarthropod, Cryptopygus antarcticus, to warming and contrasting precipitation regimes in Antarctic tundra. Glob Chang Biol 15(7):1640–1651. https://doi.org/10.1111/j.1365-2486.2009.01919.x
Dial RJ, Scott Smeltz T, Sullivan PF, Rinas CL, Timm K, Geck JE et al (2016) Shrubline but not treeline advance matches climate velocity in montane ecosystems of south-central Alaska. Glob Chang Biol 22(5):1841–1856. https://doi.org/10.1111/gcb.13207
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73(1):69–86. https://doi.org/10.1890/0012-9615(2003)073[0069:SMFPRT]2.0.CO;2
Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Bjorkman AD, Callaghan TV et al (2012a) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15(2):164–175. https://doi.org/10.1111/j.1461-0248.2011.01716.x
Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Boulanger-Lapointe N, Cooper EJ et al (2012b) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim 2(6):453–457. https://doi.org/10.1038/nclimate1465
Flombaum P, Yahdjian L, Sala OE (2017) Global-change drivers of ecosystem functioning modulated by natural variability and saturating responses. Glob Chang Biol 23(2):503–511. https://doi.org/10.1111/gcb.13441
Fu G, Shen ZX, Sun W, Zhong ZM, Zhang XZ, Zhou YT (2015) A meta-analysis of the effects of experimental warming on plant physiology and growth on the Tibetan plateau. J Plant Growth Regul 34(1):57–65. https://doi.org/10.1007/s00344-014-9442-0
Gallagher RV, Hughes L, Leishman MR (2009) Phenological trends among Australian alpine species: using herbarium records to identify climate-change indicators. Aus J Bot 57(1):1–9. https://doi.org/10.1071/BT08051
Gardes M, Dahlberg A (1996) Mycorrhizal diversity in arctic and alpine tundra: an open question. New Phytol 133:147–157. https://doi.org/10.1111/j.1469-8137.1996.tb04350.x
Ghalambor CK, McKAY JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21(3):394–407. https://doi.org/10.1111/j.1365-2435.2007.01283.x
Gottfried M, Pauli H, Futschik A, Akhalkatsi M, Barančok P, Alonso JL, Coldea G, Dick J, Erschbamer B, Kazakis G, Krajči J (2012) Continent-wide response of mountain vegetation to climate change. Nat Clim 2:111–115
Grabherr G, Gottfried M, Pauli H (2010) Climate change impacts in alpine environments. Geogr Compass 4(8):1133–1153. https://doi.org/10.1111/j.1749-8198.2010.00356.x
Guittar J, Goldberg D, Klanderud K, Telford RJ, Vandvik V (2016) Can trait patterns along gradients predict plant community responses to climate change? Ecology 97:2791–2801. https://doi.org/10.1002/ecy.1500
Harsch MA, Hulme PE, McGlone MS, Duncan RP (2009) Are Treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol Lett 12(10):1040–1049. https://doi.org/10.1111/j.1461-0248.2009.01355.x
Harte J, Saleska SR, Levy C (2015) Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate-soil carbon feedback. Glob Chang Biol 21(6):2349–2356. https://doi.org/10.1111/gcb.12831
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, San Diego
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80(4):1150–1156. https://doi.org/10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2
Hollister RD, Webber PJ (2000) Biotic validation of small open-top chambers in a tundra ecosystem. Glob Chang Biol 6(7):835–842
Hollister RD, Webber PJ, Nelson FE, Tweedie CE (2006) Soil thaw and temperature response to air warming varies by plant community: results from an open-top chamber experiment in northern Alaska. Arct Antarct Alp Res 38(2):206–215
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362. https://doi.org/10.1890/06-2128.1
IPCC-Intergovernmental Panel on Climate Change (2013) In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge and New York
Jump AS, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8(9):1010–1020. https://doi.org/10.1111/j.1461-0248.2005.00796.x
Jurasinski G, Kreyling J (2007) Upward shift of alpine plants increases floristic similarity of mountain summits. J Veg Sci 18(5):711–718. https://doi.org/10.1111/j.1654-1103.2007.tb02585.x
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017) Climatologies at high resolution for the earth’s land surface areas. Sci Data 4:170122. https://doi.org/10.1038/sdata.2017.122
Kiełtyk P (2018) Variation of vegetative and floral traits in the alpine plant Solidago minuta: evidence for local optimum along an elevational gradient. Alp Bot 128(1):47–57. https://doi.org/10.1007/s00035-017-0197-7
Klady RA, Henry GHR, Lemay V (2011) Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Glob Chang Biol 17(4):1611–1624. https://doi.org/10.1111/j.1365-2486.2010.02319.x
Körner CH (1995) Alpine plant diversity: a global survey and functional interpretations. In: Arctic and alpine biodiversity: patterns, causes and ecosystem consequences 1995. Springer, Berlin, pp 45–62
Körner C (2003) Alpine plant life, functional plant ecology of high mountain ecosystems. Springer, New York
Körner C, Hiltbrunner E (2018) The 90 ways to describe plant temperature. Perspect Plant Ecol Syst 30(SI):16–21. https://doi.org/10.1016/j.ppees.2017.04.004
Kulonen A, Imboden RA, Rixen C, Maier SB, Wipf S (2018) Enough space in a warmer world? Microhabitat diversity and small-scale distribution of alpine plants on mountain summits. Divers Distrib 24(2):252–261. https://doi.org/10.1111/ddi.12673
Lande R (2014) Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J Evol Biol 27(5):866–875. https://doi.org/10.1111/jeb.12360
Lembrechts JJ, Lenoir J, Nuñez MA, Pauchard A, Geron C, Bussé G, Milbau A, Nijs I (2018) Microclimate variability in alpine ecosystems as stepping stones for non-native plant establishment above their current elevational limit. Ecography 41:900–909. https://doi.org/10.1111/ecog.03263
Lesica P, McCune B (2004) Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming. J Veg Sci 15:679–690. https://doi.org/10.1111/j.1654-1103.2004.tb02310.x
Li H, Yu K, Ratajczak Z, Nippert JB, Tondrob D, Xu D, Li W, Du G (2016) When variability outperforms the mean: trait plasticity predicts plant cover and biomass in an alpine wetland. Plant Soil 407(1–2):401–415. https://doi.org/10.1007/s11104-016-2898-x
Matesanz S, Gianoli E, Valladares F (2010) Global change and the evolution of phenotypic plasticity in plants. Ann N Y Acad Sci 1206:35–55. https://doi.org/10.1111/j.1749-6632.2010.05704.x
Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Levesque E, Molau U, Molgaard P, Parsons AN et al (1997) Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob Chang Biol 3(S1):20–32. https://doi.org/10.1111/j.1365-2486.1997.gcb136.x
McVicar TR, Körner C (2013) On the use of elevation, altitude, and height in the ecological and climatological literature. Oecologia 171(2):335–337
Mondoni A, Rossi G, Orsenigo S, Probert RJ (2012) Climate warming could shift the timing of seed germination in alpine plants. Ann Bot 110(1):155–164. https://doi.org/10.1093/aob/mcs097
Myers-Smith I (2009) Shrub line advance in alpine tundra of the Kluane region: mechanisms of expansion and ecosystem impacts. Arctic 60(4):447–455. https://doi.org/10.14430/arctic208
Myers-Smith IH, Hik DS (2018) Climate warming as a driver of tundra shrubline advance. J Ecol 106(2):547–560. https://doi.org/10.1111/1365-2745.12817
Myers-Smith IH, Thomas HJD, Bjorkman AD (2019) Plant traits inform predictions of tundra responses to global change. New Phytol 221(4):1742–1748. https://doi.org/10.1111/nph.15592
Nakagawa S, Santos ESA (2012) Methodological issues and advances in biological meta-analysis. Evol Ecol 26:1253–1274. https://doi.org/10.1007/s10682-012-9555-5
Nogués-Bravo D, Araújo MB, Errea MP, Martínez-Rica JP (2007) Exposure of Global Mountain systems to climate warming during the 21st century. Glob Environ Chang 17(3–4):420–428. https://doi.org/10.1016/j.gloenvcha.2006.11.007
Olson ME, Soriano D, Rosell JA, Anfodillo T, Donoghue MJ, Edwards EJ, Leon-Gomez C, Dawson T, Martinez JJC, Castorena M et al (2018) Plant height and hydraulic vulnerability to drought and cold. PNAS 115(29):7551–7556. https://doi.org/10.1073/pnas.1721728115
Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G (2007) Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA* master site Schrankogel, Tyrol, Austria. Glob Chang Biol 13:147–156
Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Calzado R et al (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336(6079):353–355. https://doi.org/10.1126/science.1219033
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421(6918):37–42. https://doi.org/10.1038/nature01286
Peng F, Xue X, Xu M, You Q, Jian G, Ma SX (2017) Warming-induced shift towards forbs and grasses and its relation to the carbon sequestration in an alpine meadow. Environ Res Lett 12(4):044010. https://doi.org/10.1088/1748-9326/aa6508
Peng S, Kinlock NL, Gurevitch J, Peng S (2019) Correlation of native and exotic species richness: a global meta-analysis finds no invasion paradox across scales. Ecology 100(1):e02552. https://doi.org/10.1002/ecy.2552
Prevéy JS, Rixen C, Rüger N, Høye TT, Bjorkman AD, Myers-Smith IH, Elmendorf SC, Ashton IW, Cannone N, Chisholm CL et al (2019) Warming shortens flowering seasons of tundra plant communities. Nat Ecol Evol 3(1):45–52. https://doi.org/10.1038/s41559-018-0745-6
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981e993. https://doi.org/10.1111/j.1461-0248.2006.00950.x
Rosbakh S, Bernhardt-Römermann M, Poschlod P (2014) Elevation matters: contrasting effects of climate change on the vegetation development at different elevations in the Bavarian Alps. Alp Bot 124(2):143–154. https://doi.org/10.1007/s00035-014-0139-6
Rumpf SB, Hülber K, Klonner G, Moser D, Schütz M, Wessely J, Willner W, Zimmermann NE, Dullinger S (2018) Range dynamics of mountain plants decrease with elevation. Proc Natl Acad Sci U S A 115(8):1848–1853. https://doi.org/10.1073/pnas.1713936115
Rühland KM, Paterson AM, Keller W, Michelutti N, Smol JP (2013) Global warming triggers the loss of a key Arctic refugium. Proc R Soc Lond B Biol Sci 280:20131887. https://doi.org/10.1098/rspb.2013.1887
Scharnagl K, Johnson D, Ebert-May D (2019) Shrub expansion and alpine plant community change: 40-year record from Niwot Ridge, Colorado. Plant Ecol Divers:1–10. https://doi.org/10.1080/17550874.2019.1641757
Scherrer D, Körner C (2010) Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J Biogeogr 38:406–416
Semenchuk PR, Elberling B, Cooper EJ (2013) Snow cover and extreme winter warming events control flower abundance of some, but not all species in high arctic Svalbard. Ecol Evol 3(8):2586–2599. https://doi.org/10.1002/ece3.648
Sierra-Almeida A, Cavieres LA (2010) Summer freezing resistance decreased in high-elevation plants exposed to experimental warming in the central Chilean Andes. Oecologia 163(1):267–276. https://doi.org/10.1007/s00442-010-1592-6
Sierra-Almeida A, Cavieres LA, Bravo LA (2018) Warmer temperatures affect the in situ freezing resistance of the Antarctic vascular plants. Front Plant Sci 9:1456
Smith JG, Sconiers W, Spasojevic MJ, Ashton IW, Suding KN (2012) Phenological changes in Alpine plants in response to increased snowpack, temperature, and nitrogen. Arct Antarct Alp Res 44(1):135–142. https://doi.org/10.1657/1938-4246-44.1.135
Steinbauer MJ, Grytnes JA, Jurasinski G, Kulonen A, Lenoir J, Pauli H, Rixen C, Winkler M, Bardy-Durchhalter M, Barni E et al (2018) Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556(7700):231–234. https://doi.org/10.1038/s41586-018-0005-6
Theurillat JP, Guisan A (2001) A potential impact of climate change on vegetation in the European Alps: a review. Clim Chang 50(1–2):77–109. https://doi.org/10.1023/A:1010632015572
Totland Ø (1999) Effects of temperature on performance and phenotypic selection on plant traits in alpine Ranunculus acris. Oecologia 120(2):242–251. https://doi.org/10.1007/s004420050854
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11(12):1351–1363. https://doi.org/10.1111/j.1461-0248.2008.01250.x
Valladares F, Wright S, Lasso E, Kitajima K (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81:1925–1936. https://doi.org/10.2307/177282
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Venn S, Pickering C, Green K (2014) Spatial and temporal functional changes in alpine summit vegetation are driven by increases in shrubs and graminoids. AoB Plants 6:plu008. https://doi.org/10.1093/aobpla/plu008
Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, Callaghan TV, Carroll AB et al (2006) Plant community response to experimental warming across the tundra biome. PNAS 103(5):1342–1346. https://doi.org/10.1073/pnas.0503198103
Walther G, Beißner S, Burga CA (2005) Trends in the upward shift of alpine plants. J Veg Sci 16:541–548. https://doi.org/10.1111/j.1654-1103.2005.tb02394.x
Wilson SD, Nilsson C (2009) Arctic alpine vegetation change over 20 years. Glob Chang Biol 15(7):1676–1684. https://doi.org/10.1111/j.1365-2486.2009.01896.x
Windmaißer T, Reisch C (2013) Long-term study of an alpine grassland: local constancy in times of global change. Alp Bot 123(1):1–6. https://doi.org/10.1007/s00035-013-0112-9
Wipf S, Stöckli V, Herz K, Rixen C (2013) The oldest monitoring site of the Alps revisited: accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecol Divers 6(3–4):447–455. https://doi.org/10.1080/17550874.2013.764943
Yue K, Fornara DA, Yang W, Peng Y, Peng C, Liu Z, Wu F (2017) Influence of multiple global change drivers on terrestrial carbon storage: additive effects are common. Ecol Lett 20(5):663–672. https://doi.org/10.1111/ele.12767
Acknowledgments
The authors appreciate and thank the anonymous reviewers for their constructive comments that have improved the manuscript.
Author information
Authors and Affiliations
Contributions
Fatih Fazlioglu conceived the study, wrote the original draft, collected data, conducted the analyses, and created the figures. Justin SH Wan wrote and edited the draft, collected data, created the figures, and conducted the analyses.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 67 kb)
Rights and permissions
About this article
Cite this article
Fazlioglu, F., Wan, J.S.H. Warming matters: alpine plant responses to experimental warming. Climatic Change 164, 56 (2021). https://doi.org/10.1007/s10584-021-02996-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10584-021-02996-3