Abstract
Biological invasions have severe social, economic and ecological repercussions. Central Chile is a biodiversity hot spot but also a highly disturbed area containing many invasive plant species such as Chrysanthemoides monilifera (L.) Norl. This shrub is present close to the coast and is considered an aggressive invasive species with allelopathic effects in other countries. Despite being ubiquitous, its invasive potential and ecological impact has not yet been evaluated in Chile. Here, we aim to determine the effect of the invasive C. monilifera on local plant communities and test for possible allelopathic effects on the germination of native and other invasive species. To do this, we analyzed plant diversity in patches with and without the invader in two sites in Valparaíso, Chile. Additionally, we conducted an experiment testing the effect of different concentrations (0, 25, 50 and 100%) of the aqueous extract of the leaves on the germination of both a native and an invasive shrub. We found that C. monilifera negatively impacts local plant diversity, especially endemic species. Also, its leaf extract inhibited the germination of the native shrub, especially at higher concentrations (50 and 100%), but do not inhibit the germination of the invasive shrub. This will likely result in a rapid change in the plant community, with negative impacts on the native species and an increase in invasive and introduced species, further degrading this already altered ecosystem. Developing strategies for the control of C. monilifera are urgent to limit its spread and negative ecological impact in Chile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Invasive species are a main component of global change (GC) that has become a major threat for biodiversity worldwide (Hobbs 2000; Mack et al. 2000; Bradley et al. 2011). Biological invasions not only have ecological impacts, but can have social and economic repercussions as well (Mack et al. 2000; Shackleton et al. 2018). In Chile, invasive plant species are a serious threat to biodiversity (Arroyo et al. 2000; Fuentes et al. 2008), but there are no current specific legislation to manage them. These species belong mainly to the Poaceae, Fabaceae or Asteraceae families, and many have been reported as invasive elsewhere (Fuentes et al. 2013). Central Chile is where most introduced and naturalized species are found (Castro et al. 2005; Fuentes et al. 2020). This is mainly due to higher population density, agriculture, cattle and forestry, and other anthropic disturbances (Fuentes et al. 2008; Figueroa et al. 2011). Moreover, Central Chile has been largely affected by deforestation and the original native vegetation only exists in very small and fragmented sites. Large areas have been replaced by exotic species like Eucalyptus spp., Pinus spp. and Acacia spp. (Becerra 2006). All those species are highly pyrogenic, and added to the summer drought typical of this area, large fires of anthropogenic origin occur regularly, further affecting native flora (McWethy et al. 2018).
The Valparaíso region in Central Chile is one of the most negatively affected zones in the country by the recent wildfires. These fires are, as mentioned above, not of natural origin (McWethy et al. 2018). It also has a long history of port activity and host many dangerous invasive species. Valparaíso has been invaded by Genista monspessulana (L.) L.A.S. Johnson, Cytisus sp., Rosa spp., Rubus ulmifolius Schott and Ulex europaeus L. (Fuentes et al. 2014), among others. Ports are known to have a high risk of biological invasions due to the high traffic and trade with other parts of the world (Hulme 2021). Additionally, fires promote the germination of introduced, often invasive, species and can negatively impact native and endemic plants (Pauchard et al. 2008; Gómez-González et al. 2009; 2011; 2017) which lack adaptations to survive intense fires. This can result in a positive feedback loop further increasing the likelihood of a fire in an area. For example, the shrub Genista monspessulana, an invasive Fabaceae present in Central Chile, regenerates vigorously after fires and this, in turn, increases the risk of a subsequent fire (Pauchard et al. 2008). Valparaíso is part of the Mediterranean biodiversity hot spot (Arroyo et al. 2008) and includes many endemic plant species (Rodríguez et al. 2018), some of them with severe conservation problems. In the disturbed areas close to Valparaíso and Viña del Mar, the vegetation is usually dominated by native shrubs and herbaceous species, including the critically endangered Chloraea disoides Lindl. (Atala et al. 2017) and the vulnerable endemic palm Jubaea chilensis (Rodríguez et al. 2018). Taking into account the frequent fires, the role of Valparaíso as a main Chilean port is essential to assess any further risk for the highly endemic biodiversity of Valparaíso, particularly the possible negative impact of new invasive plant species.
The shrub Chrysanthemoides monilifera (L.) Norl., commonly known as boneseed, is an Asteraceae native to South Africa. It is considered to be an aggressive invasive species in Australia (Adair 1992). It grows abundantly, forming dense canopies that negatively impact abundance and diversity of native species (Thomas et al. 2005). There is evidence that this plant thrives after fires (Bray 2006) and its control could be very difficult due to its high seed output (personal observation). These traits put C. monilifera as a highly risky invader in Central Chile according to the criteria in Fuentes et al. (2014). To our knowledge, there are no records of how it was brought to Chile and established the first populations, but it is likely that it may be related to port activity and/or tourism. This species is present in Valparaíso, especially in degraded areas. Its risk as an invader has not been evaluated nor the mechanisms related to its success. The native plant diversity of Valparaíso could be threatened by the presence of invasive plant species such as C. monilifera and its impact should be evaluated in order to establish future control measures.
Invasive species usually thrive in invaded areas due to several mechanisms like the ability to show a wide range of phenotypes (Molina-Montenegro et al. 2013), high reproductive output, high competitive ability and the production of allelopathic compounds (Thorp and Lynch 2000; Rudman 2001; Weiss et al. 2008; Al-Harun et al. 2014). These compounds negatively affect other plant species, reducing seed germination and/or seedling growth (i.e., Gómez-González et al. 2009; Al-Harun et al. 2014). Plants can introduce the allelochemicals into the environment through leaf lixiviation, through root exudates or through the incorporation of plant residues in the soil (Inderjit and Duke 2003). C. monilifera has been shown to have an allelopathic effect on Lactuca sativa L. and Isotoma axillaris Lindl. (Al-Harun et al. 2014), possibly due to a high phenols concentration. Aqueous extract of C. monilifera reduced germination in these species, even at low concentrations (Al-Harun et al. 2014). This high allelopathic effect could partially explain the high invasiveness of this species and its success in Valparaíso, especially in disturbed areas.
The present study aims to assess the impacts of an invasive species on the local plant community. To achieve this, we characterized the plant community where C. monilifera is present and compared its diversity with patches without the invader. Additionally, we tested the existence of a possible allelopathic effect that this invasive plant could have on a coexisting native and exotic shrub species. This information could be essential for future management and control initiatives and for the conservation of the unique biodiversity of Valparaíso.
2 Materials and methods
Effect of C. monilifera on plant diversity
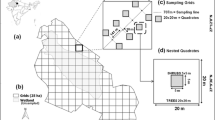
The study site was located in Valparaíso Region and was selected on the basis of the presence of C. monilifera subsp. monilifera and accessibility. This site was previously studied and is the natural habitat of some native orchids, including species classified as critically endangered (Atala et al. 2017) by Ministry of Environment (Ministerio del Medio Ambiente 2011) from Chilean government. The site is anthropically disturbed and dominated by shrubs and with abundant herbaceous cover, especially in the spring. Here, patches with (Chr +) and without (Chr−) C. monilifera were selected and 1 × 1 m randomly put plots were used to study plant diversity and abundance following standard methods (see Atala et al. 2017). Sampling was conducted in late spring to ensure most plant species are in the reproductive stage in order to facilitate identification. We applied a simulation-based sampling protocol to assess the adequacy of our sampling effort for plots with the presence of C. monilifera and without the species, as implemented in the SSP package (v. 1.0.1, Guerra-Castro et al. 2021) in R (R Core Team 2022). A rarefaction analysis was used to estimate sample completeness. Rarefaction analysis compares the accumulated number of species as a response to plot number (Chen et al. 2014; Chao et al. 2020). When the curve becomes asymptotical, it is assumed that all (or almost all) species diversity in that site has been recorded, and that it determines the minimal number of plots required to appropriately estimate such diversity. In each plot, we registered vascular plant species present. A diversity index and rarefaction analyses were used to compare plant diversity between patches in both sites. We also compared the % of native and introduced plant species in different patches (with and without C. monilifera). Rarefaction were constructed in R using the iNEXT package (Hsieh et al. 2016). This package allows extrapolation of sample completeness, while estimating standard error. Rarefaction analyses were run with 1000 bootstrap pseudoreplicates with 95% confidence interval.
Allelopathic effect of aqueous extract of C. monilifera
Leaves of C. monilifera were collected from the field in “Palmar el Salto”, Valparaíso (33°3′58.1″ S, 71°31′54.6″ W). We collected leaves of the invasive plant from at least 10 different individuals and were then pooled together. Samples were then taken to the laboratory, dried at room temperature for 48 h and then grounded with a manual mixer, adding 300 mL of distilled water per 300 g of leaves to facilitate the grounding. The obtained solution was strained using a metal strainer and then filtered using filter paper. The final filtered solution was stored in glass flasks in the dark at 4 °C for the subsequent experiment. From the stock solution, 3 dilutions with sterile distilled water were used in the experiment, 100, 50 and 25% of the obtained filtered solution. Additionally, we used a control of only distilled water (0% stock solution). All of these solutions, and the control, were stored as described above.
From natural populations, seeds of the native shrub, Baccharis linearis (Ruiz & Pav.) Pers., and the invader tree, G. monspessulana, were collected, aiming for at least 100 seeds per species from individuals near C. monilifera. We chose these species because both coexist in the same habitat where C. monilifera is found. Additionally, both species are shrubs and relatively similar in size to C. monilifera. This allowed us to assess potential allelopathic effects between exotic and native species with the same growth habit, and compare a model native and introduced species. Seeds were superficially disinfected by immersion in a 70% ethanol solution for 30 s, followed by immersion in a 1% NaOCl solution with two drops of Tween 20 for 5 min under constant agitation (see Pereira et al. 2015). This was done to avoid external fungal contamination. Then, seeds were washed 5 times with sterile distilled water. Seeds, then, were put in distilled water for 12 h to initiate imbibition. Afterward, 20 seeds of B. linearis and 30 seeds of G. monspessulana were put in Petri dishes with tissue paper at the bottom and were assigned to one of the following treatments: (1) control treatment, where dishes were watered with 1 mL of distilled water every 2 days; (2) 100% where dishes were watered with 1 mL of the 100% extract solution; (3) 50%, where plates were watered with 1 mL of the 50% solution v/v; and 4) 25%, where dishes were watered with 1 mL of the 25% solution v/v. We used 25 Petri dishes for B. linearis per treatment and 27 for G. monspessulana per treatment (i.e., n = 25 and 27, respectively). Petri dishes were put in the laboratory at room temperature and natural light. Seed germination was recorded every 2–3 days until 41 days. After that time, no further germination was detected.
Initial exploration of germination data under the different treatments was carried out with a time to event analysis, which has been used in other seed germination studies (Onofri et al. 2011; Ritz et al. 20132019). The data were analyzed using the R environment (version 4.2.0, R Core Team 2022). For each Petri dish, the response was defined as the daily proportional cumulative germination curve (propCum), expressed as the number of germinated seeds. The analysis was conducted using the different treatments as predictors against germination. The “time event approach” is available in drcSeedGerm R package (Onofri et al. 2018), and the function makeDrm was used to calculate the response. The distributional assumption for the germination times considered was the three-parameter log-logistic, a shifted log-logistic distribution (Ritz et al. 2015) where ED50 was used as a parameter with lower limit at 0. We compared the curves per treatment using nonparametric Kruskal–Wallis one-way analysis of variance using stats R package, as our data were not normal distributed (Shapiro–Wilk normality test, p < 0.001). This was followed by Dunn test post hoc analysis, available from FSA R library (Ogle et al. 2022). Differences were considered significant at p < 0.05.
3 Results
C. monilifera negatively impacts on a natural plant community
The invasive C. monilifera negatively impacted plant diversity in patches where it was present (Chr +) compared to patches without the species (Chr−) in the same site (Fig. 1). This was also evident when comparing Shannon–Weaver diversity index, 2.92 vs 3.41 in Chr + and Chr− patches, respectively (Table 1). Additionally, Chr + patches had a higher proportion of introduced species and a lower proportion of native and endemic species, compared to Chr− patches (Table 1). The differences in the proportion of introduced and endemic species were statistically significant between patches (z-score test, p < 0.05, Table 1). No statistical differences were found in the proportion of native species between Chr + and Chr− patches (z-score test, p > 0.05, Table 1). In both patches, most species belonged to the Asteraceae family (Table S1), including B. linearis, followed by Poaceae.
Rarefaction curves showed that both conditions (Chr + and Chr−) reach the asymptote within a 95% confidence interval, but with an overlap in their extrapolated confidence, modeled up to 80 sampling sites in each condition. These results also shows that Chr + sites present a lower total richness, with an observed total of 32 species and an estimated of ~ 44 species, with a standard error of 9.5 species and a sample coverage of ~ 94%. On the other hand, Chr− sites showed a total of 42 species observed and an estimated of ~ 50 species, with a standard error of ~ 6 species and a sample coverage of ~ 93%. These results are supported by our simulated sampling (data not shown), indicating that with an average of 18 samples points per conditions we can reach a descriptive precision above 67% for either community.
Allelopathic effect of C. monilifera
A log-logistic model was fitted to compare time to event analysis, which showed statistical differences in treatment effect in all models for B. linearis and G. monspessulana (Table S2, Time to event analysis, p < 0.001 in both cases). Our results showed that an increase in the solution concentration negatively affected the germination in B. linearis (Fig. 2a). Although a 25% solution was not significantly different from the control, the addition of a 50% or 100% solution of leaf extract significantly reduced germination (Dunn test, p < 0.001, Table 2). On the other hand, we observed no negative impact on G. monspessulana germination and even a slight positive effect (Fig. 2b). All treatments have higher germination compared with the control (Table 2, Dunn test, p < 0.001), but there were no significant differences between different concentrations of the extract (Dunn test, p > 0.05). Finally, after close to 40 days, seeds did not further germinate in any treatment for both species.
4 Discussion
Invasive species are a severe threat to global biodiversity (Bradley et al. 2011; Linders et al. 2020; Pyšek et al. 2020), but despite their ecological and socioeconomic impact they are seldom considered in predictive global models (Roura-Pascual et al. 2021). Here we showed that C. monilifera can negatively impact the plant community when present in a site in Central Chile. To our knowledge, this is the first report on the impact of this invasive species in Chile. Particularly, patches with the invasive species have a lower richness and a lower proportion of endemic species and higher proportion of introduced species. There is evidence of the negative impact of invasive species on biodiversity (Pyšek et al. 2012; Haider et al. 2018; Pauchard et al. 2018). However, some authors suggest that the impact of invasive plants on native plant communities is low and/or very slow, even that invasive species can add to the overall species pool of a region, increasing local diversity (Houlahan and Findlay 2004; Ellis et al. 2012; Gilbert and Levine 2013; Thomas and Palmer 2015; Corlett 2016). This may likely depend on the spatial scale and on the particular site.
Our results indicate a negative impact at a local scale in Central Chile. Moreover, among the introduced species present in patches with C. monilifera are other invasive plants such as G. monspessulana, which may further degrade the community. In fact, patches with the invader looked very homogeneous compared to patches without C. monilifera (personal observation). Furthermore, the presence of invasive species reduced the plant richness, as seen in the comparison between patches with (Chr +) and without (Chr−) C. monilifera. Endemic species in particular were more severely affected compared to native species. Endemic species were represented by less than 10% of the total richness in Chr + patches, whereas endemic species richness was close to 25% in Chr− patches. This is consistent with other studies where invasive species reduce the richness in the communities where they live, specially impacting endemic and/or rare species (Thomson 2005; Hardman et al. 2012). In addition, the presence of native and endemic species in patches without C. monilifera may serve as a barrier for the introduction of this plant (see Reinhardt Adams and Galatowitsch 2008; Vargas-Gaete et al. 2018). On the other hand, the study site show evidence of frequent disturbances (see Atala et al. 2017). It is likely that disturbances, such as fires and/or cattle, can alter patches without the invader, allowing seeds to germinate and slowly colonize new patches. We suggest that this species should be monitored to avoid its spread into nearby protected areas, where many unique endemic plant species are found, being a biodiversity hot spot and a biosphere reserve (Arroyo et al. 2008; Moreira-Muñoz and Borsdorf 2014). This could have severe consequences for the conservation of these endemic species, which can have restricted distributions and small populations. Such is the case of Chloraea disoides, a critically endangered endemic species recorded close to the study site which has few existing populations (Atala et al. 2017).
Leaf aqueous extract of C. monilifera inhibited the germination of a native shrub (B. linearis), especially at higher concentration of the extract. Low concentrations (25%) had no significant effect on seed germination on laboratory conditions. Previous studies have shown the allelopathic potential of this invasive species, reducing the germination of other plant species (Al-Harun et al. 2014, 2015a). This was attributed to a high phenolic content of the plant tissues (Al-Harun et al. 2014). This effect seems to be dose-dependent and is stronger in roots and leaves, compared to stems (Al-Harun et al. 2015b). Other invasive species present in Central Chile, such as Cytisus scoparius (L.) Link and Centaurea solstitialis L., can also have allelopathic effects on native species (Gómez-González et al. 2009; Grove et al. 2012). These mechanisms could explain the success of C. monilifera in coastal Central Chile and should be taken into account for future control initiatives. However, direct application of aqueous extract may not exactly mimic natural conditions in the soil and further studies should be conducted in the field and laboratory using C. monilifera litter to confirm its allelopathic effect on Baccharis spp. and other native Chilean plants. In the field, below C. monilifera individuals, there are large amounts of shed leaves, forming a thick layer in the soil. This could also form a physical barrier, as well as a chemical barrier, for the germination of native species. Photoblastic seeds may not receive sufficient light to germinate, added to the litter weight and potential chemical inhibition of the seeds. Some Asteraceae species have been reported as having photosensitive seeds (Schutz et al. 2002; Luo and Cardina 2012), varying in their response to light with seed traits such as seed weight and size (Schutz et al. 2002). In the field, only C. monilifera seedlings are found under the canopy of the invader (personal observation, data not shown). Thus, further studies are required to unravel the full extent of the effect of C. monilifera litter on the soil and on other plant species.
Contrary to the native shrub, leaf extract of C. monilifera did not affect and even slightly promoted the germination of the invasive shrub G. monspessulana. This negative effect on native species and neutral (or even positive) effect on other invasive species could severely deteriorate the plant community, resulting in an invasion meltdown (see Simberloff and Von Holle 1999; Collins et al. 2020). Other authors, however, disagree with the concept and use the term “secondary invasion” (O'Loughlin and Green 2017) for a facilitated invasion of an invader species due to the previous presence of another. The obtained germination % in G. monspessulana was relatively low, close to 10%. In this species, fire can increase germination to 40–50%, whereas unburnt seeds reach only 10% (García et al. 2010), which may explain the obtained low germination. There is evidence that fire also can increase germination of C. monilifera (Bray 2006). Anthropically caused wildfires have affected Central Chile, and Valparaíso in particular, especially during the dry summers (Peña and Valenzuela 2008; Atala et al. 2017). These fires could have further negative impacts on the non-adapted native species (Gómez-González and Cavieres 2009) and potentiate the increase and dominance of invasive species such as C. monilifera and G. monspessulana.
Central Chile includes a Mediterranean biodiversity hot spot (Arroyo et al. 2008) that has been largely altered by human activity. Here many unique endemic plant species can be found that are vulnerable to these anthropic disturbances and the possible negative effects of plant invasions. Based on our results, we suggest that urgent actions are needed to control the spread of C. monilifera in the country. This, especially considering that it can have a negative impact of endemic flora, already threatened by urban development, agriculture, wildfires, and other plant and animal invasive species (Castro et al. 2005; Fuentes et al. 2008; Pauchard et al. 2008; Figueroa et al. 2011; Gómez-González et al. 2011). Since its distribution in Chile is still relatively small, we suggest that manual control can be implemented as a first step to manage this invader, especially close to protected natural areas such as the biosphere reserve La Campana–Peñuelas (Moreira-Muñoz and Borsdorf 2014). In the mid-long term, other strategies such as biological control or the use of herbicides could also be applied to control its spread as used in other countries such as Australia (Brougham 2006; Melland and Preston 2008; Melland 2009). Additionally, future studies could also address possible effects of this invader on soil microorganisms, its regeneration after the frequent summer fires of the areas and its possible effects on pollinators and other animals that could indirectly impact the native and endemic flora.
References
Adair RJ (1992) Biological control of boneseed and bitou bush (Chrysanthemoides monilifera) in Australia. Vic Ent 22:61–68
Al Harun MAY, Johnson J, Uddin MN, Robinson RW (2015) Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 10:e0139992. https://doi.org/10.1371/journal.pone.0139992
Al-Harun MAY, Robinson RW, Johnson J, Uddin MN (2014) Allelopathic potential of Chrysanthemoides monilifera subsp. monilifera (boneseed): a novel weapon in the invasion processes. S Afr J Bot 93:157–166
Al-Harun MAY, Johnson J, Robinson RW (2015) The contribution of volatilization and exudation to the allelopathic phytotoxicity of invasive Chrysanthemoides monilifera subsp. monilifera (boneseed). Biol Invasions 17:3609–3624
Arroyo MTK, Marticorena C, Matthei O, Cavieres L (2000) Plant invasions in Chile: present patterns and future predictions. In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world, 1st edn. Island Press, Washington DC, pp 385–421
Arroyo MTK, Marquet P, Marticorena C, Simonetti JA, Cavieres L, Squeo FA, Rozzi R, Massardo F (2008) El Hotspot chileno, prioridad mundial para la conservación. In: Conama (ed) Biodiversidad de Chile, patrimonio y desafíos, 2nd edn. Ocho Libros editores, Santiago de Chile, pp 90–95
Atala C, Muñoz-Tapia M, Pereira G, Romero C, Vargas R, Acuña-Rodriguez IS, Molina-Montenegro MA, Brito E (2017) The effect of future climate change on the conservation of Chloraea disoides Lindl. (Orchidaceae) in Chile. Braz J Bot 40:353–360
Becerra PI (2006) Invasión de árboles alóctonos en una cuenca pre-andina de Chile central. Gayana Bot 63:161–174
Bradley AB, Blumenthal DM, Wilcove DS, Ziska LH (2011) Predicting plant invasions in an era of global change. Trends Ecol Evol 25:310–318
Bray C (2006) Fire leads to extreme boneseed invasion. In: Brougham KJ, Cherry H, Downey PO (eds) Boneseed management manual: current management and control options for boneseed (Chrysanthemoides monilifera spp. monilifera) in Australia, 1st end. Department of Environment and Conservation NSW, Sydney, Australia, pp 65–67
Brougham KJ, Cherry H, Downey PO (2006) Boneseed Management Manual: current management and control options for boneseed (Chrysanthemoides monilifera spp. monilifera) in Australia. Department of Environment and Conservation NSW, Sydney Australia, p 45
Castro SA, Figueroa JA, Muñoz-Schick M, Jaksic FM (2005) Minimum residence time, biogeographical origin, and life cycle as determinants of the geographical extent of naturalized plants in continental Chile. Divers Distrib 11:183–191
Chao A, Kubota Y, Zelený D, Chiu C, Li C-F, Kusumoto B, Yasuhara M, Thorn S, Wei C, Costello MJ, Colwell RK (2020) Quantifying sample completeness and comparing diversities among assemblages. Ecol Res 35:292–314
Chen J, Yang Y, Stöcklin J, Cavieres LA, Peng D, Li Z, Sun H (2014) Soil nutrient availability determines the facilitative effects of cushion plants on other plant species at high elevations in the South-eastern Himalayas. Plant Ecol Divers 8:199–210
Collins RJ, Copenheaver CA, Barney JN, Radtke PJ (2020) Using invasional meltdown theory to understand patterns of invasive richness and abundance in forests of the northeastern USA. Nat Areas J 40:336–344
Corlett RT (2016) Plant diversity in a changing world: status, trends, and conservation needs. Plant Divers 38:10–16
Ellis EC, Antill EC, Kreft H (2012) All is not loss: plant biodiversity in the anthropocene. PLoS ONE 7:e30535. https://doi.org/10.1371/journal.pone.0030535
Figueroa J, Teillier S, Castro SA (2011) Diversity patterns and composition of native and exotic floras in central Chile. Acta Oecol (montrouge) 37:103–109
Fuentes N, Ugarte E, Kühn I, Klotz S (2008) Alien plants in Chile: inferring invasion periods from herbarium records. Biol Invasions 10:649–657
Fuentes N, Pauchard A, Sánchez P, Esquivel J, Marticorena A (2013) A new comprehensive database of alien plant species in Chile based on herbarium records. Biol Invasions 15:847–858
Fuentes N, Sánchez P, Pauchard A, Urrutia J, Cavieres LA, Marticorena A (2014) Plantas invasoras del centro-sur de Chile: una guía de campo. Concepción, Chile
Fuentes N, Marticorena A, Saldaña A, Jerez V, Ortiz JC, Victoriano P, Moreno RA, Larraín J, Villaseñor-Parada C, Palfner G, Sánchez P, Pauchard A (2020) Multi-taxa inventory of naturalized species in Chile. NeoBiota 60:25–41
García RA, Pauchard A, Cavieres LA, Peña E, Rodríguez MF (2010) El fuego favorece la invasión de Teline monspessulana (Fabaceae) al aumentar su germinación. Rev Chil Hist Nat 83:443–452
Gilbert B, Levine JM (2013) Plant invasions and extinction debts. Natl A Sci USA 110:1744–1749
Gómez-González S, Cavieres LA (2009) Litter burning does not equally affect emergence on native and alien species of the mediterranean-type chilean matorral. Int J Wildland Fire 18:213–221
Gómez-González S, Cavieres LA, Torres P, Torres-Díaz C (2009) Competitive effects of the alien invasive Centaurea solstitialis L. on two chilean Baccharis species at different life-cycle stages. Gayana Bot 66:71–83
Gómez-González S, Torres-Díaz C, Valencia G, Torres-Morales P, Cavieres L, Pausas G (2011) Anthropogenic fires increase alien and native annual species in the chilean coastal matorral. Divers Distrib 17:58–67
Gómez-González S, Paula S, Cavieres LA, Pausas JG (2017) Postfire responses of the woody flora of Central Chile: Insights from a germination experiment. PLoS ONE 12:e0180661
Grove S, Haubensak KA, Parker IM (2012) Direct and indirect effects of allelopathy in the soil legacy of an exotic plant invasion. Plant Ecol 213:1869–1882
Guerra-Castro EJ, Cajas JC, Simões N, Cruz-Motta JJ, Mascaró M (2021) SSP: an R package to estimate sampling effort in studies of ecological communities. Ecography 44:1–13
Haider S, Kueffer C, Bruelheide H, Seipel T, Alexander JM, Rewearing LJ, Arévalo JR, Cavieres LA, Milbau A, Naylor BJ, Special K, Pauchard A (2018) Mountain roads and non-native species modify elevational patterns of plant diversity. Glob Ecol Biogeogr 27:667–678
Hardman C, Williams S, Manco B, Hamilton M (2012) Predicting the potential threat of Casuarina equisetifolia to three endemic plant species on the Turks and Caicos Islands. Oryx 46:204–212
Hobbs RJ (2000) Land-use changes and invasions. In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world, 1st edn. Island Press, Washington DC, pp 31–54
Houlahan JE, Findlay CS (2004) Effect of invasive plant species on temperate wetland plant diversity. Conserv Biol 18:1132–1138
Hsieh TC, Ma KH, Chao A (2016) iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. http://chao.stat.nthu.edu.tw/wordpress/software-download/
Hulme PE (2021) Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 4:666–679
Inderjit DSO (2003) Ecophysiological aspects of allelopathy. Planta 217:529–539
Linders TE, Bekele K, Schaffner U, Allan E, Alamirew T, Choge SK, Eckert S, Haji J, Muturi G, Mbaabu PR (2020) The impact of invasive species on social–ecological systems: relating supply and use of selected provisioning ecosystem services. Ecosyst Serv 41:101055
Luo J, Cardina J (2012) Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Res 52:112–121
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
McWethy DB, Pauchard A, García RA, Holz A, González ME, Veblen TT, Stahl J, Currey B (2018) Correction: landscape drivers of recent fire activity (2001–2017) in south-central Chile. PLoS ONE 13:e0205287. https://doi.org/10.1371/journal.pone.0205287
Melland RL (2009) Management of boneseed (Chrysanthemoides monilifera ssp. monilifera) (L.) T. Norl. using fire, herbicides and other techniques in Australian woodlands. University of Adelaide, Adelaide
Melland R, Preston C (2008) The role of fire in integrated management of boneseed (Chrysanthemoides monilifera subsp. monilifera). Plant Prot Q 23:32–33
Ministerio del Medio Ambiente (2011) Reglamento de clasificación de especies. Sexto proceso de clasificación de especies silvestres según categoría de conservación. Decreto Supremo N°41/2011, MMA
Molina-Montenegro MA, Palma-Rojas C, Alcayaga-Olivares Y, Oses R, Corcuera LJ, Cavieres LA, Gianoli E (2013) Ecophysiological plasticity and local differentiation help explain the invasion success of Taraxacum officinale (dandelion) in South America. Ecography 36:718–730
Moreira-Muñoz A, Borsdorf A (2014) Reservas de la biosfera de Chile: laboratorios para la sustentabilidad. Instituto de Geografía UC, Santiago de Chile
Ogle DH, Doll JC, Wheeler P, Dinno A (2022) FSA: fisheries stock analysis. R package version 0.9.3. https://github.com/fishR-Core-Team/FSA
O’Loughlin LS, Green PT (2017) Secondary invasion: when invasion success is contingent on other invaders altering the properties of recipient ecosystems. Ecol Evol 7:7628–7637
Onofri A, Mesgaran MB, Tei F, Cousens RD (2011) The cure model: an improved way to describe seed germination? Weed Res 51:516–524
Onofri A, Benincasa P, Mesgaran MB, Ritz C (2018) Hydrothermal-time-to-event models for seed germination. Eur J Agron 101:129–139
Onofri A, Piepho H-P, Kozak M (2019) Analysing censored data in agricultural research: a review with examples and software tips. Ann Appl Biol 174:3–13
Pauchard A, García RA, Peña E, Bustamante-Araya R, González C, Cavieres L (2008) Positive feedbacks between plant invasions and fire regimes: Teline monspessulana (L.) K. Koch (Fabaceae) in central Chile. Biol Invasions 10:547–553
Pauchard A, Meyerson LA, Bacher S, Blackburn TM, Brundu G, Cadotte MW, Courchamp F, Essl F, Genovesi P, Haider S, Holmes ND, Hulme PE, Jeschke JM, Lockwood JL, Novoa A, Nummer MA, Pelztier DA, Pyšek P, Richardson DM, Simberloff D, Smith K, van Wilgen BW, Vilà M, Wilson JRU, Winter M, Zenii RD (2018) Biodiversity assessments: origin matters. PLoS Biol 16:e2006686. https://doi.org/10.1371/journal.pbio.2006686
Pereira G, Albornoz V, Muñoz-Tapia L, Romero C, Atala C (2015) Asymbiotic germination of Bipinnula fimbriata (Orchidaceae) seeds in different culture media. Seed Sci Technol 43(3):367–377. https://doi.org/10.15258/sst.2015.43.3.01
Peña E, Valenzuela L (2008) Incremento de los incendios forestales en bosques naturales y plantaciones forestales en Chile. In: González-Cabán A (ed) Memorias del segundo simposio internacional sobre políticas, planificación y economía de los programas de protección contra incendios forestales: una visión global, 1st edn. Department of Agriculture, Albany, pp 595–612
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM (2020) Scientist’s warning on invasive alien species. Biol Rev 95:1511–1534
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reinhardt Adams C, Galatowitsch SM (2008) The transition from invasive species control to native species promotion and its dependence on seed density thresholds. Appl Veg Sci 11:131–138
Ritz C, Pipper CB, Streibig JC (2013) Analysis of germination data from agricultural experiments. Eur J Agron 45:1–6
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:e0146021. https://doi.org/10.1371/journal.pone.0146021
Rodríguez R, Marticorena C, Alarcón D, Baeza C, Cavieres L, Finot VL, Kiessling A, Mihoc M, Pauchard A, Ruiz E, Sánchez P, Marticorena A (2018) Catálogo de las plantas vasculares de Chile. Gayana Bot 75:1–430
Roura-Pascual N, Leung B, Rabitsch W, Rutting L, Vervoort J, Bacher S, Dullinger S, Erb KH, Jeschke JM, Katsanevakis S, Kühn I, Lenzner B, Liebhold AM, Obersteiner M, Pauchard A, Peterson GD, Roy HE, Seebens H, Winter M, Burgman MA, Genovesi P, Hulme PE, Keller RP, Latombe G, McGeoch MA, Ruiz GM, Scalera R, Springborn MR, von Holle B, Essl F (2021) Alternative futures for global biological invasions. Sustain Sci 16:1637–1650
Rudman T (2001) Tasmanian weed status report: boneseed (Chrysanthemoides monilifera subsp. monilifera). Hobart nature conservation branch, dept of primary industries, water and environment. Nature Conservation Report (Tasmania), Hobart Australia, p 15
Schutz W, Milbert P, Lamont BB (2002) Seed dormancy, after-ripening and light requirements of four annual Asteraceae in south-western Australia. Ann Bot 90:707–714
Shackleton RT, Biggs R, Richardson DM, Larson BMH (2018) Social-ecological drivers and impacts of invasion-related regime shifts: consequences for ecosystem services and human wellbeing. Environ Sci Policy 80:300–314
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Thomas CD, Palmer G (2015) Non-native plants add to the British flora without negative consequences for native diversity. Natl A Sci USA 112:4387–4392
Thomas PB, Possingham H, Roush R (2005) Effects of boneseed (Chrysanthemoides monilifera (L.) Norl. ssp. monilifera) on the composition of the vegetation and the soil seed bank of an open eucalypt woodland. Plant Prot Q 20:74–80
Thomson D (2005) Measuring the effects of invasive species on the demography of a rare endemic plant. Biol Invasions 7:615–624
Thorp J, Lynch R (2000) The determination of weeds of national significance. National Weeds Strategy Executive Committee, Launceston
Vargas-Gaete R, Salas-Eljatib C, Gärtner SM, Vidal OJ, Bannister JR, Pauchard A (2018) Invasive plant species thresholds in the forests of Robinson Crusoe Island Chile. Plant Ecol Divers 11:205–215
Weiss PW, Adair RJ, Edwards PB, Winkler MA, Downey PO (2008) Chrysanthemoides monilifera subsp monilifera (L.) T. Norl. and subsp. rotundata (DC.) T. Norl. Plant Prot Q 23:3–14
Acknowledgements
This study was funded by DI emergente 039.478/2020 (PUCV). SAR and OJ-C were funded by Vicerrectoría Académica from Pontificia Universidad Católica de Valparaíso. The authors thank Felipe Cacciuttolo for his assistance in the field.
Author information
Authors and Affiliations
Contributions
CA was involved in conceptualization, resources, writing—original draft and writing—reviewing and editing. SAR was responsible for formal analysis, data curation, writing—reviewing and editing, and visualization. JO carried out investigation and data curation. OJ-C participated in data curation, investigation and visualization. RV contributed to formal analysis, writing—reviewing and editing, and visualization.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atala, C., Reyes, S.A., Osses, J. et al. The invader shrub Chrysanthemoides monilifera (boneseed) negatively impacts native plant communities in a Mediterranean zone in Central Chile. Braz. J. Bot 46, 695–703 (2023). https://doi.org/10.1007/s40415-023-00905-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-023-00905-9