Abstract
Cyanobacteria are photosynthetic oxygen-evolving prokaryotes that are distributed in diverse habitats. They synthesize the ultraviolet (UV)-screening pigments, scytonemin (SCY) and mycosporine-like amino acids (MAAs), located in the exopolysaccharide (EPS) matrix. Multiple roles for both pigments have gradually been recognized, such as sunscreen ability, antioxidant activity, and heat dissipation from absorbed UV radiation. In this study, a filamentous terrestrial cyanobacterium Nostoc flagelliforme was used to evaluate the potential stabilizing role of SCY on the EPS matrix. SCY (∼3.7 %) was partially removed from N. flagelliforme filaments by rinsing with 100 % acetone for 5 s. The physiological damage to cells resulting from this treatment, in terms of photosystem II activity parameter Fv/Fm, was repaired after culturing the sample for 40 h. The physiologically recovered sample was further desiccated by natural or rapid drying and then allowed to recovery for 24 h. Compared with the normal sample, a relatively slower Fv/Fm recovery was observed in the SCY-partially removed sample, suggesting that the decreased SCY concentration in the EPS matrix caused cells to suffer further damage upon desiccation. In addition, the SCY-partially removed sample could allow the release of MAAs (∼25 %) from the EPS matrix, while the normal sample did not. Therefore, damage caused by drying of the former resulted from at least the reduction of structural stability of the EPS matrix as well as the loss of partial antioxidant compounds. Considering that an approximately 4 % loss of SCY led to this significant effect, the structurally stabilizing potential of SCY on the EPS matrix is crucial for terrestrial cyanobacteria survival in complex environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cyanobacteria are primitive photosynthetic oxygen-evolving prokaryotes that appeared on Earth when the ozone shield was absent [1]. Today, they are ubiquitous in terrestrial, freshwater, and marine habitats, having adapted to various extreme environments. Many cyanobacteria are surrounded by a matrix of exopolysaccharides (EPSs) and synthesize ultraviolet (UV)-screening pigments, including mycosporine-like amino acids (MAAs) and scytonemin (SCY) [2–4]. Both pigments can accumulate within the EPS matrix, with the latter distributed in the peripheral region [4–6]. SCY is mainly induced by UV-A radiation and can prevent up to 90 % of incident UV-A from entering the cells [7]. In some tested cyanobacteria, MAA concentrations ranged from 0.61 up to 8.23 mg/g dry weight (DW) and SCY ranged from 0.76 to 79.84 mg/g DW [8]. In the terrestrial cyanobacterium Nostoc commune, DW percentages were estimated to be >50, 4, and 0.4 % for EPS, MAAs, and SCY, respectively [6]. With the accumulation of research data, the multi-functionality of both pigments has been recognized, such as sunscreen ability, antioxidant activity, and heat dissipation from absorbed UV radiation [9–11]. The last role may facilitate the optimization of photosynthesis by increasing the surface temperature of cyanobacteria grown in cold environments. In addition, the significant stability of SCY has been recognized [12, 13]. Because of its abundance and peripheral distribution in the EPS matrix of cyanobacteria, as well as its molecular stability, we speculate that SCY may play an extra eco-physiological role in stabilizing the EPS matrix.
The terrestrial cyanobacterium Nostoc flagelliforme, found in the arid or semi-arid steppes of some countries, has a hair-like colony form (filament) of 0.2–1 mm in diameter [14]. It undergoes frequent cycles of desiccation and rehydration in its native habitats. When fully rehydrated, the filaments expand 2–4-fold in diameter. During rehydration and desiccation, the cells embedded in the dense EPS matrix remain intact [15]. Light microscopy of N. flagelliforme sections clearly shows the peripheral distribution of SCY in the filaments [5]. Thus, this species serves as an ideal material for investigating the potential role of SCY in stabilizing the EPS matrix. The chemical reagent acetone was often used for SCY extraction [7, 13]. When fully rehydrated filaments were rinsed in 100 % acetone for 5 s, the loss of ∼3.7 % of total SCY was observed (Fig. 1a). SCY in the N. flagelliforme sample was calculated to be 0.21 mg/g fresh weight (FW), according to the extinction coefficient of 112.6 L g−1 cm−1 at 384 nm [16]. Meanwhile, this rapid rinsing process also destroyed some cells, as implied by the tiny peak of chlorophyll a (Chl a) observed (Fig. 1a). However, this peak only accounted for ∼0.3 % of total Chl a content (0.27 mg/g FW), as calculated by the extinction coefficient of 92.6 L g−1 cm−1 at 663 nm [16]. The peripheral distribution of SCY was also implied by the higher SCY/Chl a ratio (12.3) than the average ratio (0.78). As shown in Fig. 1b, SCY-partially removed sample showed physiological damage but fully recovered its physiological activity after a 40-h culture period, in terms of the widely used photosystem II (PSII) activity parameter Fv/Fm [17–19]. Cyanobacterial Fv/Fm is very indicative to quickly detect stressful conditions and monitor adaptation responses to surrounding environments [19]. The recovered samples were further desiccated by natural or rapid drying with hot air followed by 24 h of culture for recovery (Fig. 1c). The SCY concentration did not change during these short-term drying treatments or the recovery processes (data not shown). A relatively slower physiological recovery for SCY-partially removed sample, especially after rapid drying, was observed compared with the normal sample (Fig. 1c), implying that the removal of SCY could cause additional damage to cells subjected to desiccation stress.
The partial removal of SCY in the N. flagelliforme sample and the physiological recovery of the resulting material following desiccation stress. a The full wavelength scanning of the acetone extracts. The physiologically recovered sample of 1 g FW was fully extracted with 100 % acetone as described by [13] or extracted by rapid rinsing for 5 s. b The SCY-partially removed sample was incubated in BG110 solution for physiological recovery. The sample was rinsed as described above, but was immediately washed three times with plenty of water. c Recovery in BG110 solution for 24 h after the samples (samples at 40 h in b) were respectively subjected to natural drying (22 °C, 50 % relative humidity, ∼4 h) or rapid drying by hot air (70 °C, 30 min). Samples were incubated at 20 μmol photons m−2 s−1 at 22 °C. PSII activity parameter Fv/Fm, as a physiological activity indicator, was determined using a plant efficiency analyzer as previously described [17]. Normal S normal sample, −SCY S SCY-partially removed sample, SCY scytonemin, Carot carotenoids, Chl a chlorophyll a. Data are shown as means ± SD (n = 5–6). *Significant difference (P < 0.05); **significant difference (P < 0.01), student’s t test
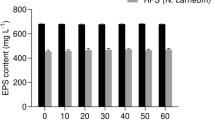
Modulation of the structure and function of the dense EPS matrix has been taken to be crucial for desiccation resistance in terrestrial cyanobacteria [6]. The shrinking process of N. flagelliforme filaments upon desiccation can have two effects on the EPS matrix, a change in the structural rigidity/elasticity and an oxidative burst [6, 15, 20]. The secreted WSPA protein putatively plays an important role in maintaining dynamic structural stability [6]. Extracellular superoxide dismutase, MAAs, SCY, and even EPS itself are thought to scavenge reactive oxygen species [2, 21]. The effect of decreased SCY on the structural stability of the EPS matrix was evaluated by the release of MAAs (Fig. 2). Normal sample does not obviously release MAAs from the EPS matrix during the rehydration process, although the EPS matrix is expanded and also the dissociation of oligosaccharide-linked MAAs from the EPS cannot be excluded. n-acetylcysteine (NAC) can efficiently destabilize the EPS matrix of N. flagelliforme to allow the release of MAAs [5], an effect used for comparison purposes in this study. Acidic NAC solution may mediate the process of EPS hydrolysis to release MAAs, due to its acid hydrolysis effect. As shown in Fig. 2a, SCY-partially removed sample released partial MAAs following 1-h incubation in both solutions (BG110, BG110 supplemented with 10 mM NAC), and an additive effect was also observed for it in the NAC solution. Therefore, a structural relaxation of the EPS matrix was caused by the partial removal of SCY. Following incubation for 16 h (Fig. 2b), the maximum release of MAAs was calculated to be 2.03 mg/g FW for samples in the NAC solution, according to the extinction coefficient of 17 L g−1 cm−1 at 312 nm [22]. At this time, approximately, a quarter of total MAAs was released from the SCY-partially removed sample. These results implied that the aforementioned drying damage caused to the SCY-partially removed sample resulted at least from the altered structural stability of the EPS matrix as well as the partial loss of antioxidant compounds. Considering that approximately 4 % loss of SCY led to such a significant effect, the structurally stabilizing potential of SCY on the EPS matrix appears crucial for terrestrial cyanobacteria survival in arid regions.
Through rapid rinsing of N. flagelliforme samples with acetone, the potential stabilizing role of SCY on the EPS matrix was uncovered in this study. Due to its indispensability, this role cannot readily be uncoupled from its other key roles. Its hydrophobic feature, specific molecular structure, or even its oxidized state [23] might be associated with this novel function. However, a more appropriate experimental system is required to evaluate the functional mechanism of SCY with respect to the stabilizing of EPS matrix. This finding is consistent with our previous report showing that coating of aquatic-living N. flagelliforme colonies (which has lost a natural colony form) with the polymer polyvinylpyrrolidone, to potentially confer stability, endowed cells with desiccation resistance [20]. In contrast to N. flagelliforme, non-colonial cyanobacteria, such as Lyngbya sp. [24], contain a single trichome in their individual filaments. To avoid a potentially lethal crushing effect on the trichome upon acute desiccation, abundant SCY in the EPS matrix may play a more crucial role in maintaining the rigidity of the matrix. In general, multiple roles of secondary metabolites, including SCY, have provided crucial protection for cyanobacteria surviving in complex environments with simultaneous multiple stresses.
References
Tomitani A, Knoll AH, Cavanaugh CM, Ohno T (2006) The evolutionary diversification of cyanobacteria: molecular phylogenetic and paleontological perspectives. Proc Natl Acad Sci U S A 103:5442–5447
Rastogi RP, Sonani RR, Madamwar D (2015) Cyanobacterial sunscreen scytonemin: role in photoprotection and biomedical research. Appl Biochem Biotechnol 176:1551–1563
Llewellyn CA, White DA, Martinez-Vincente V, Tarran G, Smyth TJ (2012) Distribution of mycosporine-like amino acids along a surface water meridional transect of the Atlantic. Microb Ecol 64:320–333
Proteau PJ, Gerwick WH, Garcia-Pichel F, Castenholz R (1993) The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 49:825–829
Ferroni L, Klisch M, Pancaldi S, Häder D-P (2010) Complementary UV-absorption of mycosporine-like amino acids and scytonemin is responsible for the UV-insensitivity of photosynthesis in Nostoc flagelliforme. Mar Drugs 8:106–121
Wright DJ, Smith SC, Joardar V, Scherer S, Jervis J, Warren A, Helm RF, Potts M (2005) UV irradiation and desiccation modulate the three-dimensional extracellular matrix of Nostoc commune (Cyanobacteria). J Biol Chem 280:40271–40281
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implication of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409
Büdel B, Karsten U, Garcia-Pichel F (1997) Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivatives in exposed, rock-inhabiting cyanobacterial linchens. Oecologia 112:165–172
Conde FR, Churio MS, Previtali CM (2000) The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J Photochem Photobiol B 56:139–144
Wada K, Sakamoto T, Matsugo S (2013) Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 3:463–483
Couradeau E, Karaoz U, Lim HC, Nunes da Rocha U, Northen T, Brodie E, Garcia-Pichel F (2016) Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat Commun 7:10373
Leavitt PR, Vinebrooke RD, Donald DB, Smol JP, Schindler DW (1997) Past ultraviolet radiation environments in lakes derived from fossil pigments. Nature 388:457–459
Fleming ED, Castenholz RW (2007) Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ Microbiol 9:1448–1455
Gao KS (1998) Chinese studies on the edible blue-green alga, Nostoc flagelliforme: a review. J Appl Phycol 10:37–49
Liang W, Zhou Y, Wang L, You X, Zhang Y, Cheng CL, Chen W (2012) Ultrastructural, physiological and proteomic analysis of Nostoc flagelliforme in response to dehydration and rehydration. J Proteome 75:5604–5627
Garcia-Pichel F, Sherry ND, Castenholz RW (1992) Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol 56:17–23
Liu YH, Yu L, Ke WT, Gao X, Qiu BS (2010) Photosynthetic recovery of Nostoc flagelliforme (Cyanophyceae) upon rehydration after 2 years and 8 years dry storage. Phycologia 49:429–437
Lan S, Wu L, Zhang D, Hu C (2014) Desiccation provides photosynthetic protection for crust cyanobacteria Microcoleus vaginatus from high temperature. Physiol Plant 152:345–354
Schuurmans RM, van Alphen P, Schuurmans JM, Matthijs HCP, Hellingwerf KJ (2015) Comparison of the photosynthetic yield of cyanobacteria and green algae: different methods give different answers. PLoS ONE 10:e0139061
Gao X, Yang YW, Cui LJ, Zhou DB, Qiu BS (2015) Preparation of desiccation-resistant aquatic-living Nostoc flagelliforme (Cyanophyceae) for potential ecological application. Microb Biotechnol 8:1006–1012
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5:1218–1238
Böhm GA, Pfleiderer W, Böger P, Scherer S (1995) Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J Biol Chem 270:8536–8539
Chen J, Zhao L, Xu J, Yang R, He S, Yan X (2013) Determination of oxidized scytonemin in Nostoc commune Vauch cultured on different conditions by high performance liquid chromatography coupled with triple quadrupole mass spectrometry. J Appl Phycol 25:1001–1007
Rastogi RP, Incharoensakdi A (2014) Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol Ecol 87:244–256
Acknowledgments
This work has been supported in part by the Fundamental Research Funds for the Central Universities (No. CCNU16A02007) and the National Natural Science Foundation of China (No. 31670104). I also thank group members for some help in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X. Scytonemin Plays a Potential Role in Stabilizing the Exopolysaccharidic Matrix in Terrestrial Cyanobacteria. Microb Ecol 73, 255–258 (2017). https://doi.org/10.1007/s00248-016-0851-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0851-4