Abstract
We aimed to evaluate the structure of periphytic algae communities, the trait distribution and the patterns of functional diversity in the last non-dammed stretch of the Upper Paraná River floodplain. We hypothesize that there is an increase in the functional diversity and a change o f the traits and environmental variables structure along this floodplain. We expected an increase in the functional diversity due to the increase in tributaries propagule input leading to a functional divergence, provided by deterministic processes. The sampling was in channels, lakes and rivers in a floodplain area covering 230 km of extension of the main river. The periphyton was obtained from scraping petioles of the floating macrophyte Eichhornia azurea (Sw.) Kunth. The traits evaluated were life form and strength and form of adherence to the substrate. The functional diversity was calculated from a functional dendrogram and assembly rules. The hypotheses proposed in this study were partially accepted, since there was no increase in functional diversity along the sampled area, but we observed a change in the structure of the functional traits along the different stretches of the floodplain. The results showed diatoms as the dominant algae, and functional convergence as the assembly rule prevalent for this community. There was a large difference between local environmental factors along the floodplain. The protected areas housed the greatest functional diversity values, which was apparently influenced by the increase in functional diversity, which can be explained by the limnological factors and the input of propagules from the tributaries to the Paraná River. We noted the importance of the protected areas and local factors for assembly of this community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biotic communities are organized by the combination of abiotic factors, biotic interactions and dispersal processes (Soininen 2012; Padial et al. 2014). To explain those mechanisms in species composition, several models and alternative theories have been proposed (Chase et al. 2005). One of the theory proposed for microorganisms is based on niche models, in which mechanisms are deterministic, with two main processes affecting community composition: limiting similarity—assuming that biotic interactions tend to disfavour the coexistence of species with similar niches, leading to a functional divergence; and environmental filters—which imply that communities are primarily structured by local environmental control (Hillebrand and Blenckner 2002; Pavoine et al. 2011; Soininen 2012). One of the patterns resulting from environmental filtering is convergence, in which coexisting species display ecological strategies more similar than expected by chance (Weiher and Keddy 1995). This point of view recovers the theory of Baas-Becking (1934), which states that for microorganisms “everything is everywhere, but the environments selects”.
Understanding which factors are more relevant in structuring communities (biotic or abiotic), e.g. community assembly rules, can help predict community structure (Weiher and Keddy 1995) and ecosystem functioning (Díaz et al. 2007). However, the roles of geographic distance (restricting through dispersal), and the environment affecting the community structure of microorganisms remain controversial. One of the tools facilitating the quantification of patterns and processes along environmental and spatial gradients is the use of species functional traits and functional diversity (Díaz and Cabido 2001).
Functional diversity plays an important role, since it measures the values and range of species traits capable of influencing ecosystem functioning (Tilman et al. 2001). That is, it measures the magnitude of functional trait dissimilarity among species (Petchey and Gaston 2002). It is directly related to community ecological processes and the maintenance of ecosystem functioning (Balvanera et al. 2006; Petchey and Gaston 2006; Díaz et al. 2007; Sobral and Cianciaruso 2012; Lavorel et al. 2013).
The knowledge of the temporal and spatial dynamics of functional strategies of periphytic algae contributes to predicting, generalizing and understanding processes structuring these communities (Dunck et al. 2013; Cibils et al. 2015; Cibils-Martina et al. 2017). Because they have a sessile life style (for the most part) and short life cycle, periphytic algae respond rapidly to environmental changes and are directly influenced by environmental alterations in temperature, water velocity, light and nutrient availability (Biggs 1996; Stevenson 1996; Bourassa and Cattaneo 2000; Moresco et al. 2009; Ferragut and Bicudo 2010; Lange et al. 2011; Larson and Passy 2012; Algarte et al. 2013; Dunck et al. 2013; Moresco and Rodrigues 2013; Rodrigues et al. 2013; Zanon et al. 2013). They exhibit different biological traits, varying in size, life form (unicellular, filamentous and colonial) and forms of adherence to the substrate (loosely attached and firmly attached). Moreover, these algae are responsible for high rates of aquatic primary productivity (Moschini-Carlos 1999; Rodrigues et al. 2003) and are considered good indicators for the analysis of environmental conditions in highly dynamic aquatic ecosystems, such as river-floodplain systems.
River-floodplain systems are important ecosystems, since they harbour high diversity in aquatic and adjacent terrestrial areas (Agostinho et al. 2005). However, an interruption in this natural gradient can often occur by anthropogenic influences, such as the disposal of chemical waste or reservoir construction (Straškraba et al. 1993; Malmqvist and Rundle 2002), whose effects potentially alter the patterns and processes of abiotic and biotic factors in ecosystems (Ward and Stanford 1983). However, due to lateral, vertical and latitudinal gradients (wetland and tributaries from conservation units) in a floodplain, the river can recover from the effects caused by the damming (e.g. increasing in the water column transparency and nutrients levels, see Roberto et al. 2009), which would tend to decrease with increasing distance from the reservoir (Stanford and Ward 2001). Although the Paraná River is a system with a fluvial dynamic altered by reservoir construction, it exhibits a large non-dammed area, such as the region of the Upper Paraná River floodplain. In environments located in the fluvial plains, the integrity of the system is dependent on the connectivity between the channels and the wetland and this connectivity is represented by the flood pulse (Junk et al. 1989; Tundisi and Matsumura-Tundisi 2008). This area encompasses three large conservation units (the Área de Proteção Ambiental das Ilhas e Várzeas do Rio Paraná, the Parque Nacional de Ilha Grande and the Parque Estadual do Ivinhema) covering around 230 km extension, with numerous secondary channels, lakes and rivers (Baía, Ivinhema, Amambaí and Iguatemi rivers in the right margin, and Paranapanema, Ivaí and Piquiri rivers in the left margin) (Souza-Filho and Stevaux 1997).
In this paper, we aimed to evaluate the structure of periphytic algae communities and trait distribution downstream from a dam in the last non-dammed stretch of the Upper Paraná River floodplain. In addition, considering that the functional diversity is a tool to quantification the changes in environment gradients, we assessed the patterns of the functional diversity of these communities (functional convergence or divergence). Specifically, we hypothesize that there is an increase in functional diversity of periphytic algae along this stretch, as well as a change in the structure of the functional traits and their relations with environmental variables. We expect the occurrence of functional divergence, provided by deterministic processes, a positive relationship between the increase in the functional diversity with distance from the dam, due to the input of propagules from the tributaries.
2 Materials and methods
Study area
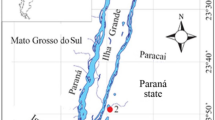
– The Upper Paraná River encompasses the stretch between downstream from Porto Primavera reservoir, situated between Mato Grosso do Sul and São Paulo states, and upstream from Itaipu reservoir, between Mato Grosso do Sul and Paraná states (Fig. 1), and constitutes the last non-dammed stretch of the river in Brazilian territory (Souza-Filho and Stevaux 1997), between the coordinates 22°38′–22°57′S and 53°05′-53°36′O.
Location of the spatial gradient along the corresponding study area to the upper Paraná River floodplain. Sampling points: (1) Garças Lake; (2) Baía River; (3) Xirica Lake; (4) Ivinhema River; (5) Ivinhema River (medium); (6) Ivinhema Lake; (7) Ivinhema River (bass); (8) Amambaí River; (9) Paraná River 1; (10) Paraná River 2; (11) Iguatemi River and (12) Paraná River 3
Its floodplain is 20 km wide, with numerous secondary channels, lakes and rivers. Among them, Baía, Ivinhema, Amambaí and Iguatemi rivers are on the right margin, and Paranapanema, Ivaí and Piquiri rivers on the left margin (Souza-Filho and Stevaux 1997).
Samplings
– Samplings were performed in November 2014, period considered high waters, and the sampling sites were distributed on the right side of the plain, as follows: three are located in the Paraná River main channel (P9, P10 and P12), six located at the mouth of the main tributaries [Baía (P2), Ivinhema Complex (P4, P5 and P7), Amambaí (P8) and Iguatemi rivers (P11)], and three are lentic environments [Garças (P1), Xirica (P3) and Ivinhema lakes (P6)] (Fig. 1). In all sampling sites, the aquatic macrophyte Eichhornia azurea (Sw.) Kunth is the dominant species, which was a substrate to obtain periphytic algal community. We also measured the geographic coordinates of each sampling site.
Limnological variables
– At each sampling site, the following variables were measured in the field: depth (m) (digital sonar—HawkEye); water temperature (°C) (digital oximeter—YSI55A); dissolved oxygen (% saturation and mg L−1) (digital oximeter—YSI55A); pH (digital portable pH meter—Digimed); conductivity (µS/cm) (digital portable conductivity meter—Digimed); transparency (m) (Secchi disc); turbidity (NTU) (digital turbidimeter—LaMotte2020e); alkalinity (µEq L−1) (Carmouze 1994); nitrate (Giné et al. 1980), orthophosphate (Mackereth et al. 1978), total nitrogen and total phosphorus (μg L−1) (Bergamin et al. 1978; Mackereth et al. 1978). To analyse dissolved nutrients and determine suspended materials (Teixeira et al. 1965), samples were filtered under low pressure (< 0.5 atm) using Whatman GF/F filters and kept cool for further laboratory analyses (Supplementary Material, Table S1).
Biological variables
– Samples for the periphytic algal community were obtained of the aquatic macrophyte E. azurea, always in a mature stage. For each sampling site, two petioles were collected in different macrophyte mats and represented a regular sample. The petioles were placed in Wheaton bottles (150 mL) and transported to the laboratory in Styrofoam box containing ice. The periphytic material was removed scraping the surface of the petioles with stainless-steel blade wrapped in aluminium foil and jets of distilled water. A total of 24 samples were analysed.
The removed material designated to the quantitative analyses was preserved in 5% acetic Lugol solution and in 1:1 Transeau solution for qualitative analyses, according to Bicudo and Menezes (2006). The length and diameter of the petioles were measured to calculate the area of periphytic colonization.
The quantification of periphytic algae was performed according to the Utermöhl (1958) method, using an inverted microscope (Olympus® M021) in sedimentation chambers at 400× magnification. To analyse the samples, horizontal and vertical transects were defined, until reaching at least 100 individuals of the predominant taxon and the stabilization of the species accumulation curve (Bicudo 1990).
Qualitative analyses were performed by mounting temporary slides and analysing them in a binocular optical microscope with ocular micrometres at 400× and 1000× magnification (Bicudo and Menezes 2006). For diatom species identification, the material was oxidized according to the technique proposed by Simonsen (1974), modified by Moreira-Filho and Valente-Moreira (1981), and further mounted on permanent slides. Identification was performed to the lowest taxonomic level possible (species), according to classical literature and regional studies, following classification proposed by Round (1965).
Community attributes
– The equation for density calculation followed that proposed by Ros (1979), adapted to the substrate area. Results were expressed per area unit (individuals per square centimetre—ind/cm2). Species richness was obtained through the quantitative analysis.
Selection of periphytic algae functional traits comprised three different aspects of species niche: life form, strength of adherence and form of adherence to the substrate (Biggs et al. 1998; Burliga and Schwarzbold 2013). The functional matrix of species was composed of three algal functional traits distributed in 14 categories: life form (unicellular, filamentous and colonial), strength of adherence (firmly, loosely and mobile), form of adherence (mobile, prostrate, entangled, stalked, mucilaginous pad, mucilage tubes, heterotrichous and differentiated basal cell). These functional traits were analysed directly in each species during sample counting and identification.
The choice of traits was based on studies on periphytic algae approaching functional traits (Ferragut and Bicudo 2010; Passy and Larson 2011; Schneck and Melo 2012; Dunck et al. 2013, 2015a, 2016; Algarte et al. 2014; Lange et al. 2016).
Data analyses
– Values of functional diversity were calculated for the environments through a measure proposed by Faith (1992) (PD), which is similar to the measure of mean pairwise distance (MPD) proposed by Webb (2000). PD values are calculated through the sum of the branch length of a functional dendrogram generated from a matrix of functional traits (Webb 2000). The relationship between functional diversity and geographic distance among environments was tested through a simple linear regression.
We tested the community assembly (functional convergence and divergence) through the analysis described by Pillar et al. (2009). This analysis also evaluates the correlation between communities based on functional traits and ecological gradients and distinguishes the fractions representing the trait-convergence assembly patterns (TCAP), trait-divergence assembly patterns (TDAP) and the interaction of assembly patterns (TCAP * TDAP) through a partial correlation. This method was performed using three matrices: W matrix, containing species abundance; B matrix containing species functional traits; and E matrix containing environmental variables at each site (for more robust data, we used the scores of axis 1 of a principal coordinate analysis (PCoA), representing the environmental gradient). Correlations between matrices were tested through permutations (1000) for measures of trait-convergence and trait-divergence. Null model used to measure TDAP preserves trait-convergence in fuzzy types (fuzzy weighting), community abundance and autocorrelation. It is noteworthy that abundance was log transformed prior to this analysis and environmental matrices were standardized by centering each variable by its mean and scaling each variable by its standard deviation.
To verify the relationship between the functional traits, selected by the prior analysis, and environmental variables, we performed a RLQ analysis (Dolédec et al. 1996). This multivariate technique is based on the ordination of three independent matrices: R matrix, containing environmental variables of each site; L matrix, containing species abundances; and Q matrix, containing species functional traits. The result of this analysis is a linear combination of R and Q matrices, which maximizes covariance between groups of variables, mediated by the L matrix. This analysis was performed in two steps: (a) a PCA was performed for matrix R; and (b) a correspondence analysis (CA) was performed for matrix L. R and L matrices were standardized prior to the analysis, whereas Q matrix was not subject to any transformations. We further evaluated the degree of significance of correlation between matrices through a Monte Carlo test (9999 permutations). Permutations were performed using model 6 (Dray et al. 2014), which is a combined model (models 2 and 4), which allow lower rates of type I error, if compared to the use of only one of the models or using them separately (Braak et al. 2012).
All analyses were performed using software R (R Development Core Team 2015). Packages ade4 (Chessel et al. 2004) and picante (Kembel et al. 2010) were used to construct the functional distance matrix and dendrogram; ade4 was also used to perform RLQ analysis and picante used to calculate functional diversity. Package SYNCSA (Debastiani and Pillar 2012) was used to test for metacommunity assembly patterns. Graphs were constructed using software Statistica, version 7.1 (StatSoft 2005).
3 Results
Periphytic community
– A total of 358 species were registered during the study. The periphytic community present in the tributaries corresponded to 179 species divided in eight classes (Bacillariophyceae, Chlamydophyceae, Chlorophyceae, Chrysophyceae, Cyanophyceae, Oedogoniophyceae, Xanthophyceae and Zygnemaphyceae). Lakes were represented by 159 species divided in nine classes (Bacillariophyceae, Chlorophyceae, Chrysophyceae, Cyanophyceae, Dinophyceae, Euglenophyceae, Oedogoniophyceae, Xanthophyceae and Zygnemaphyceae). Sampling sites of the main channel of the Paraná River showed 139 species divided in nine classes (Bacillariophyceae, Chlorophyceae, Chrysophyceae, Craspedomonadophyceae, Cyanophyceae, Oedogoniophyceae, Rhodophyceae, Xanthophyceae and Zygnemaphyceae). Bacillariophyceae showed the highest richness in all the studied environments, with 174 species. Considering species density, Bacillariophyceae was the most abundant class too and dominant in all environments (Supplementary material, Figs. S1 and S2), except at P12, where it represented around 40% of total density. Among the species found in the sampling sites, only two, belonging to class Bacillariophyceae, were common to all environments: Achnanthidium minutissimum (Kützing) Czarnecki complex and Eunotia incisa W. Smith ex W. Gregory complex.
Functional diversity
– Results of the simple regression did not show an increase in PD values along the area (F1,10 = 3.14, P = 0.107, r2 = 0.163). The evaluation of convergence and divergence traits demonstrated higher ρ values of optimal subset of traits for the trait-convergence patterns, and for the interaction between trait-convergence and trait-divergence, and selected life form (LF) and form of adherence (FA) as the best traits explaining metacommunity assembly of periphytic algae in this study (Table 1).
Relationship between functional traits and environmental variables
– RLQ analysis showed significant correlations between species functional traits and environmental variables determining community composition and species distribution. Patterns of PCoA demonstrated the same tendency of distribution of environments, which were ordinated according to their locality inside the stretch (Fig. 2a). Results of both RLQ models were significant (model 2, P = 0.0027; model 4, P = 0.0001 Monte Carlo test).
RLQ analysis. a Analysis of principal coordinates (PCoA); b correlation between functional traits and environmental variables. Black frames: significant positive correlation; Gray frames: significant negative correlation. CON conductivity, DO: dissolved oxygen, NO3 nitrate, PO4 orthophosphate, TEM temperature, TUR turbidity, Col colonial, Fil filamentous, Uni unicellular, Dcb differentiated basal cell, Ent entangled, Het heterotrichous, Mob mobile, Sta stalked, Mup mucilaginous pad, Pro prostrate, Mut mucilage tubes, (1) Garças Lake, (2) Baía River, (3) Xirica Lake, (4) Ivinhema River, (5) Ivinhema River (medium), (6) Ivinhema Lake, (7) Ivinhema River (bass), (8) Amambaí River, (9) Paraná River 1, (10) Paraná River 2, (11) Iguatemi River, (12) Paraná River 3
RLQ analysis also demonstrated significant correlations between life form and form of adherence (differentiated basal cell, mobile and prostrate) in relation to environmental variables. Turbidity showed negative correlations for colonial species, species with differentiated basal cells and mobile species, and positive correlations for unicellular species. Temperature showed positive correlations for species with differentiated basal cells. Conductivity showed positive correlations for colonial and filamentous species, with differentiated basal cells or that are mobile. On the other hand, conductivity showed negative correlations for unicellular prostrate species. Nitrate showed negative correlations for colonial species and for forms of adherence mobile and differentiated basal cells, whereas unicellular species were positively correlated with this variable (Fig. 2b).
P1 and P3 exhibited the higher percentages of species with colonial life form and differentiated basal cells and mobile forms of adherence (Fig. 3). Sampling sites P2 and P11 showed higher abundances of unicellular prostrate species (Fig. 3). Sites P8 and P12 showed higher abundances of filamentous and entangled species (Fig. 3). There was also an increase in entangled and stalked species with an increase in the distance from the reservoir, while there was a reduction in prostrate species (Fig. 3).
Relative density of the functional traits in the sampling points. Life form (a) and form of adherence (b). Col colonial, Fil filamentous, Uni unicellular, Dcb differentiated basal cell, Ent entangled, Het heterotrichous, Mob mobile, Sta stalked, Mup mucilaginous pad, Pro prostrate, Mut mucilage tubes, (1) Garças Lake, (2) Baía River, (3) Xirica Lake, (4) Ivinhema River, (5) Ivinhema River (medium), (6) Ivinhema Lake, (7) Ivinhema River (bass), (8) Amambaí River, (9) Paraná River 1, (10) Paraná River 2, (11) Iguatemi River, (12) Paraná River 3
4 Discussion
The results supported partially the hypothesis, and there was a relationship between functional traits and environmental variables, such as life forms and adherence traits with turbidity, temperature, conductivity and nitrate. Functional diversity was related to trait-convergence pattern generated by deterministic processes (abiotic filters provided by the large variation among local environmental factors). This indicates that the periphytic algal community respond to changes in environmental variables, emphasizing the importance of local factors for assembly of the periphyton community.
The presence of upstream damming provides a series of changes as the increasing in the water column transparency and nutrients levels, in the fluvial system (Ward and Stanford 1983; Roberto et al. 2009). Both PCoA and RLQ results showed a tendency of regional segregation relative to the distance from the reservoir. These patterns may be attributed for two possible explanations, first to the proximity of the sample sites in each group, which share similar environmental characteristics. Second, by the influence of the tributaries and their flow regimes which, contrary to the Paraná River, do not have their hydrological regime controlled by cascading reservoirs (Agostinho et al. 2008).
Results show a difference in the functional assembly occupied by periphytic algal communities among sampling sites; however, an increase in the functional diversity along the sampled area was not observed. According to Vanormelingen et al. (2008), local environmental factors are fundamental to determine patterns of community distribution with high turnover rates, such as periphytic algae. Additionally, under the effect of environmental filters, species more adapted to a certain habitat are more prone to share similar functional traits, resulting in functional convergence (Petchey et al. 2007).
Functional convergence is usually related to environmental filters, which are assembly rules that represent the set of environmental conditions that restrict the establishment of species with unsuited trait values (Cornwell and Ackerly 2009), and this could reflect the importance of abiotic local forces in the structuring of these communities. The results of assembly rules showed functional convergence of these communities (also known as functional clustering), with co-occurring species with functional traits more similar than expected by chance.
We observed a change among the functional traits in the sampled area. Two functional traits were most important in explaining species distribution along the study area: life form and form of adherence to the substrate. The RLQ analysis showed a correlation between higher percentage of colonial species, species with differentiated basal cells and mobile species and low turbidity, low nitrate concentrations and high values of conductivity in Lakes located in the beginning of the stretch (P1 and P3). Colonial species usually have a mucilage, which controls mobility and colony suspension (Reynolds 2006; Dunck et al. 2013), favouring a competitive advantage for light capture and in environments with low nutrient concentrations (Kruk et al. 2010). Species with differentiated basal cells (Oedogoniophyceae) are excellent competitors for light and space, and are associated with high values of conductivity, intermediate levels of nutrients and low water flow (Biggs 1996; Biggs et al. 1998; Cavati and Fernandes 2008; Bichoff et al. 2016). Mobile forms have greater adaptive advantage in oligotrophic environments, since their movement within the periphytic matrix allows access to different sources of resource (Ferragut and Bicudo 2010).
Sites P8 and P12, where conductivity values were intermediate, showed higher abundances of heterotrichous, filamentous and entangled species. This could be associated with an increase of turbidity and reduction of light penetration in the water column. Entangled species are more subject to disturbances, since they do not possess any structures to adhere to the substrate, remaining more superficially in the periphytic matrix, usually stuck to stalked species (Tuji 2000; Dunck et al. 2013). Filamentous species are favoured by their vertical growth, which contributes to the access to light and nutrients, rapid growth in size and biovolume maintenance (Margalef 1983).
Bacillariophyceae, whose species dominated in all sites, usually have a higher capacity of resisting physical perturbations than other groups and are almost always the first algae colonizing the substrate (Schneck and Melo 2012). They are excellent strategists and have adaptive advantages which allow better adhesion to the substrate. Specialized structures, such as mucilaginous peduncles (short or long), allow species to reach the interface of the periphytic matrix, where there is higher light and nutrient availability (Hoagland et al. 1982; Hudon and Legendre 1987).
Species complex A. minutissimum and E. incisa are the only organisms present in all sampling sites. A. minutissimum is among the most registered diatoms in the world and may occur in acid or alkaline environments, and in oligotrophic to eutrophic environments (Round 2004; Dunck et al. 2015b; Bichoff et al. 2016). Eunotia incisa is common in acid habitats, with great variation in conductivity values (Ortiz-Lerín and Cambra 2007); however, in this study, it was found in oligotrophic to mesotrophic environments.
It was evident the importance of local factors in the fragmentation of the functional traits of periphytic algae. Considering that research has indicated that low values of functional diversity cause a reduction in ecosystem services and function (Díaz and Cabido 2001; Moretti et al. 2013), monitoring biological diversity is a viable manner to assure a long-term maintenance of several ecosystem services (Duffy 2009). Thus, knowing the components of periphytic algae biodiversity is a fundamental step for studies of ecosystem management and conservancy.
References
Agostinho AA, Thomaz SM, Gomes LC (2005) Conservação da biodiversidade em águas continentais do Brasil. Megadiversidade 1:70–78
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68:1119–1132
Algarte VM, Siqueira NS, Rodrigues L (2013) Desiccation and recovery of periphyton biomass and density in a subtropical lentic ecosystem. Acta Sci Biol Sci 35:311–318
Algarte VM, Rodrigues L, Landeiro VL, Siqueira T, Bini LM (2014) Variance partitioning of deconstructed periphyton communities: does the use of biological traits matter? Hydrobiologia 722:279–290
Baas-Becking LGM (1934) Geobiologie of inleiding tot de milieukunde. WP Van Stockum & Zoon, Netherlands
Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156
Bergamin H, Reis BF, Zagatto EAG (1978) A new device for improving sensitivity and stabilization in flow injection analysis. Anal Chim Acta 97:63–70
Bichoff A, Osório NC, Dunck B, Rodrigues L (2016) Periphytic algae in a floodplain lake and river under low water conditions. Biota Neotrop 16:e20160159
Bicudo DC (1990) Considerações sobre metodologias de contagem de algas do perifíton. Acta Limnol Bras 3:459–475
Bicudo CEM, Menezes M (2006) Gêneros de algas de águas continentais do Brasil: chaves para identificação e descrições. Rima, São Carlos
Biggs BJF (1996) Patterns in benthic algae of streams. In: Stevenson RJ, Bothwell ML (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 31–56
Biggs BJF, Stevenson RJ, Lowe RL (1998) A habitat matrix conceptual model for stream periphyton. Arch Hydrobiol 143:21–56
Bourassa N, Cattaneo A (2000) Responses of a lake outlet community to light and nutrient manipulation: effects on periphyton and invertebrate biomass and composition. Freshw Biol 44:629–639
Braak CJF, Cormont A, Dray S (2012) Improved testing of species traits–environment relationships in the fourth-corner problem. Ecology 93:1525–1526
Burliga AL, Schwarzbold A (2013) Perifíton: Diversidade taxonômica e morfológica. In: Schwarzbold A, Burliga AL (eds) Ecologia do Perifíton. Rima, São Carlos, pp 1–6
Carmouze JP (1994) O metabolismo dos ecossistemas aquáticos: fundamentos teóricos, métodos de estudo e análises químicas. Edgard Blücher/Fapesp, São Paulo
Cavati B, Fernandes VO (2008) Algas perifíticas em dois ambientes do baixo rio Doce (lagoa Juparanã e rio Pequeno-Linhares, Estado do Espírito Santo, Brasil): variação espacial e temporal. Acta Sci Biol Sci 30:439–448
Chase JM, Amarasekare P, Cottenie K, Gonzalez A, Holt RD, Holyoak M, Hoopes MF, Leibold MA, Loreau M, Mouquet N, Shurin JB, Tilman D (2005) Competing theories for competitive metacommunities. In: Holyoak M, Leibold MA (eds) Metacommunities: spatial dynamics and ecological communities. The University of Chicago Press, Chicago, pp 335–354
Chessel D, Dufour AB, Thioulouse J (2004) The ade4 package-I—one-table methods. R News 4:5–10
Cibils L, Principe R, Márquez J, Gari N, Albariño R (2015) Functional diversity of algal communities from headwater grassland streams: how does it change following afforestation? Aquat Ecol 49:453–466
Cibils-Martina L, Principe RE, Márquez JA, Gari EN, Albariño RJ (2017) Succession of algal communities in headwaters: a comparison of pine afforested and natural grassland streams. Ecol Res 32:423–434
Cornwell WK, Ackerly DD (2009) Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol Monogr 79:109–126
Debastiani VJ, Pillar VD (2012) SYNCSA-R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics 28:2067–2068
Development Core Team R (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Díaz S, Lavorel S, Chapin FS II, Tecco PA, Gurvich DE, Grigulis K (2007) Functional diversity at the crossroads between ecosystem functioning and environmental filters. In: Canadell JG, Pitelka LF (eds) Terrestrial ecosystems in a changing world. The IGBP Series. Springer, Berlin, pp 103–113
Dolédec S, Chessel D, ter Braak CJF, Champely S (1996) Matching species traits to environmental variables: a new three-table ordination method. Environ Ecol Stat 3:143–166
Dray S, Choler P, Dolédec S, Peres-Neto PR, Thuiller W, Pavoine S (2014) Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95:14–21
Duffy JE (2009) Why biodiversity is important to the functioning of real-world ecosystems. Front Ecol Environ 7:437–444
Dunck B, Bortolini JC, Rodrigues LC, Jati S, Train S, Rodrigues L (2013) Flood pulse drives functional diversity and adaptative strategies of planktonic and periphytic algae in isolated tropical floodplain lake (Brazil). Rev Bras Bot 36:257–266
Dunck B, Lima-Fernandes E, Cássio F, Cunha A, Rodrigues L, Pascoal C (2015a) Responses of primary production, leaf litter decomposition and associated communities to stream eutrophication. Environ Pollut 202:32–40
Dunck B, Rodrigues L, Bicudo DC (2015b) Functional diversity and functional traits of periphytic algae during a short-term successional process in a Neotropical floodplain lake. Braz J Biol 75:587–597
Dunck B, Schneck F, Rodrigues L (2016) Patterns in species and functional dissimilarity: insights from periphytic algae in subtropical floodplain lakes. Hydrobiologia 763:237–247
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10
Ferragut C, Bicudo DC (2010) Periphytic algal community adaptive strategies in N and P enriched experiments in a tropical oligotrophic reservoir. Hydrobiologia 646:295–309
Giné MF, Bergamin FH, Zagatto EAG, Reis BF (1980) Simultaneous determination of nitrate and nitrite by flow injection analysis. Anal Chim Acta 114:191–197
Hillebrand H, Blenckner T (2002) Regional and local impact on species diversity–from pattern to processes. Oecologia 132:479–491
Hoagland KD, Roemer SC, Rosowski JR (1982) Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). Am J Bot 69:188–213
Hudon C, Legendre P (1987) The ecological implications of growth forms in epibenthic diatoms. J Phycol 23:434–441
Inc Statsoft (2005) Statistica (data analysis software system). Version 7:1
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can J Fish Aquat Sci 106:110–127
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kruk C, Huszar VLM, Peeters ETHM, Bonilla S, Costa L, Lurling M, Reynolds CS, Scheffer M (2010) A morphological classification capturing functional variation in phytoplankton. Freshw Biol 55:614–627
Lange K, Liess A, Piggott JJ, Townsend CR, Matthaei CD (2011) Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshw Biol 56:264–278
Lange K, Townsend CR, Matthaei CD (2016) A trait-based framework for stream algal communities. Trends Ecol Evol 6:23–36
Larson CA, Passy SI (2012) Taxonomic and functional composition of the algal benthos exhibits similar successional trends in response to nutrient supply and current velocity. Microb Ecol 80:352–362
Lavorel S, Storkey J, Bardgett RD, De Bello F, Berg MP, Le Roux X, Moretti M, Mulder C, Pakeman RJ, Díaz S, Harrington R (2013) A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. J Veg Sci 24:942–948
Mackereth FYH, Heron J, Talling JF (1978) Water analysis: some revised methods for limnologists. Freshw Biol Assoc 36:1–120
Malmqvist B, Rundle S (2002) Threats to the running water ecosystems of the world. Environ Conserv 29:134–153
Margalef R (1983) Limnologia. Ediciones Omega, Barcelona
Moreira-Filho H, Valente-Moreira IM (1981) Avaliação taxonômica e ecológica das diatomáceas (Bacillariophyceae) epífitas em algas pluricelulares obtidas nos litorais dos estados do Paraná, Santa Catarina e São Paulo. Boletim do Museu Botânico Municipal 47:1–17
Moresco C, Rodrigues L (2013) O perifíton como bioindicador em rios. In: Schwarzbold A, Burliga AL (eds) Ecologia do Perifíton. Rima, São Carlos, pp 147–156
Moresco C, Algarte VM, Rodrigues L (2009) Biomonitoramento utilizando algas perifíticas. In: Lansac-Tôha FA, Benedito E (eds) Contribuições da história da ciência e das teorias ecológicas para a limnologia. EDUEM, Maringá, pp 477–495
Moretti M, De Bello F, Ibanez S, Fontana S, Pezzatti GB, Dziock F, Rixen C, Lavorel S (2013) Linking traits between plants and invertebrate herbivores to track functional effects of land-use changes. J Veg Sci 24:949–962
Moschini-Carlos V (1999) Importância, estrutura e dinâmica da comunidade perifítica nos ecossistemas aquáticos continentais. In: Pompêo MLM (ed) Perspectivas na Limnologia Brasileira. Gráfica e Editora União, São Luis, pp 91–103
Ortiz-Lerín R, Cambra J (2007) Distribution and taxonomic notes of Eunotia Ehrenberg 1837 (Bacillariophyceae) in rivers and streams of Northern Spain. Limnetica 26:415–434
Padial AA, Ceschin F, Declerck SAJ, De Meester L, Bonecker CC, Lansac-Tôha FA, Rodrigues L, Rodrigues LC, Train S, Machado-Velho LF, Bini LM (2014) Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9:e111227
Passy S, Larson CA (2011) Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microb Ecol 62:414–424
Pavoine S, Vela E, Gachet S, Bélair G, Bonsall MB (2011) Linking patterns in phylogeny, traits, abiotic variables and space: a novel approach to linking environmental filtering and plant community assembly. J Ecol 99:165–175
Petchey OL, Gaston KJ (2002) Functional diversity (FD), species richness, and community composition. Ecol Lett 5:402–411
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Lett 9:741–758
Petchey OL, Evans KL, Fishburn IS, Gaston KJ (2007) Low functional diversity and no redundancy in British avian assemblages. J Anim Ecol 76:977–985
Pillar VD, Duarte LDS, Sosinski EE Jr, Joner F (2009) Discriminating trait-convergence and trait-divergence assembly patterns in ecological community gradients. J Veg Sci 20:334–348
Reynolds CS (2006) Ecology of phytoplankton. Cambridge University Press, Cambridge
Roberto MC, Santana NF, Thomaz NF (2009) Limnology in the Upper Parana River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Braz J Biol 69:717–725
Rodrigues L, Bicudo DC, Moschini-Carlos V (2003) O papel do perifíton em áreas alagáveis e nos diagnósticos ambientais. In: Thomaz SM (eds) Ecologia e manejo de macrófitas aquáticas. EDUEM, Maringá, pp 211–229
Rodrigues L, Algarte VM, Siqueira NS, Machado EMN (2013) Fatores envolvidos na distribuição e abundância do perifíton e principais padrões encontrados em ambientes da planície de inundação. In: Schwarzbold A, Burliga AL (eds) Ecologia do Perifíton. Rima, São Carlos, pp 131–145
Ros J (1979) Práticas de ecologia. Omega, Barcelona
Round FE (1965) The biology of the algae. Edward Arnold, London
Round FE (2004) pH scaling and diatom distribution. Diatom 20:9–12
Schneck F, Melo AS (2012) Hydrological disturbance overrides the effect of substratum roughness on the resistance and resilience of stream benthic algae. Freshw Biol 57:1678–1688
Simonsen R (1974) The diatom plankton of the Indian Ocean Expedition of R/V—Meteor, Meteor Forschungsergbnisse. Reihe D-Biol 19:1–66
Sobral FL, Cianciaruso MV (2012) Phylogenetic and functional assembly structure: (re)assembling the community ecology on different spatial scales. Biosci J 4:617–631
Soininen J (2012) Macroecology of unicellular organisms—patterns and processes. Environ Microbiol Rep 4:10–22
Souza-Filho EE, Stevaux JC (1997) Geologia e geomorfologia do complexo rio Baía, Curutuba, Ivinhema. In: Vazzoler AEAM, Agostinho AA (eds) A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. EDUEM, Maringá, pp 3–46
Stanford JA, Ward JV (2001) Revisiting the serial discontinuity concept. Regul Rivers Res Manag 17:303–310
Stevenson RJ (1996) An introduction to algae ecology in freshwater benthic habitats. In: Stevenson RJ, Bothwell ML (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 3–30
Straškraba M, Tundisi JG, Duncan A (1993) State-of-the-art of reservoir limnology and water quality management. In: Straškraba M, Tundisi JG (eds) Comparative reservoir limnology and water quality management. Kluwer Academic, Dordrecht, pp 213–288
Teixeira C, Tundisi JG, Kutner MB (1965) Plankton studies in a mangrove II: the standing-stock and some ecological factors. Bol Inst Oceanogr 24:23–41
Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C (2001) Diversity and productivity in a long-term grassland experiment. Science 294:843–845
Tuji A (2000) Observation of developmental processes in loosely attached diatom (Bacillariophyceae) communities. Phycol Res 48:75–84
Tundisi JG, Matsumura-Tundisi T (2008) Limnologia. Oficina de Textos, São Paulo, p 632
Utermöhl H (1958) ZurVervollkommnung der quantitativen phytoplankton methodic. Mitt Int Ver Theor Angew Limnol 9:1–39
Vanormelingen P, Cottenie K, Michels E, Muylaert K, Vyverman W, De Meester L (2008) The relative importance of dispersal and local processes in structuring phytoplankton communities in a set of highly interconnected ponds. Freshw Biol 53:2170–2183
Ward JV, Stanford JA (1983) The serial discontinuity concept of lotic ecosystems. In: Fontaine TD (eds) Dynamics of lotic ecosystems. Ann Arbor Science, Michigan, pp 29–42
Webb CO (2000) Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am Nat 156:145–155
Weiher E, Keddy P (1995) The assembly of experimental wetland plant communities. Oikos 73:323–335
Zanon JE, Simões NR, Rodrigues L (2013) Effects of recurrent disturbances on the periphyton community downstream of a dammed watercourse. Braz J Biol 73:253–258
Acknowledgements
This study was funded by the Long-Term Ecological Research program (PELD-CNPq) and Ilha Grande project. We would like to thank the COORDINATION FOR THE IMPROVEMENT OF HIGHER EDUCATION PERSONNEL (CAPES) for providing master scholarships to Andressa Bichoff and Daiane Trevisan Ruwer, and post-doctoral scholarships to Bárbara Dunck; the BRAZILIAN NATIONAL COUNCIL OF TECHNOLOGICAL AND SCIENTIFIC DEVELOPMENT (CNPq) for providing a Masters scholarship to Nicolli Cristina Osório and research productivity scholarships to Liliana Rodrigues. We also thank Dr. Hugo Message and M.Sc. Oscar Peláez Zapata for the support in statistical analyses; Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia) for technical and logistic support; and Nupélia’s Laboratory of Limnology for all limnological analyses.
Author information
Authors and Affiliations
Contributions
Bichoff, A. was responsible for the analysis of biological material and formulation of goals and hypothesis. Bichoff, A. Osório, N.C., and Ruwer, D.T. contributed with the article conceptualization, including ideas, and all the writing structure of the manuscript. Bichoff, A., and Dunck, B. applied the statistics, and Dunck, B reviewed the article. Rodrigues, L. supervised, and was responsible for the research activity planning and execution.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bichoff, A., Osório, N.C., Ruwer, D.T. et al. Trait structure and functional diversity of periphytic algae in a floodplain conservation area. Braz. J. Bot 41, 601–610 (2018). https://doi.org/10.1007/s40415-018-0467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-018-0467-7