Abstract
Bread wheat quality is mainly correlated with protein quality, particularly the glutenin content and high molecular weight glutenin subunits (HMW-GS) of grain endosperm. The number of HMW-GS alleles and loci are limited in bread wheat cultivars, though ideally, a large amount of HMW-GS alleles in wheat-related grasses should be exploited. In this study, a novel wheat-Dasypyrum villosum GP005 translocation line, NAU425, carrying a pair of T1VS·6BL translocation chromosomes was developed and assessed via molecular cytogenetic analysis. Grain quality analysis indicated that NAU425 has a positive effect on protein content, Zeleny sedimentation value, wet gluten content, and the rheological characteristics of wheat flour dough compared to the same qualities of recurrent parent ‘Chinese Spring’ attributed to the additional HMW-GS donated by 1VS of D. villosum. The protein content of T1VS·6BL was significantly improved compared to ‘Chinese Spring’ likely owing to the dramatic increase in glutenin content. Considering the importance of glutenin content for bread wheat end-use quality, and T1VS·6BL line with good plant vigor, full fertility and cytogenetic stability, NAU425 may be valuable in bread wheat quality improvement. Our results presented here may provide an approach to improve bread wheat quality through additional alien HMW-GS introgression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In wheat and its relatives, seed storage proteins are mainly comprised of glutenins and gliadins. Gliadins are monomeric proteins that affect the extensibility of gluten dough which can be divided into four structural types: α-, β-, ω-, and γ-gliadins (Shewry and Halford 2002). The glutenin fraction contains high molecular weight glutenin subunits (HMW-GSs) and low molecular weight glutenin subunits (LMW-GSs) which are linked by inter-chain disulfide bonds to form polymers. HMW-GS and LMW-GS vary from 6 to 10% and from 60 to 80%, respectively, of the total glutenins. Their concentration and composition are relevant factors in determining the resultant cooking proprieties of wheat dough (Branlard and Dardevet 1985). The HMW-GSs play an especially pivotal role in determining the viscoelastic properties and bread-making qualities of dough (Shewry et al. 1992). They are located at the Glu-1 loci on the long arms of bread wheat chromosomes 1A, 1B, and 1D (Lawrence and Shepherd 1980; Payne 1987). Although the HMW-GSs are very important determinants of processing quality, the number of subunits associated with high quality remains limited (Garg et al. 2009a). Screening the positive effects of HMW-GSs in wheat-related species is necessary to secure new methods for improving bread-making quality. Previous studies have shown that the introgression of alien genes, even from tertiary gene pool species, can increase genetic diversity and affect the grain quality of cultivated wheat through addition or substitution lines, but not translocation lines (Liu et al. 2007; Jiang et al. 2010; Li et al. 2013; Garg et al. 2009b).
Dasypyrum villosum GP005 (2n = 2x = 14, VV) (formerly known as Haynaldia villosa GP005 ‘Schur’), a wild relative of wheat, has been identified as an important gene resource for wheat improvement. It provides the resistance to several serious diseases, as well as tolerance to drought and frost, tillability, high grain protein content, and a functional reservoir of genetic diversity for seed storage proteins (Gradzielewska 2006). Chromosome 1V has loci for storage proteins similar to the wheat HMW glutenins (Glu-V1, homoeologous to wheat Glu-1), ω- and γ-gliadins (Gli-V1, homoeologous to wheat Gli-1), and LMW glutenins (Glu-V3, homoeologous to wheat Glu-3) (Zhang et al. 2014; Zhao et al. 2010), so the polymorphism loci of D. villosum can provide useful alleles to improve wheat processing qualities. To date, storage protein genes of D. villosum have been successfully transferred into common wheat via compensating Robertsonian translocations (Zhang et al. 2014; Zhao et al. 2010). The homoeologous group 1 chromosomes of the HMW subunit of glutenin, a major protein of wheat endosperm, also has a profound influence on wheat dough baking quality (Zhang et al. 2014). In effect, there are many HMW-GSs on the chromosomes 1A, 1B, and 1D, which have detrimental effect if any chromosomes or chromosomal segments in group 1 are missing (Yang et al. 2014). The creation of wheat-alien compensating translocation lines is related to simple substitutions of different alleles, which limits the extent to which wheat quality can be improved. Developing T. aestivum Chinese Spring–D. villosum 1V translocation lines with assembled quality alleles of seed storage proteins may be more effective than compensating translocation lines as far as successful wheat breeding.
In the present study, we used cytogenetic methods, molecular markers, and measured quality indicators to characterize the T. aestivum–D. villosum translocation line T1VS·6BL, and then evaluated its effect on wheat grain quality. This new breeding material may prove valuable for improving the quality of bread wheat. Further, this study may represent an approach to quality improvement within the Triticeae tribe.
Materials and methods
Plant materials – To obtain wheat-D. villosum translocations, the pollen of T. durum–D. villosum amphiploid (AABBVV) was irradiated by 60Co-γ ray and pollinated to the ‘Chinese Spring’ (CS) genetic background (Bie et al. 2007). The M1 plants were then backcrossed successively to CS three to four times and progenies were identified by genomic in situ hybridization (GISH). D. villosum accession (DP005) was originally introduced from the Cambridge Botanical Garden, UK. The common wheat cultivar CS and CS-D. villosum disomic 1V addition line were maintained at the Cytogenetics Institute, Nanjing Agricultural University (CINAU), China. The CS nulli-tetrasomic (NT) lines N6AT6D, N6BT6D, and N6DT6A that we used in this study were kindly provided by Dr. Bikram S. Gill.
Cytogenetic analysis – Genomic in situ hybridization (GISH) and fluorescence in situ hybridization (FISH) were conducted according to a previous study (Zhang et al. 2004). The total genomic DNA of D. villosum labeled with fluorescein-12-dUTP was used to detect segments of chromosome 6V. Clone pSc119.2, a 120 bp tandem-repeat sequence from rye inserted in the plasmid pBR322 (Mukai et al. 1993) and labeled with Digoxigenin-11-dUTP, was used to identify wheat B-genome chromosomes. The repetitive sequence clones 45S rDNA (Zhang et al. 2014), labeled with biotin-16-dUTP, were used as a FISH probe for further identification. Both FISH processes were conducted by counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). After hybridization with the probes, the chromosomes were observed under an Olympus BX60 fluorescence microscope (Olympus, Japan) and photographed with a SPOT cooled color digital camera.
DNA isolation and PCR amplification – Genomic DNA was isolated from young leaves according to instructions accompanying the DNA Secure Plant Kit (Tiangen Biotech Co., Ltd., Beijing). STS marker Xcinau15 −902 (Cao et al. 2006) was adopted to analyze D. villosum, ‘Chinese Spring’ and T. aestivum–D. villosum alien chromosome lines to identify the short arms of 6A, 6B, and 6D. 1VS-specific molecular marker CINAU27 (Wang et al. 2007) was used to check the lines with the 1VS translocated chromosome. The PCR amplification procedure we used was first described by Zhang et al. (2014).
Protein extraction from grain and SDS-PAGE – Protein was extracted from the grain following the procedure described by Garg et al. (2009b), making use of one half-seed endosperm (with the other half as an embryo possessing germination for genetic analysis). A 10-μl sample was loaded onto an SDS-PAGE gel formed by a 10% gradient separating gel (pH 8.5) and a 4% stacking gel (pH 6.8). Separation was carried out at a constant current of 6 mA for 14–16 h. The gel was stained in 0.1% w/v Coomassie Brilliant Blue R250 and 12.5% trichloroacetic acid for 10 h then rinsed with distilled water.
Agronomic traits and grain quality – All genetic stocks were grown in the greenhouse at Jiangpu Experimental Station, Nanjing Agricultural University, during the 2014–2015 growing season in a randomized plot design. Each plot (with three replications) had five 1.2-m rows with 0.25-m spaces between rows and 0.1-m spaces between plants within the rows. Before planting, 150 kg carbamide and 375 kg compound fertilizer per hectare were applied as base manure. Other field management was conducted in accordance with local practices.
Plant height (PH, cm), spike length (SL, cm), spikelets per spike (SS), grains per spike (GS), and 1000-kernel weight (TKW, g) data were collected at maturity from a 1-m section in a central row of each plot. Grain samples were harvested and cleaned prior to conditioning and milling. Grain protein content was calculated as 5.7× the nitrogen concentration determined via micro-Kjeldahl method (Zhao et al. 2010) and the separation and extraction of protein compositions was performed according to He (1985). Zeleny sedimentation tests were run according to the AACC method 56-61A (AACC 1995). The dough was washed and remaining gluten was collected and weighed to determine wet gluten yield; then, mixograph analysis (10 g) was evaluated according to AACC method 54-40A (AACC 1995) based on a 10-g sample with a National Manufacturing Mixograph instrument. Analysis of variance and multiple comparisons among original agronomic traits data and resulting grain quality were performed in SPSS 16.0.
Results
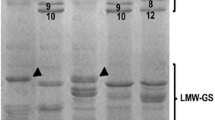
Wheat- D. villosum T1VS·6BL translocation line – The backcross progenies derived from the pollen irradiation treatment of T. durum ZY1286–D. villosum amphiploids were detected by GISH and 1VS-specific molecular markers. One translocation line with the 1VS-specific molecular marker bands, designated NAU425 was further identified by molecular cytogenetic analysis. GISH results at the mitotic metaphase indicated that a pair of CS chromosome arms were translocated by D. villosum and the chromosome number of NAU425 was 2n = 42 (Fig. 1). Twenty-one bivalents, including a pair of translocated chromosomes, had formed in PMC during meiosis I (MI) indicating that the translocation line NAU425 was homozygous (Fig. 2). Marker analysis showed that the diagnostic band of 1VS-specific marker CINAU27 was present in line NAU425 (Fig. 5a), indicating that this translocation chromosome could be T1VS·W. According to the dual-color FISH result with DNA clone psc119.2 and the total genomic DNA of D. villosum as probes, the translocated chromosome was verified to be composed of D. villosum 1VS and wheat 6BL (Mukai et al. 1993) (Fig. 3). The repetitive sequence clone 45S rDNA also generated strong hybridization signals on the translocated chromosome of 1VS (Fig. 4). All this leads up to NAU425 was a homozygous translocation line, T1VS·6BL. Marker analysis showed that the diagnostic band of 6BS-specific marker CINAU15 was absent in line NAU425 (Fig. 5b), which confirmed the cytogenetic identification results. SDS-PAGE analysis of the HMW-GS revealed that translocation line NAU425 and CS-D. villosum 1V disomic addition line DA1V all had one extra HMW-GS of D. villosum, which was a fast-moving HMW-GS and below the 1Bx7 of CS (Fig. 6) and further confirmed that the alien chromosome arm in NAU425 was 1VS.
GISH and FISH analysis of wheat-Dasypyrum villosum translocation line NAU425. 1 GISH patterns on RTC of NAU425 (2n = 42). Total genomic DNA of D. villosum was labeled with fluorescein-12-dUTP, visualized with green fluorescence; wheat chromosomes were counterstained with propidium iodide (PI) and fluoresced red. 2 Meiotic MI chromosomes of translocation line (2n = 21II); homoeologous pairing occurred between wheat and alien chromosome shown by the formation of bivalent ring marked with an arrow. 3 FISH patterns of wheat-D. villosum recombinant chromosomes. Repetitive sequence clone pSc119.2 was labeled with digoxigenin-11-dUTP and visualized with red fluorescence, the total genomic DNA of D. villosum was labeled with biotin-16-dUTP and visualized with green fluorescence; wheat chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and fluoresced blue. 4 FISH using the repetitive sequence clones, 45S rDNA labeled with biotin-16-dUTP and detected by yellow fluorescence. (Color figure online)
Agronomic traits and grain quality of NAU425 – Agronomic comparisons between T1VS·6BL and CS are presented in Table 1. There were no significant differences among main traits, including plant height (PH, cm), spike length (SL, cm), spikelet per spike (SS), grains per spike (GS), 1000-kernel weight (TKW, g), and heading data, suggesting that the T1VS·6BL translocated chromosome may had no significant effect on the main yield characteristics. However, the grain quality values revealed that except peak height (PH), other indexes Zeleny sedimentation value (ZSV), wet gluten content (WGC), peak time (PT), peak width (PW), and area under midline peak (AMP) of the translocation line NAU425 were all considerably higher than those of its recurrent parent CS (Table 1), suggesting that the translocation chromosome significantly increased the grain quality. The content analysis of total protein and its components revealed that total protein concentration, globulin, gliadin, glutenin, and albumin contents of the translocation line T1VS·6BL all significantly increased over those of CS (Fig. 7). Interestingly, the globulin content of NAU425 (4.46%) increased more than twice than that of CS (2.05%), which may be the key factor that enhanced NAU425 grain quality.
Discussion
Common wheat speciation has been domesticated by polyploidy containing A, B, and D genomes. There are three mechanisms known to affect the fate of orthologous genes in polyploids: (1) The majority of homoeologous genes are co-expressed, (2) some duplicate genes are lost, mutate or diverge, and (3) epigenetic changes may reprogram gene expression and developmental patterns of new allopolyploids (Z. Jeffrey Chen 2007). In other words, the additive gene will be rapidly deleted, if its expression has a disadvantageous effect on the organism. Conversely, the additive gene will be conserved, if its expression has a beneficial effect (Li et al. 2008). In common wheat, HMW-GSs are encoded by complex Glu-1 loci that are located on the long arms of homoeologous group 1 chromosomes, respectively, called Glu-1A, Glu-1B, and Glu-1D. Each Glu-1 locus encodes the x- and the y-types of HMW-GSs conserved from their diploid ancestor species (Shewry et al. 2003). The unique bread-making property of common wheat especially lies in its proteins and glutenin; and the HMW-GSs have been found to have a large effect on bread-making quality (Shewry et al. 2003). Therefore, the multiple loci and co-expression of HMW-GSs we observed (Fig. 6) suggest that wheat end-use quality may be enhanced through the efforts to elevate the dosage of HMW-GS genes.
In the present study, in addition to the Glu-1A, Glu-1B, and Glu-1D loci, the developed T1VS·6BL translocation line NAU425 carried an additional HMW-GS local Glu-V1 of D. villosum. Glu-V1 can co-express with Glu-1A, Glu-1B, and Glu-1D loci in the CS background. The size of the special HMW-GS subunit encoded in this local lies between 14x and 15y subunits (Fig. 6). This novel subunit introduced into common wheat may contribute to enhance protein content and quality. Mixograph studies of NAU425 showed significant increase in the peak time and peak bandwidth compared to CS. The increase in peak time indicates the positive effect of the seed storage proteins derived from D. villosum on protein quality. This was further supported by increases in Zeleny sedimentation value and wet gluten content which directly indicated higher protein quality in NAU425 than in recipient CS. Protein analysis showed that T1VS·6BL translocation line NAU425 was significantly increased in total protein and four compositions, glutenin, gliadin, globulin, and albumin concentrations compared to CS. By contrast, the glutenin content of NAU425 (4.46%) dramatically increased to more than twice that of CS (2.05%), which may be the key factor in enhancing NAU425 grain quality. In addition to Glu-V1, the low molecular weight subunit local Glu-V3 of D. villosum located on chromosome arm 1VS likely contributed to the glutenin content.
The principal series of genes which encode storage proteins that determine bread-making quality in hexaploid wheat are mainly located on the chromosomes of homoeologous groups 1 and 6. Glu-1, Glu-3, and Gli-1 loci were located on the chromosomes of group 1, while Gli-2 encodes α-gliadins, most of the β-gliadins, and some of the γ-gliadins were located on group 6 short arms (McIntosh et al. 2008). Based on their effect on grain quality, the homoeologous group chromosomes were stratified as follows: 1D > 1B > 1A and 6A > 6B = 6D. The relationship of chromosome dosage with quality was especially linear for the chromosomes of 1A, 1B, 1D, and 6A, but not for 6B or 6D (Rogers et al. 1990). We presumed that the substitution of chromosome arm 1VS for 6BS may be better for grain quality than for 1BS, 1DS, or 1AS. Similar studies have revealed that the seed protein content of compensation translocation lines T1VS·1BL (Zhang et al. 2014) and T1VS·1DL (Zhao et al. 2010) is not significantly different than those of recurrent parent CS, though we found that the T1VS·6BL line had considerably more total protein and glutenin than CS. Therefore, non-compensation translocations should be considered to transform alien HMW-GS into common wheat. Unfortunately, the agronomy characteristics of most non-compensation translocations are not suitable for wheat breeding programmes due to the unbalanced genetic systems. The main agronomic traits of NAU425 (T1VS·6BL), however, were not significantly different from the recipient CS (Table 1). This may be due to the fact that the genetic absence of 6BS was compensated by 6AS or 6DS, as common wheat is an allohexaploid species. Also, chromosome arm 1VS of D. villosum may have partially compensated for 6BS, similar to the NOR region and Gli loci. However, the poor fertility of T1VS-6BS·6BL developed previously (Zhang et al. 2014) was observed although it also carried the alien HMW-GS and NOR region of D. villosum. The agronomic differences between T1VS·6BL and T1VS-6BS·6BL lines indicated that other chromosome(s) in the T1VS-6BS·6BL line may have been damaged by the 60Co-γ ray toward the material DA1V. As a result, this T1VS·6BL translocation line NAU425 may provide a useful resource for future studies on the quality and effects on wheat flour processing among different wheat genetic backgrounds. By creating various combinations of HMW-GSs, we may be able to effectively discern the structure and function of the Glu-V1 of D. villosum.
References
AACC (1995) Approved methods of the American association of cereal chemists, 9th edn. AACC, St. Paul
Bie TD, Cao YP, Chen PD (2007) Mass production of intergeneric chromosomal translocations through pollen irradiation of Triticum durum–Haynaldia villosa amphiploid. J Integr Plant Biol 49:1619–1626. doi:10.1111/j.1774-7909.2007.00578.x
Branlard G, Dardevet M (1985) Diversity of grain protein and bread wheat quality. II. Correlation between high molecular weight subunits of glutenin and flour quality characteristics. J Cereal Sci 3:345–354
Cao AZ, Wang XE, Chen YP, Zou XW, Chen PD (2006) A sequence-specific PCR marker linked with Pm21 distinguish chromosome 6AS, 6BS and 6DS of Triticum aestivum and 6VS of Haynaldia villosa. Plant Breed 125:201–205
Garg M, Tanaka H, Tsujimoto H (2009a) Exploration of Triticeae seed storage proteins for improvement of wheat end product quality. Breed Sci 59:519–528
Garg M, Tanaka H, Ishikawa N, Takata K, Yanaka M, Tsujimoto H (2009b) Agropyron elongatum HMW-glutenins have a potential to improve wheat end-product quality through targeted chromosome introgression. J Cereal Sci 50:358–363. doi:10.1016/j.jcs.2009.06.012
Gradzielewska A (2006) The genus Dasypyrum—part 2. Dasypyrum villosum—a wild species used in wheat improvement. Euphytica 152:441–454. doi:10.1007/s10681-006-9245-x
He ZF (1985) Grain quality of cereals, oils and its analysis technique. China Agricultural Press, Beijing, pp 290–294
Jeffrey Chen Z (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58:377–406. doi:10.1146/annurev.arplant.58.032806.103835
Jiang QT, Wei YM, Lu ZX, Liu T, Wang JR, Pu ZE, Lan XJ, Zheng YL (2010) Characterization of a novel variant HMW-glutenin gene from Elymus canadensis. Genes Genomics 32:361–367. doi:10.1007/s13258-010-0028-3
Lawrence GJ, Shepherd KW (1980) Variation in gluten in protein subunits of wheat. Aust J Biol Sci 33:221–233
Li WL, Huang L, Gill BS (2008) Recurrent deletions of puroindoline genes at the grain hardness locus in four independent lineages of polyploid wheat. Plant Physiol 146:200–212. doi:10.1104/pp.107.108852
Li GR, Liu C, Li CH, Zhao JM, Zhou L, Dai G, Yang EN, Yang ZJ (2013) Introgression of a novel Thinopyrum intermedium St-chromosome-specific HMW-GS gene into wheat. Mol Breed 31:843–853. doi:10.1007/s11032-013-9838-8
Liu SW, Zhao SY, Chen FG, Xia GM (2007) Generation of novel high quality HMW-GS genes in two introgression lines of Triticum aestivum/Agropyron elongatum. BMC Evol Biol 7:1471–2148. doi:10.1186/1471-2148-7-76
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris CF, Somers DJ (2008) Catalogue of gene symbols for wheat: Proceedings of the 11th International Wheat Genetic Symp. Sydney University of Sydney Press, Australia
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol 38:141–153
Rogers WJ, Rickatson JM, Sayers EJ, Law CN (1990) Dosage effects of chromosomes of homoeologous groups 1 and 6 upon bread-making quality in hexaploid wheat. Theor Appl Genet 80:281–287
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958
Shewry PR, Halford NG, Tatham AS (1992) The high molecular weight subunits of wheat glutenin. J Cereal Sci 15:105–120
Shewry PR, Halford NG, Tatham AS, Popineau Y, Lafiandra D, Belton PS (2003) The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv Food Nutr Res 45:219–302
Wang CM, Feng YG, Zhuang LF, Cao YP, Qi ZJ, Bie TD, Cao AZ, Chen PD (2007) Screening of chromosome-specific markers for chromosome 1R of Secale cereale, 1V of Haynaldia villosa and 1Rk# 1 of Roegneria kamoji. Acta Agron Sin 33:1741–1747
Yang YS, Li SM, Zhang KP, Dong ZY, Li YW, An XL, Chen J, Chen QF, Jiao Z, Liu X, Qin HJ, Wang DW (2014) Efficient isolation of ion beam-induced mutants for homoeologous loci in common wheat and comparison of the contributions of Glu-1 loci to gluten functionality. Theor Appl Genet 127:359–372. doi:10.1007/s00122-013-2224-4
Zhang P, Li WL, Friebe B, Gill BS (2004) Simultaneous painting of three genomes in hexaploid wheat by BAC-FISH. Genome 47:979–987. doi:10.1139/G04-042
Zhang RQ, Zhang MY, Wang XE, Chen PD (2014) Introduction of chromosome segment carrying the seed storage protein genes from chromosome 1V of Dasypyrum villosum showed positive effect on bread-making quality of common wheat. Theor Appl Genet 127:523–533. doi:10.1007/s00122-013-2244-0
Zhao WC, Qi LL, Gao X, Zhang GS, Dong J, Chen QJ, Friebe B, Gill BS (2010) Development and characterization of two new Triticum aestivum–Dasypyrum villosum Robertsonian translocation lines T1DS·1V#3L and T1Dl·1V#3S and their effect on grain quality. Euphytica 175:343–350. doi:10.1007/s10681-010-0177-0
Acknowledgements
We would like to thank Dr. Zhongwei Tian of the College of Agronomy, Nanjing Agricultural University, China, Nanjing, for quality evaluation. This study was sponsored by the Special Fund for Independent Innovation of Agricultural Science and Technology in Jiangsu, China (No. CX(14)5082) and the National Natural Science Foundation of China (30871519, 31471497, 31101142).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mingxing Wen and Yigao Feng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wen, M., Feng, Y., Chen, J. et al. Characterization of a Triticum aestivum–Dasypyrum villosum T1VS·6BL translocation line and its effect on wheat quality. Braz. J. Bot 40, 371–377 (2017). https://doi.org/10.1007/s40415-016-0352-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0352-1