Abstract
Soil salinity and Zn deficiency are among the major limiting factors for growth and improvement of pistachio trees in central Iran. Using plant growth promoting rhizobacteria (PGPR) is new strategy to reduce the destructive effects of salinity and improvement of nutrient availability. This study investigated the individual and the interactive effects of the PGPR and Zn treatments on some physiological and biochemical parameters, antioxidant enzymes activities and alleviation of salinity stress in pistachio seedlings. The treatments include isolates of fluorescent pseudomonads as PGPR [control (non-inoculated), pf1, pf2 and pf3], Zn (0 and 5 mg kg−1 soil) and salinity (0, 1000 and 2000 mg NaCl kg−1 soil). The results indicated that salinity increased the proline, soluble sugars and H2O2 concentrations in the seedling leaves and roots. Inoculation with PGPR efficiently enhanced the concentrations of proline (35 and 16 %) and the soluble sugars (25 and 22 %), whereas, reduced the H2O2 levels (16 and 18 %) in the leaves and roots. Increasing the concentration of NaCl to 2000 mg kg−1 significantly decreased the dry weight, Zn concentration, and chlorophyll and carotenoids contents. At the same salinity level, the PGPR and Zn alone increased these parameters compared to the untreated soil, but furthest by the combined PGPR and Zn treatments. Also, the combined application of PGPR and Zn significantly increased the antioxidant enzyme activities and protein concentration relative to their sole usage in the pistachio seedling leaves and roots, especially at the higher salinity levels. The isolates containing ACC-deaminase activity were more efficient to alleviate salt stress and develop seedling improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the important ecological and agronomical concerns in the arid regions. It reduces the growth and productivity of the agricultural crops through its influence on plant metabolism such as osmotic effects, nutrient uptake, photosynthesis, enzyme activity and hormonal imbalance (Munns 2002).
Plants accumulate various organic compounds in the cytoplasm known as compatible solutes, such as amino acids, sugars, inorganic ion and organic acids to increase their tolerance against water loss under salinity. Proline is an amino acid, which plays a key role in preserving the subcellular structures, macromolecules and scavenging free radicals. Proline may also buffer cellular redox potential under environmental stresses (Porcel et al. 2012). It is also reported that the chlorophyll and carotenoids concentration of the leaves decreased under saline conditions (Parida and Das 2005).
Reactive oxygen species (ROS) are normal cell metabolism by-products of plants, but the abiotic stresses disturb the balance between the production and elimination of ROS in plants cellular components (Karuppanapandian et al. 2011). Superoxide radicals (O2·−), hydrogen peroxide (H2O2), hydroxyl radicals (OH·) and singlet oxygen (1O2) are the most common ROS in the plant (Lopez-Huertas and Del Río 2014). The ROS are responsible for different types of stress-induced damage to the macromolecules, including the photosynthetic pigments, protein, lipids and ultimately the cellular structure (Gill and Tuteja 2010). Several plant antioxidant enzymes like peroxidase (POX), superoxide dismutase (SOD), polyphenol oxidase (PPO) and catalase (CAT) are responsible for maintaining the plants under unfavorable conditions, which scavenge the ROS. SOD is the first-line defense against the toxic effects of O2·− by catalyzing the disproportionation of the O2·− radicals into H2O2 and O2 (Halliwell and Gutteridge 2007). Also, H2O2 decomposes into water and oxygen by CAT or POX. PPO oxidizes the phenolic compounds when the plant tissue is damaged (Silva and Koblitz 2010). There are significant correlations between the activity of antioxidant enzymes and the salt tolerance of plants (Ding et al. 2010). Habibi et al. (2014) reported that the activity of antioxidant enzymes and the H2O2 level significantly increased in the leaves of pistachio under salinity stress.
Zinc is one of the essential micronutrients whose deficiency in plants grown on calcareous and salt-affected soils with high pH values is observed. Plant species differ in their sensitivity to Zn deficiency. The critical deficiency levels of Zn in the leaves of higher plants are approximately 15–20 mg kg−1 dry weights (Marschner 1995). Zinc deficiency causes significant damage to the ultrastructure of the chloroplasts because of its interaction with the phospholipids and sulfhydryl groups of the membrane proteins (Dang et al. 2010). Zinc is one of the main components of many enzymes such as hydrogenase and Cu/Zn SOD, a structural stabilizer for the proteins and needed for their structural integrity (Marschner 1995; Aravind and Prasad 2004). Zinc deficiency reduces net photosynthesis by 50–70 % depending on the deficiency intensity and the plant species (Alloway 2008). It facilitates the detoxification of O2·− to O2 and H2O2 via the enhancement of the ascorbic acid concentration. The role of Zn in the improvement of pistachio seedlings under salt stress has been reported by Tavallali et al. (2009). Also, Shahriaripour et al. (2010) showed that application of 5 mg Zn kg−1 soil significantly enhanced the pistachio seedlings growth under saline conditions. In general, they reported that higher levels of Zn had no significant effect on the studied parameters.
The use of salt-tolerant beneficial microbes associated with plant roots can improve fertility of salt-affected soils and enhance the plants’ resistance to environmental stresses (Egamberdieva and Kucharova 2009). The fluorescent pseudomonads rhizobacteria, one of the most effective strains of pseudomonas, are suitable to be used as plant growth promoting agents due to production of various secondary metabolites and stimulation of plant growth in saline soils (Mishra et al. 2010). This rhizobacteria can actively colonize plant roots and improve seed germination, root and shoot growth, nutrient uptake and plant stress tolerance by different mechanisms. Production of plant growth hormones, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, siderophore, indole acetic acid (IAA), increasing the availability of immobile nutrients such as P, Fe and Zn and controlling the plant pathogens are some of the mechanisms involved (Abbas-Zadeh et al. 2010). The ability of pseudomonas strains to survive and colonize plant roots in the arid and saline soils have been reported (Paul and Nair 2008). ACC-deaminase plays a significant role in the regulation of a plant hormone and ethylene. It regulates the ethylene production by metabolizing ACC into α-ketobutyrate and ammonia. Plant growth promoting rhizobacteria (PGPR) with ACC-deaminase activity can contribute to the growth and improvement of plants under conditions of stress by reducing stress-induced ethylene production (Saleem et al. 2007). Also, Saravanakumar and Samiyappan (2007) concluded that Pseudomonas fluorescens strain with ACC-deaminase activity increased plant growth parameters and the salt stress resistance of groundnut seedling as compared to plants inoculated with strains of pseudomonas bacteria lacking ACC-deaminase activity. In another study, Stefan et al. (2013) reported that the PGPR strains drastically enhanced the activity of SOD and POX enzymes. Furthermore, Han and Lee (2005) showed that salt stress drastically increased the activity of enzyme in the leaves of lettuce inoculated by PGPR.
In Iran, pistachio is an important crop, and crop yields suffer from high salinity. The reduction of pistachio growth in saline condition has been reported by various workers (Shahriaripour et al. 2010; Habibi et al. 2014). Besides soil salinity, Zn deficiency is one of the major limiting factors for the growth and yield of the pistachio trees in many regions of Iran, especially in the Rafsanjan area because of the high CaCO3 concentration in the soil, high soil pH value and soil salinity as well as irrigation water (Mozaffari and Malakouti 2006). Zinc may be present in soil as unavailable form for plant use. Thus, the use of PGPR could be a more effective perspective for improvement of soil Zn availability by production of various metabolites, particularly in saline conditions. However, it appears that indigenous PGPR can help to establish and increase the tolerance of these trees to salt stress. Little is known about the interactive effects of PGPR and Zn on the pistachio physiology and antioxidant enzyme activity under salinity stress. Thus, the aim of this study was to investigate the effect of the PGPR and Zn on the biochemical and physiological changes and antioxidant enzyme activity of pistachio seedlings under salinity conditions.
Materials and methods
Isolation and determination of plant growth promoting (PGP) activities of rhizobacteria
Eighty soil samples with various salinity levels (EC = 2–18 dS m−1) were collected from the rhizosphere of the pistachio trees in the Rafsanjan (30°24′24″N and 55°59′38″E) located in Iran. Based on radiation under fluorescence in the UV light, 32 fluorescent pseudomonads isolates were selected and purified in King B medium and then some of their PGP activities including the production of auxins, siderophore, HCN, ACC-deaminase activity and phosphorous (Ca3 (PO4)2) and zinc (ZnCO3) solubilization were studied by the standard methods as described by Abbas-Zadeh et al. (2010). Three isolates were selected for the greenhouse experiment based on their PGP activities (ACC-deaminase activity, solubilization of insoluble Zn compounds and production of IAA as well as siderophore) (Table 1).
Pot experiment
A greenhouse experiment was set up as a factorial experiment based on a completely randomized design with three replications. The soil sample with electrical conductivity (EC) of 1.1 dS m−1 was collected from the pistachio orchards of Rafsanjan area and air-dried and crushed to pass through a 4-mm sieve. Selected physical and chemical properties of the soil used in this experiment are shown in Table 2. The Zn treatments were mixed uniformly with the soil at the concentration of 0 and 5 mg kg−1 soil as zinc sulfate (ZnSO4·7H2O). Also, nitrogen at the concentration of 50 mg kg−1 soil, phosphorous at the concentration of 20 mg kg−1 soil, iron at the concentration of 10 mg kg−1 soil and manganese at the concentration of 5 mg kg−1 soil were added uniformly to all the pots as urea, KH2PO4, Fe-EDTA and MnSO4·H2O, respectively. At the first, eight pistachio (P. vera L. cv. Badami) germinated seeds were placed in each pot containing 5 kg soil. For inoculation, pistachio seeds were treated with bacterial suspensions (1 mL for each seed with CFU = 108 cell mL−1) [control (non-inoculated), pf1, pf2 and pf3]. Four weeks after planting, the number of pistachio seedlings was reduced to 5 and then salt treatments (0, 1000 and 2000 mg NaCl kg−1 soil) were applied to pots using irrigation water. Plants were irrigated with distilled water to maintain the soil at field capacity for a period of 168 days. Twenty weeks after the salt treatments, the pistachio seedlings were collected and measurements were recorded. Also, the content of selected soil properties in the seedlings rhizosphere soils was determined (Sparks 1996).
Determination of Zn concentration and leaf relative water content (RWC)
Zn concentrations in the seedlings were measured by atomic absorption spectrophotometer (GBC Avanta ver.1.33, GBC Scientific, Dandenong, Australia) following acid digestion (0.5 g dried and ground plant material was digested with 5 mL of 2 N HCl) (Chapman and Pratt 1961). Also, the RWC was determined by incubating leaf samples in the deionized water and calculated from the following equation (Gonzalez and Gonzalez-Vilar 2003):
Determination of chlorophyll and carotenoids contents
For chlorophyll and carotenoids determination, the leaf sample (0.25 g) was ground in 80 % acetone using a pestle and mortar. After measurement of absorbance, the concentration of chlorophyll (Porra 2002) and carotenoids (Lichtenthaler and Wellburn 1983) were calculated as follows:
where OD470, OD646.6 and OD663.6 represent absorbance values measured at 470, 646.6 and 663.6 nm wavelengths, respectively.
Also, the chlorophyll fluorescence index (F v/F m) was measured using the chlorophyll fluorescence meter (Hansatech LTD Pocket PEA, UK).
Determination of the concentration of proline, soluble sugars and hydrogen peroxide (H2O2)
Free proline concentration in the leaf and root was determined following the method of Paquin and Lechasseur (1979). Leaf and root samples (0.5 g) were homogenized in 5 mL of 95 % ethanol using a mortar and pestle. After centrifugation for 10 min at 3500 rpm, about 1 mL of extract was taken in a test tube and to it deionized water (10 mL), glacial acetic acid (5 mL) and ninhydrin reagent (5 mL) were added. The reaction mixture was boiled in a water bath (100 °C) for 45 min. After cooling, 10 mL of benzene was added to the reaction mixture. After thorough mixing, the absorbance was read at 515 nm by a spectrophotometer against benzene blank. The proline concentration was measured by referring to the standard curve of proline.
For determination of the soluble sugars, 0.1 mL of the extract (extracted by 95 % ethanol) was mixed with 3 mL anthrone and was boiled in a water bath (100 °C) for 10 min. The absorbance was read at 625 nm by a spectrophotometer and the concentration of the soluble sugars was estimated by referring to the standard curve of glucose (Irigoyen et al. 1992).
For determination of H2O2, the leaf and root samples (0.25 g) were homogenized with 0.1 % Trichloroacetic acid (5 mL) in an ice bath and the homogenate was centrifuged for 15 min at 12,000 rpm. The supernatant (0.5 mL) was then added to 0.5 mL of the 10 mM potassium phosphate buffer (pH 7) and 1 mL of 1 M KI. After incubation for 1 h under dark conditions, the absorbance was read at 390 nm (Velikova et al. 2000).
Assay of the antioxidant enzymes activities and protein concentration in the leaves and roots
For determination of the antioxidant enzymes activities, the leaf and root samples (0.5 g) were homogenized in ice-cold 1 mM phosphate buffer containing 1 mM EDTA and 2 g L−1 PVPP with pre-chilled pestle and mortar. The homogenate was centrifuged at 4 °C in a refrigerated centrifuge for 20 min at 12,000 rpm and the supernatants were used as enzyme extract. All operations were performed at 4 °C.
For the determination of peroxidase (POX; EC 1.11.1.7) activity, 0.1 mL of enzyme extract was added to 1.9 mL of the phosphate buffer (60 mM, pH 6.1) and 0.5 mL of guaiacol (28 mM) and the increasing absorbance measured at 470 nm every 30 s for 120 s after adding 0.5 mL of H2O2 (5 mM). The activity of POX was described as U/mg protein min, where U = ∆A 470 (Pandolfini et al. 1992).
To determine the polyphenol oxidase (PPO; EC 1.10.3.1) activity, the substrate was composed of 2.3 mL of phosphate buffer (50 mM; pH 7.0) and 0.6 mL of pyrocatechol solution (100 mM). The reaction was started by adding 0.1 mL of the enzyme extract and the increase in absorbance measured at 425 nm after 3 min of reaction and compared with a control cell in which the enzyme extract was substituted by water. One unit of PPO activity was defined as the amount of enzyme required to induce an increase of 0.001 in the absorption for each minute of reaction time. The PPO activity was expressed as U/mg protein min, where U = ∆A 425 (Silva and Koblitz 2010).
To determine the catalase (CAT; EC 1.11.1.6) activity, the reaction mixture used contained 2 mL phosphate buffer (50 mM, pH 7.0), 0.5 mL H2O2 (40 mM) and 0.5 mL enzyme extract. The H2O2 decomposition was measured by the reduction in absorbance at 240 nm at 25◦C. The activity of CAT was described as U/mg protein min, where U = ∆A 240 (Cakmak and Marschner 1992).
To determine the activity of superoxide dismutase (SOD; EC 1.15.1.1), the reaction mixture contained 1.5 M sodium carbonate (0.1 mL), 200 mM methionine (0.2 mL), 2.25 mM nitro-blue tetrazolium (NBT) (0.1 mL), 3 mM EDTA (0.1 mL), 100 mM potassium phosphate buffer (1.5 mL), deionized water (1 mL) and enzyme extract (0.05 mL). Two tubes without enzyme extract were used as control. The reaction was initiated by the addition of 0.1 mL riboflavin (60 mM) and keeping the tubes under two 15 W florescent lamps. The reaction was stopped after 15 min by switching off the lights. The tubes were covered with a black cloth and absorbance was recorded at 560 nm. Tubes without the enzyme developed the maximum color. Identical solutions maintained in the dark served as blanks. One unit of SOD activity was defined as the amount of enzyme necessary to inhibit the NBT reduction by 50 % at 560 nm. The activity of SOD was described as U/mg protein, where U = ∆A 560 (Dhindsa et al. 1981). The protein concentration in the leaf and root was determined using bovine serum albumin as the standard. The absorbance was read at 595 nm (Bradford 1976).
Results
Properties of rhizosphere soils
The application of PGPR, Zn and NaCl increased the soluble Zn concentration, but reduced pH value in comparison with the control (Table 3). The highest soluble Zn concentration was related to Zn application. The application of 1000 and 2000 mg NaCl kg−1 soil enhanced the concentration of soluble Zn by 18 and 81 % as compared to the control. Also, the inoculation by pf1, pf2 and pf3 isolates increased soluble Zn concentration by 41, 25 and 20 % in comparison with the control, respectively. The application of 1000 and 2000 mg NaCl kg−1 soil decreased pH value to 7.49 and 7.42. Generally, inoculation by pf1, pf2 and pf3 isolates tended to decrease pH value to 7.61, 7.65 and 7.51. Also, the application of PGPR, Zn and NaCl enhanced EC contents as compared to the control. The application of 1000 and 2000 mg NaCl kg−1 soil increased EC to 7.32 and 12.0 dS m−1, respectively. The inoculation by PGPR and the application of NaCl increased Na, Mg, Ca, SO −24 , HCO -3 , Cl and SAR concentrations relative to the control (Table 3).
Shoot and root dry weight
The analysis of variance revealed that the PGPR, salinity, Zn and PGPR × salinity × Zn interaction showed a significant effect on the shoot and root dry weight of the pistachio seedlings (p ≤ 0.05).
The results revealed that salt stress significantly reduced the shoot and root dry weight of the pistachio seedlings (Table 4). The enhancement of salt stress to 1000 and 2000 mg NaCl kg−1 soil, reduced shoot dry weight by 7 and 29 % and root dry weight by 24 and 54 % as compared to the control. The application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly enhanced the shoot dry weight by 38, 19 and 18 %, and root dry weight by 11, 16 and 44 % in comparison with the control at the same salinity levels, respectively. Also, the inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil increased the dry weight of shoot by 12, 16 and 15 %, and dry weight of root by 5, 12 and 27 % in comparison with the control at the same salinity levels, respectively. However, the combined application of PGPR and Zn in the enhancement of shoot and root dry weight was more effective than that of their sole usage (Table 4).
Shoot and root Zn concentration
The concentration of Zn in the pistachio seedlings shoot and root was significantly (p ≤ 0.05) affected by the PGPR inoculation, NaCl levels, Zn application and the interaction of PGPR × salinity × Zn.
The results indicated that the concentration of Zn in the shoot was decreased with increase in the NaCl levels (Table 4). With application of 2000 mg NaCl kg−1 soil, the Zn concentration of shoot decreased by 44 %, while the Zn concentration of root in the highest NaCl rates (2000 mg NaCl kg−1 soil) increased by 21 % as compared to the control. The application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly increased the Zn concentration by 29, 10 and 25 % in the shoot, and 34, 41 and 23 % in the root in comparison with the control at the same salinity levels, respectively. Furthermore, the inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil increased the Zn concentration by 34, 38 and 48 % in the shoot, and 12, 13 and 10 % in the root compared to the non-inoculated control at the same salinity levels, respectively. Also, in the non-saline (0 mg NaCl kg−1 soil), low-saline (1000 mg NaCl kg−1 soil) and high-saline (2000 mg NaCl kg−1 soil) conditions, the simultaneous application of the PGPR and Zn significantly enhanced the shoot Zn concentration by 56, 49 and 89 % in comparison with the control, respectively. At these salinity levels, the root Zn concentration in the combined treatment with PGPR and Zn significantly increased by 46, 54 and 48 % compared to the control (Table 4).
Leaf relative water content (RWC)
The variance analysis showed that the PGPR, salinity, Zn and interaction of salinity × Zn showed a significant effect on the leaf RWC content of the pistachio seedlings (p ≤ 0.05).
The leaf RWC of the seedlings inoculated by pf1, pf2 and pf3 isolates recorded increase of 8, 4 and 6 % compared to the control, respectively (Fig. 1a). The leaf RWC of the pistachio seedlings significantly decreased by 5 and 11 % at the 1000 and 2000 mg NaCl kg−1 soil as compared to the control, respectively. Treatment with Zn significantly increased this parameter at all the salinity levels (Fig. 1b).
The main effect of PGPR (a) and the interactive effect of salinity × Zn (b) on RWC of the pistachio seedling leaves 24 weeks after sowing. pf fluorescent pseudomonads isolates. The error bars in the graphs are standard errors. Within each graph, values followed by the letter are not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
Proline, soluble sugars and the H2O2 concentrations in leaf and root
Proline, soluble sugars and the H2O2 concentrations in the pistachio seedlings leaf were significantly influenced by the PGPR, salinity, Zn and PGPR × salinity × Zn interaction. Also, the root proline concentration was affected by the PGPR, salinity, Zn and PGPR × salinity interaction, while the root soluble sugars and the H2O2 concentrations were significantly influenced by the PGPR, salinity, Zn and salinity × Zn interaction.
The results showed that the proline concentration of leaves showed about a twofold and fivefold increase at the 1000 and 2000 mg NaCl kg−1 soil salinity levels relative to the control (Table 5). Also, the inoculation of the pistachio seedlings by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil enhanced this parameter by 26, 69 and 14 % compared to the non-inoculated control at the same salinity levels, respectively. Furthermore, the application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly increased the concentration of proline in the leaves by 56, 97 and 24 % as compared to the control. However, the highest value of the leaf proline concentration was observed after the simultaneous application of PGPR, salinity and Zn (53.9 μmol g−1 FW).
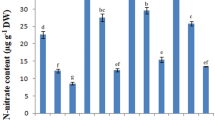
The results illustrated that application of Zn significantly increased the root proline concentration by 15 % compared to the control (Fig. 2a). Also, application of 1000 and 2000 mg NaCl kg−1 soil significantly increased this parameter by 18 and 31 % relative to the control. Furthermore, in the inoculated seedlings by pf1, pf2 and pf3 isolates, the root proline concentration increased by 21, 15 and 11 % compared to the control, respectively. Generally, the root proline concentration was higher in the inoculated seedlings under high (2000 mg NaCl kg−1 soil) salinity levels (Fig. 2b).
The main effect of Zn (a) and the interactive effect of PGPR × salinity (b) on the root proline concentration, and the main effect of PGPR (c) and interactive effect of salinity × Zn (d) on the root soluble sugars concentration, and the main effect of PGPR (e) and the interactive effect of salinity × Zn (f) on the root H2O2 concentration of the pistachio seedlings 24 weeks after sowing. pf fluorescent pseudomonads isolates. The error bars in the graphs are standard errors. Within each graph, values followed by the same letter are not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
Increasing the levels of salinity enhanced the concentration of leaf soluble sugars. The application of PGPR and Zn increased this parameter at all the salinity levels (Table 5). The application of 1000 and 2000 mg NaCl kg−1 soil drastically increased this parameter by 1.5-fold and 2.2-fold compared to the control, respectively. Also, the inoculation of the pistachio seedlings by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly increased this parameter by 25, 38 and 12 % in comparison with the non-inoculated control at the same salinity levels. Furthermore, the application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil increased the concentration of leaf soluble sugars by 34, 27 and 10 % as compared to the control, respectively. However, the combined application of PGPR and Zn in the enhancement of this parameter was more effective than that of their sole usage (Table 5).
The results indicated that inoculation by PGPR isolates significantly increased root soluble sugars (20-31 %) compared to the control (Fig. 2c). Also, the application of 2000 mg NaCl kg−1 soil significantly enhanced the root soluble sugars concentration by 29 % compared to the control. However, the application of Zn enhanced this parameter at all the salinity levels (Fig. 2d).
The H2O2 concentration significantly increased in the pistachio seedling leaves under salinity stress (Table 5). In comparison with the control, the leaf H2O2 concentration increased by 14 and 70 % at the 1000 and 2000 mg NaCl kg−1 soil, respectively. The application of the PGPR and Zn significantly decreased this parameter under both normal and salinity-stressed conditions. The inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil reduced the leaf H2O2 concentration by 14, 7 and 12 % compared to the non-inoculated control at the same salinity levels. Also, the application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly reduced the concentration of leaf H2O2 by 12, 11 and 17 % in comparison with the control, respectively. Compared with the single application of PGPR or Zn, their combined application significantly decreased the leaf H2O2 concentration at all the salinity levels. The highest concentration of the leaf H2O2 (40.1 μmol g−1 FW) was observed in the use of 2000 mg NaCl kg−1 soil.
The concentration of root H2O2 in the inoculated seedlings by pf1, pf2 and pf3 isolates reduced by 14, 10 and 17 % compared to the control, respectively (Fig. 2e). However, the salinity significantly enhanced the root H2O2 concentration (20–68 %). On the other hand, the Zn treatment significantly reduced this parameter (15 %) only at the high NaCl level (Fig. 2f).
Chlorophyll and carotenoids concentrations in leaves
The chlorophyll a and carotenoids concentrations were affected by PGPR, salinity, Zn and the salinity × Zn interaction, the chlorophyll b concentration was affected by the PGPR × salinity and salinity × Zn interactions. Also, only the chlorophyll a/b and F v/F m ratios were affected by PGPR inoculation, salinity levels and Zn application.
The concentration of chlorophyll a, chlorophyll b and carotenoids in the pistachio seedling leaves were significantly reduced with the rising salinity levels (Fig. 3b, d, f). Also, the pistachio seedlings treated with NaCl, PGPR and Zn contained significantly greater amounts of these parameters than those exposed to the salinity condition alone.
The main effect of PGPR (a) and salinity × Zn interaction (b) on the chlorophyll a (Chl. a) concentration, the interactive effect of PGPR × salinity (c) and salinity × Zn (d) on the chlorophyll b (Chl. b), and the main effect of PGPR (e) and salinity × Zn interaction (f) on the carotenoids (Car.) concentration of the pistachio seedling leaves 24 weeks after sowing. pf: fluorescent pseudomonads isolates. The error bars in the graphs are standard errors. Within each graph, values having a common letter are not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
The inoculation by pf1, pf2 and pf3 isolates significantly increased chlorophyll a concentration by 19, 14 and 19 % (Fig. 3a), and carotenoids concentration by 37, 27 and 35 %, respectively, in comparison with the control (Fig. 3e). Furthermore, supplementation with the Zn significantly increased the chlorophyll a and carotenoids concentrations at all the NaCl levels (Fig. 3b, f). The highest concentration of the carotenoids of the leaves was observed in the simultaneous use of 1000 mg NaCl kg−1 soil and 5 mg Zn kg−1 soil (Fig. 3f). In comparison with the NaCl-stressed seedlings, chlorophyll b concentration showed a significant increase under PGPR treatment. The effect of the pf1 isolate in increasing of chlorophyll b concentration was greater than that of the other isolates (Fig. 3c). Also, the application of Zn significantly increased chlorophyll b concentration at all the salinity levels. The highest chlorophyll b concentration was observed for seedlings grown in the Zn-treated soils and 0 mg NaCl kg−1 soil (Fig. 3d). The chlorophyll a/b ratio increased with a rise in the NaCl levels (Fig. 4b). The treatment with Zn significantly reduced the ratio of chlorophyll a/b (Fig. 4a). As the salinity levels increased, the F v/F m ratio reduced (Fig. 4b). When the salt stress increased to 2000 mg NaCl kg−1 soil, a 15 % reduction in the F v/F m ratio was observed. However, the application of Zn significantly enhanced this ratio by 8.5 % in comparison with the control (Fig. 4a).
The main effect of Zn (a) and salinity (b) on the F v/F m and chlorophyll a/b (Chl. a/b) ratios of the pistachio seedling leaves 24 weeks after sowing. pf fluorescent pseudomonads isolates. The error bars in the graphs are standard errors. Within each graph, values followed by the same letter are not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
Antioxidant enzymes activity
The PGPR × salinity × Zn interaction had significant effects on the SOD, PPO, POX and CAT activities in the pistachio seedling leaves and roots.
An analysis of the SOD, PPO, POX and CAT activities indicated that with the increasing salinity levels, their activities were also significantly increased in the pistachio seedling leaves and roots compared to the control. Also, the inoculation with PGPR and supplementation with the Zn enhanced these antioxidant enzyme activities in the both stressed and non-stressed seedlings.
When the salt stress increased to 2000 mg NaCl kg−1 soil, the SOD activity in the leaf and root of pistachio seedlings increased by 2.9-fold and 3.5-fold, respectively. Furthermore, the application of Zn at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly increased the SOD activity by 54, 68 and 41 % in the leaf, and 44, 63 and 38 % in the root in comparison with the control at the same salinity levels, respectively. Also, the inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil increased this enzyme activity by 28, 29 and 18 % in the leaf, and 30, 28 and 31 % in the root in comparison with the non-inoculated control at the same salinity levels, respectively (Table 6).
The application of the 1000 and 2000 mg NaCl kg−1 soil significantly increased PPO activity by 1.3-fold and 2.2-fold in the leaf, and 1.4-fold and 2.4-fold in the root in comparison with the control. Also, the application of the Zn significantly increased leaf and root PPO activity under saline conditions. Moreover, the inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil increased this enzyme activity by 21, 17 and 62 % in the leaf, and 20, 17 and 72 % in the root in comparison with the non-inoculated control at the same salinity levels, respectively. The PPO activity was at the highest with the application of the PGPR and Zn at 2000 mg NaCl kg−1 soil which was 15.2 U mg−1 protein min and 19.0 U mg−1 protein min in the leaf and root (Table 6).
According to the results, application of the 1000 and 2000 mg NaCl kg−1 soil significantly increased POX activity by 1.9-fold and 4.8-fold in the leaf, and 2.1-fold and 5.8-fold in the root in comparison with the control. Furthermore, the Zn application at the 0, 1000 and 2000 mg NaCl kg−1 soil significantly increased the POX activity by 25, 10 and 11 % in the leaf, and 36, 16 and 19 % in the root in comparison with the control at the same salinity levels, respectively. Also, the inoculation by PGPR at the 0, 1000 and 2000 mg NaCl kg−1 soil increased this enzyme activity by 66, 41 and 15 % in the leaf, and 74, 36 and 14 % in the root in comparison with the non-inoculated control at the same salinity levels, respectively. However, the highest value of the leaf and root POX activity was observed after the simultaneous application of PGPR, salinity (2000 mg NaCl kg−1 soil) and Zn (Table 6).
The application of the 1000 and 2000 mg NaCl kg−1 soil significantly increased the CAT activity by 1.6-fold and 2.4-fold in the leaf, and 1.9-fold and 2.8-fold in the root compared to the control. Also, the inoculation by PGPR and supplementation with the Zn significantly increased the leaf and root CAT activity at all the salinity levels. The activity of CAT in the leaf and root was at the highest with the simultaneous application of the PGPR and Zn at 2000 mg NaCl kg−1 soil which was 4.56 U mg−1 protein min and 5.95 mg−1 protein min, respectively (Table 6).
Leaf and root protein concentration
The leaf and root protein concentration was significantly influenced by the PGPR, salinity, Zn and interaction of salinity × Zn. Also the interaction of PGPR × salinity had only significant effect on the root protein concentration. Compared with the control, inoculation by pf1, pf2 and pf3 isolates significantly increased the leaf protein concentration by 20, 8 and 13 %, respectively (Fig. 5a). Also, the salinity at the 2000 mg NaCl kg−1 soil significantly decreased the protein concentration when compared to the other salinity levels (0 and 1000 mg NaCl kg−1 soil). However, the application of Zn significantly increased the leaf protein concentration at all the salinity levels. The highest leaf protein concentration was recorded for plants grown in the Zn-treated soils under salinity level of 1000 mg NaCl kg−1 (Fig. 5b). The results illustrated that supplementation with the Zn significantly increased the root protein concentration by 17 % compared to the control (Fig. 5c). Also, the inoculation by PGPR significantly increased the root protein concentration at all the NaCl levels. However, the efficiency of pf1 isolate in increasing of root protein concentration was more than that of the other isolates. The results revealed that application of 1000 mg NaCl kg−1 soil increased the root protein concentration (4–12 %) compared to the control. But, this parameter was decreased at the high level of NaCl (Fig. 5d).
The main effect of PGPR (a) and salinity × Zn interaction (b) on the leaf protein concentration, and the main effect of Zn (c) and the interactive effect of PGPR × salinity (d) on the root protein concentration of the pistachio seedling 24 weeks after sowing.pf fluorescent pseudomonads isolates. The error bars in the graphs are standard errors. Within each graph, values followed by the same letter are not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
Discussion
Besides soil salinity, the availability of micronutrients such as Zn in the soils of pistachio orchard of the Rafsanjan area is very low due to the high CaCO3 concentration in the soil, high soil pH value and soil salinity as well as irrigation water. Soil salinity and nutrients deficiency cause various physiological and biochemical changes in the plants such as reduction in the growth and yields, damage to cellular structure, accumulation of compatible solutes, phytotoxicity of ions including Na+ and Cl− and nutritional imbalances due to depression in uptake and/or shoot transport. This study investigated the effect of the indigenous PGPR and Zn on some growth, physiological and biochemical parameters as well as activity of the antioxidant enzymes in the pistachio seedlings growing at the different NaCl levels.
The analysis of rhizosphere soil revealed that total dissolved Zn concentration increased with application of NaCl, Zn and PGPR, but the value of pH reduced. Enhancement in the concentration of total dissolved Zn under salinity condition could be attributed to the replacement of exchangeable Zn with Na+ as well as lower pH value. Also, soil salinity may enhance the mobility of Zn in soils by the formation of soluble complexes of Zn chloride such as ZnCl+ and ZnCl2° (Khoshgoftar et al. 2004). Furthermore, soil microbe can decrease rhizosphere pH value by proton and organic acids production. Soil pH plays a key role in solubility of Zn. A negative correlation has been reported between soil pH and Zn extractability (Lindsay 1978).
Inhibition of plant growth is a common response to salt stress. According to the results, salinity significantly decreased the dry weight of pistachio seedlings, while treatment with PGPR and Zn increased this parameter. The increase in the seedlings dry weight was maximal after the combined treatment with PGPR and Zn. The reduction of growth parameters under salinity may be related to increment of osmotic stress, nutrients deficiency and destruction of some biochemical and physiological mechanisms (Alqarawi et al. 2014). A decrease in the shoot and root dry weight of pistachio seedlings due to salinity stress have been observed by Shahriaripour et al. (2010). The growth promoting ability of inoculated seedlings with PGPR may be related to their ability to produce the siderophore, the IAA, solubilization of Zn and P as well as ACC-deaminase activity as observed under in vitro conditions. PGPR with the ACC-deaminase activity can play an essential role in ameliorating abiotic stress conditions (Saleem et al. 2007). The improvement of crops growth inoculated with PGPR has been reported by Egamberdieva (2012).
Our results illustrated that leaf RWC significantly was affected by salinity levels, while the inoculation by PGPR and supplementation with the Zn increased this parameter. The RWC of leaf is one of the important plant water status indices that help in the evaluation of plants tolerance to environmental stress. Mayak et al. (2004) stated that PGPR could facilitate the rooting and growth of salt-stressed plants by improving the water use efficiency. The increments of RWC by PGPR have been reported for strawberry (Karlding et al. 2013) grown under salt stress.
NaCl stress caused significant decrease in the Zn concentration of pistachio seedlings. Decreasing Zn concentration in tissues under salinity condition could be attributed to high Na concentration and reduced availability of water to plants caused by excess soluble salts (Tavallali et al. 2009). Furthermore, the inoculation with PGPR increased Zn concentration in the seedling tissues. The accumulation of Zn in the PGPR-inoculated seedlings was due to the increased root activity and also the solubilization of Zn compounds present in the soil. Soil microbes can influence the nutrients availability such as Zn by production of organic acids, proton and humic substances (Hernandez et al. 2004). With regard to the ability of isolates in the solubilization of insoluble ZnCO3 in vitro condition, increasing the concentration of Zn in the seedlings may be related to it. Sharma et al. (2014) reported an increase in Zn concentration of rice genotypes in response to PGPR treatments. Also, the application of Zn improved the Zn concentration at all the NaCl levels. Our findings are also according to Tavallali et al. (2009) and Shahriaripour et al. (2010).
In this study, the proline as well as soluble sugars concentrations increased with increase in the NaCl level in the both PGPR-inoculated and non-inoculated seedlings. However, their concentrations were maximal after the combined treatment with PGPR and Zn. Hence, increased salt tolerance in the PGPR- and Zn-treated seedlings may be related to the higher concentrations of proline and soluble sugars. The accumulation of compatible solutes under saline condition could be a more effective procedure, which help in maintaining plants cell water status. Several PGPR strains, such as Bacillus and Arthrobacter maintain the cell water status by increasing the synthesis of proline in the stressed plants (Sziderics et al. 2007). The aggregation of proline under salt stress conditions may be related to a reduction in the level of oxidative transformation of proline to glutamate, reduction in the use of proline in protein synthesis and an increase in the proline enzymatic biosynthesis (Claussen 2005). The improvement of proline concentration in the PGPR-inoculated seedlings could be attributed to upregulation of proline biosynthesis pathway to keep it in high levels (Yoshiba et al. 1997). Enhancing the concentration of the soluble sugars with the Zn application indicates the useful effects of Zn on the enzymes which are involved in the carbohydrate metabolism.
Chlorophyll concentration in the stressed plants is a key index to predict plant’s health and photosynthesis capacity to NaCl stress. According to the results, the concentrations of chlorophyll a, chlorophyll b, carotenoids and the F v/F m ratio decreased with increase in the NaCl levels. However, the simultaneous treatment with the PGPR and Zn significantly enhanced these parameters under different salinity levels. Reduction in the chlorophyll concentration under salinity stress might also be due to the impaired biosynthesis or accelerated pigment degradation. Enhancement of the chlorophyll concentration in the Zn-treated seedlings could be a result of the Zn playing a role in the activation of the enzymes involved in the chlorophyll synthesis. The Zn deficiency induced a reduction in the chlorophyll concentration, photosynthetic capacity of leaves and destruction of the chloroplast ultrastructure (Tavallali et al. 2009). The enhancement of chlorophyll concentration by application of the PGPR and Zn has been reported by Han and Lee (2005) and Arif et al. (2012).
According to the results, the ratio of chlorophyll a/b enhanced with increase in the salinity levels. However, the application of Zn reduced this ratio. In the salt-treated plants, the chlorophyll b may be transformed into chlorophyll a, thus resulting in the enhanced chlorophyll a concentration (Eckardt 2009). Abiotic stress such as salinity reduced the quantum efficiency of the PSII photochemistry. Yaman et al. (2008) stated that the F v/F m ratio is an indicator to evaluate the damage to the chlorophyll, especially the thylakoid membrane under salt stress.
Different abiotic stresses including salinity can increase cellular damage due to increased ROS generation. In the present study, the H2O2 concentration significantly increased in the NaCl-treated pistachio seedlings. However, the H2O2 concentration was minimal after the combined treatment with the PGPR and Zn. Zinc plays an important role in reducing the production and detoxification of the ROS which can damage the sulfhydryl groups and membrane lipids (Alloway 2008). Also, the Zn is the stabilizing constituent of the biomembranes against the ROS such as H2O2. The alleviation of salt stress by PGPR could be attributed to their ability in production of auxin, organic acids, solubilization of nutrients such as Zn and enzyme ACC-deaminase. Iqbal and Ashraf (2013) indicated that pretreatment of wheat with auxin promote plant metabolism under saline condition. Also, Ali et al. (2014) observed that inoculation of the salt-stressed tomato by ACC-deaminase containing PGPR significantly increased the fresh and dry biomass and chlorophyll concentration compared to the control. Gururani et al. (2013) showed that the inoculation of potato with PGPR isolates significantly reduced the H2O2 levels under NaCl stress conditions.
The elevation of the H2O2 levels under salt stress facilitates the formation of highly active OH·, which can cause degradation of lipids and lead to membrane damage. Thus, the antioxidative defense system of plants including various enzymes plays an importance role in the plant tolerance to salt stress. It is evident from the results of the present study that the SOD, CAT, PPO and POX activities increased with increase in the salinity levels. Sairam et al. (2002) reported that salinity induced increase in the activity of SOD and CAT. High levels of superoxide radicals can inactivate the CAT activity. The SOD protects against the cell damage by catalyzing the conversion of the superoxide radicals to O2 and H2O2, and the transformation of the H2O2 into H2O and O2 facilitated by either POX, CAT (Marschner 1995; Cakmak 2000). The inoculation with the PGPR and Zn treatment (specially their combined application) significantly enhanced the activity of the antioxidant enzymes, especially under higher NaCl levels. The elevation of antioxidant enzyme activities in the salt-affected seedlings treated with PGPR and Zn indicates their potential to increase the salt tolerance of seedlings. Zinc plays a key role in the activity of many enzymes such as SOD, and in the stabilization of protein and membrane (Aravind and Prasad 2004). Cakmak (2000) stated that the Zn facilitates the biosynthesis of the antioxidant enzymes. Enhancement of the PPO activity under saline conditions indicates its ability in the control and detoxification of toxic substances such as phenolic compounds. The effect of PGPR on the plants antioxidant enzymes activity under abiotic stress has been reported by Stefan et al. (2013).
In this study, the protein concentration became reduced under high levels of salt stress. The results suggested that the treatment with the PGPR and Zn enhanced the protein concentration in the NaCl-treated pistachio seedlings, thus reducing the salt effect and improving seedling growth. Protein concentration is better indicator of osmotic adjustments in plants in response to environmental stress. Reduction in the protein concentration under conditions of high salt stress may be due to the accumulation of ROS, resulting in damage to the cellular macromolecules, including those of the protein and lipids. Qurashi and Sabri (2012) reported that application of 100 mM NaCl increased protein level in the chickpea, but, its concentration became reduced under high salinity level (200 mM NaCl). However, the inoculation by soil bacteria increased protein content at all the salinity levels. Also, Tavallali et al. (2009) reported that supplementation with the Zn significantly increased the protein concentration in the salt-stressed pistachio seedling leaves.
Conclusion
In summary, our results demonstrated that the inoculation by fluorescent pseudomonads isolates and Zn supplementation effectively protected the pistachio seedlings against the deleterious effects of salinity stress by controlling the generation of the oxidative agents such as H2O2, and the enhancement of the compatible solutes as well as activity of antioxidant enzymes. However, the combined application of fluorescent pseudomonads isolates and Zn was more effective than their sole usage. Any increase in the antioxidant enzymes activities in the Zn-treated seedlings that scavenged the reactive radicals might be related to the role of Zn in the activation of these enzymes. The isolates containing the ACC-deaminase activity were more efficient to alleviate salt stress and improve plant growth. According to the results, the ACC-deaminase could be a key enzyme for improvement of pistachio growth under salinity stress. Also, these isolates, by solubilizing the immobile forms of the nutrients such as P and Zn and synthesizing the siderophore and IAA could enhance the concentrations of the chlorophyll, protein and photosynthesis efficiency of the pistachio seedlings. The beneficial effect of fluorescent pseudomonads on plant defense system provides a very useful approach to reduce salt stress and enhancement of plant tolerance against salinity by these microorganisms.
Author contribution statement
Designing and performing the experiment: Farhad Azarmi, Vahid Mozafari, Payman Abbaszadeh Dahaji and Mohsen Hamidpour. Analysis, interpretation and discussion of data: Farhad Azarmi, Vahid Mozafari, Payman Abbaszadeh Dahaji and Mohsen Hamidpour. Paper preparation: Farhad Azarmi, Payman Abbaszadeh Dahaji and Mohsen Hamidpour. Final approval of the paper to be submitted: Farhad Azarmi, Vahid Mozafari, Payman Abbaszadeh Dahaji and Mohsen Hamidpour.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylate
- CAT:
-
Catalase
- CFU:
-
Colony forming unit
- IAA:
-
Indole acetic acid
- PGPR:
-
Plant growth promoting rhizobacteria
- POX:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
- Chl.:
-
Chlorophyll
- Car.:
-
Carotenoids
References
Abbas-Zadeh P, Saleh-Rastin N, Asadi-Rahmani H, Khavazi K, Soltani A, Shoary-Nejati R, Miransari M (2010) Plant growth-promoting activities of fluorescent pseudomonads isolated from the Iranian soils. Acta Physiol Plant 32:281–288
Ali Sh, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167
Alloway BJ (2008) Zinc in soils and crop nutrition. Second edition, published by IZA and IFA, Brussels, Belgium and Paris, France
Alqarawi AA, Hashem A, Abd Allah EF (2014) Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphyllaForssk. J Plant Interact 9:802–810
Aravind P, Prasad MNV (2004) Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllumdemersum L. a fresh water macrophyte. Plant Sci 166:1321–1327
Arif M, Shehzad MA, Bashir F, Tasneem M, Yasin G, Iqbal M (2012) Boron, zinc and microtone effects on growth, chlorophyll contents and yield attributes in rice (Oryza sativa L.) cultivar. AfrJ Biotechnol 11:10851–10858
Bradford M (1976) A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Ann Rev Biochem 72:248–254
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascrobate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Chapman HD, Pratt PF (1961) Methods of analysis for soils, plants, and waters. University of California, Riverside
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248
Dang HR, Li Y, Sun X, Zhang Y (2010) Absorption, accumulation and distribution of zinc in highly yielding winter wheat. AgricSci China 9:965–973
Dhindsa RS, Plump-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Ding M, Hou P, Shen X (2010) Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant MolBiol 73:251–269
Eckardt NA (2009) A new chlorophyll degradation pathway. Plant Cell 21:700
Egamberdieva D (2012) Pseudomonas chlororaphis:a salt-tolerant bacterial inoculants for plant growth stimulation under saline soil conditions. Acta Physiol Plant 34:751–756
Egamberdieva D, Kucharova Z (2009) Selection for root colonizing bacteria stimulating wheat growth in saline soils. BiolFertil Soil 45:563–571
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gonzalez L, Gonzalez-Vilar M (2003) Determination of relative water content. In: Reigosa MJ (ed) Handbook of plant ecophysiology techniques. Kluwer Academic, Dordrecht, pp 207–212
Gururani MA, UpadhyayaChP Basker V, Venkatesh J, Nookaraju A, Park SW (2013) Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanumtuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J Plant Growth Regul 32:245–258
Habibi GH, Norouzi F, Hajiboland R (2014) Silicon alleviates salt stress in pistachio plants. Prog Biolog Sci 4:189–202
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Han HS, Lee KD (2005) Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res J AgricBiolSci 1:210–215
Hernandez M, Kappler A, Newman D (2004) Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl Environ Microbiol 70:921–928
Iqbal M, Ashraf M (2013) Alleviation of salinity-induced perturbations in ionic and hormonal concentrations in spring wheat through seed preconditioning in synthetic auxins. Acta Physiol Plant 35:1093–1112
Irigoyen JJ, Emerich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Karlding H, Yildirim E, Turan M, Pehluvan M, Donmez F (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria × ananassa). HortSci 48:563–567
Karuppanapandian T, Moon JC, Kim Ch, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. AJCS 5:709–725
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Zee SEATM, Parker DR (2004) Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci Soc Am J 68:1885–1889
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lindsay WL (1978) Chemical equilibria in soil. Wiley, New York
Lopez-Huertas E, Del Río L (2014) Characterization of antioxidant enzymes and peroxisomes of olive (Oleaeuropaea L.) fruits. Plant Physiol 171:1463–1471
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, Londan
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant PhysiolBiochem 42:565–572
Mishra M, Kumar U, Mishra PK, Prakash P (2010) Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicerarietinum L. growth and germination under salinity. AdvBiol Res 4:92–96
Mozaffari V, Malakouti MJ (2006) An investigation of some causes of die-back disorder of Pistachio trees and its control through balanced fertilization in Iran. Acta Hort 726:247–252
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidase activity in seedling of Triticumaestivum L. Plant Cell and Environ 15:719–725
Paquin R, Lechasseur P (1979) Observation’s suruneméthode de dosage de la praline libredans les extraits de plantes. Can J Bot 57:1851–1854
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60:324–349
Paul D, Nair S (2008) Stress adaptations in a plant growth promoting Rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microb 48:1–7
Porcel R, Aroca R, Ruiz-Lozano JM (2012) Salinity stress alleviation using arbuscularmycorrhizal fungi: a review. Agron Sustain Dev 32:181–200
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Qurashi AW, Sabri AN (2012) Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz J Microbiol 43:1183–1191
Sairam RJ, Veerabhadra R, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 136:1046–1073
Saleem M, Arshad M, Hussain S, Bhatti A (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J IndMicrobiolBiotechnol 34:635–648
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J ApplMicrobiol 102:1283–1292
Shahriaripour R, Tajabadi Pour A, Mozaffari V, Dashti H, Adhami E (2010) Effects of salinity and soil zinc application on growth and chemical composition of pistachio seedlings. J Plant Nutr 33:1166–1179
Sharma A, Shankhdhar D, Sharma A, Shankhdhar SC (2014) Growth promotion of the rice genotypes by pgprs isolated from rice rhizosphere. J Soil Sci Plant Nutr 14:505–517
Silva GR, Koblitz MGB (2010) Partial characterization and inactivation of peroxidases and polyphenol-oxidases of umbu-cajá (Spondias spp.). CiêncTecnol Aliment Campinas 30:790–796
Sparks DL (1996) Methods of soil analysis. Part. 3, chemical methods.Soil science society of America, Madison, Wisconsin, USA
Stefan M, Munteanu N, Stoleru V, Mihasan M (2013) Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom Biotech Lett 18:2
Sziderics AH, Rasche F, Trognitz F, Wilhelm E, Sessitsch A (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microb 53:1195–1202
Tavallali V, Rahemi M, Maftoun M, Panahi B, Karimi S, Ramezanian A, Vaezpour M (2009) Zinc influence and salt stress on photosynthesis, water relations, and carbonic anhydrase activity in pistachio. Scientia Hort 123:272–279
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Yaman K, Taniguchi M, Kawasaki M, Miyake H (2008) Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice (Oryza sativa L.) grown under salinity. Plant Product Sci 11:139–145
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38:1095–1102
Acknowledgments
We thank to the Vali-e-Asr University of Rafsanjan research council for providing financial support (Grant Number: 4-9164455). Also, we are most grateful to two anonymous referees for providing the critical comments and helpful suggestions on the original version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Krolicka.
Rights and permissions
About this article
Cite this article
Azarmi, F., Mozafari, V., Abbaszadeh Dahaji, P. et al. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol Plant 38, 21 (2016). https://doi.org/10.1007/s11738-015-2032-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-2032-3