Abstract
Callogenesis was induced from Stylosanthes guianensis (Aubl.) Sw. cv. CIAT-184 hypocotyl explants cultured in the presence of different concentrations of NaCl. Whereas calluses formed at 50 mM NaCl did not show significant changes, concentrations in the range of 100–250 mM NaCl influenced callus formation and survival. The concentrations which reduced the callus formation and survival by 50 % with respect to the controls (I 50) were 190 and 225 mM NaCl, respectively. Callus growth was also affected being 160 mM NaCl the concentration that reduced the fresh weight gain in 50 %, in comparison to the control. The cell walls of calluses formed at higher NaCl concentrations showed a lower cellulose content, which was accompanied by an increase in protein, phenolics, and esters, as revealed by Fourier transform infrared spectroscopy. These overall cell wall modifications were accompanied by the formation of cell wall appositions enriched with homogalacturonan and rhamnogalacturonan components, and also with an increase of arabinogalactan proteins in cell walls. These results evidence the ability of cells to adjust the cell wall composition under salt stress conditions, as part of the strategy to sustain their growth in such environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stylosanthes Sw. comprises 50 species and subspecies, all of them are perennial herb plants. Due to their vigorous growth habit, deep rooting ability, and resistance to poor and infertile soils, stylosanthes species, associated with a wide range of grasses, are currently being used as protein banks and green manure and as forage crops (Partridge 1996; Lovato and Martins 1997). Also, they are currently used as feed for pasturing cattle and as a component for wasteland reclamation (Chandra 2006).
In order to extend the culture of stylosanthes plants, soil and water salinity can be regarded as one of the major abiotic stresses susceptible to reduce their growth and crop productivity worldwide. For this reason, the goal of breeding for stylosanthes plants would be to develop cultivars that can grow and produce economic yields under moderately saline conditions. Among the various species, Stylosanthes guianensis (Aubl.) Sw., taking into account its ecological plasticity, is a good candidate to extend its culture to a wide range of soil conditions, climate, and altitude, including saline environments, as it has been regarded as moderately tolerant to salinity (González et al. 2000). However, before developing a breeding program to improve the salt tolerance of this crop, it is necessary to gain knowledge concerning the genetics and physiology of its tolerance mechanisms.
Stylosanthes is considered one of the least recalcitrant legume genus regarding to regeneration via callus (Dornelas et al. 1992; Consoli et al. 1996; Valarini et al. 1997). Furthermore, organogenic calli have been referred to as a suitable strategy to regenerate stable stylosanthes transgenic plants (Quecini et al. 2006). Nevertheless, the studies about behavior under saline conditions in stylosanthes species referred only to germination (Gonela et al. 2004, Silva et al. 2014) and plant growth (Lovato and Martins 1997; González et al. 2000; Fuentes et al. 2010). In this regard, programs of in vitro selection for tolerance to abiotic stresses could be a useful tool; however, this strategy is dependent on the development of efficient and reliable callus induction and plant regeneration systems (Benderradji et al. 2007). Following this orientation, callus cultures are commonly used as an in vitro technique for biochemical and physiological studies of the response to salt and water stress at cellular level (Gandonou et al. 2006; Liu et al. 2006; Kaviani 2008). In addition, several studies have established an important role of a set of cell wall modifications produced by cells in order to cope with different environmental constraints, including saline stress (Iraki et al. 1989; McCann et al. 1994; Lamport et al. 2006). Nevertheless, changes taking place in the cell wall components in calluses under NaCl stress, are not well known yet.
The aim of this work was to study the callogenesis response of Stylosanthes guianensis CIAT-184 under a range of NaCl concentrations, and to monitor the cell wall changes produced during callogenesis process. For this purpose, after assessing the effects of NaCl concentrations on several parameters related to callogenesis, cell wall changes were monitored, using different approaches, such as FTIR spectroscopy and cellulose assay in extracted cell walls, as well as immunodetection of a set of cell wall proteins and pectins in calluses pieces.
Materials and methods
Plant material
Seeds of Stylosanthes guianensis CIAT-184, obtained from the “Indio Hatuey” Experimental Station (Cuba), were sterilized in 70 % ethanol (1 min) and 5 % commercial sodium hypochlorite solution (15 min) and subsequently rinsed three times in sterile distilled water. Then, sterile seeds were cultured in dark sterile humidity chamber on filter paper at room temperature, and 15 days later hypocotyls from seedlings were used as explants.
Calluses formation in NaCl
Hypocotyl sections of 4–6 mm were grown in Petri dishes containing Murashige and Skoog medium (1962), 2 % (w/v) sucrose, 7 g L−1 agar, 1 mg L−1 2,4-D (2,4-dichlorophenoxy-acetic acid), 2 mg L−1 6-BAP (6-benzylaminopurine), and a range of NaCl concentrations (0, 50, 100, 150, 200, or 250 mM), being pH adjusted to 5.6. After placing Petri dishes in growing chamber at 25 °C with 16 h photoperiod, calluses formation was observed after 7 days of culture.
Calluses inductive-conditions were maintained for 6 weeks in order to determine the percentage of calluses formation. The survival percentages were calculated by considering green explants or explants forming calli as live ones. Five 20-day old calluses were used for cellulose quantification, FTIR, and immunolocation analyses.
The initial fresh weight (Wi) of each callus was recorded just before the beginning of treatment, and the final fresh weight (Wf) was measured after 10, 20, and 30 days of growth. The daily increase in relative weight (RWI) during the culturing period was calculated using the following formula: RWI = (Wf − Wi) Wi−1 and the I 50 was calculated as the concentration of NaCl able to inhibit fresh weight increase by 50 % with respect to the control.
Preparation of cell walls
Calluses were homogenized to a fine powder in liquid nitrogen using a mortar and pestle and were treated with 2.5 U mL−1 α-amylase obtained from porcine pancreas (Sigma, type VI-A) in 0.01 M phosphate buffer pH 7.0 for 24 h at 37 °C. The mixture was centrifuged at 1000×g for 15 min and the pellet was washed (10 mL g−1) with water (×3), acetone (×3), methanol–chloroform (1:1 v/v, ×3), and diethyl ether (×2), and air-dried. Final pellet was considered as cell walls (Talmadge et al. 1973).
Cell wall analyses
FTIR spectroscopy
Tablets for FTIR spectroscopy were prepared in a Graseby-Specac Press, using cell walls (2 mg) mixed with KBr (1:100). Ten spectra were obtained from each tablet on a Perkin-Elmer instrument at a resolution of 1 cm−1 and the average spectrum was used thereafter. A window between 800 and 1800 cm−1, containing information on characteristic polysaccharides, was selected in order to monitor cell wall structure modifications. All spectra were normalized and baseline-corrected with the Perkin-Elmer Spectra v.5.3 software. The data were then exported to Microsoft Excel and all spectra were area-normalized.
Cellulose quantification
Cellulose in cell walls was quantified by the Updegraff method (1969) using the hydrolytic conditions described by Saeman et al. (1963) and quantifying the glucose released by the anthrone method, using a suitable calibration curve (Dische 1962).
Immunolocation of cell wall components
Callus pieces were fixed in 2.5 % paraformaldehyde in 0.1 M phosphate buffer pH 7.5 at 4 °C overnight. After washing with phosphate buffer, cells were dehydrated in an ethanol series prior to embedding in resin (LR White, London Resin, Reading, UK), then placed in gelatin capsules containing resin, and allowed to polymerize at 37 °C for 5 days. One micrometer-thick sections were obtained in a Ultracut microtome LKB 2088 (Reichert-Jung, Austria) and applied to multi-well slides (ICN Biomedicals, Cleveland, OH, USA) coated with Vectabond reagent (Vector Laboratories, Burlingame, CA, USA). Sections were incubated for 2 h with primary antibody in a 1/10 dilution of phosphate-buffered saline (PBS: 0.14 M NaCl, 2.7 mM KCl, 7.8 mM Na2HPO4·12H2O, 1.5 mM KH2PO4, pH 7.2) containing 4 % fat-free milk powder (MPBS). After being washing exhaustively with PBS, the sections were incubated in darkness for 2 h with a 1/100 dilution of an anti-rat immunoglobulin G linked to fluorescence in isothiocyanate (Sigma) in MPBS at room temperature. Sections were washed with PBS and stained in darkness with Calcofluor for 5 min. Finally, after being washed with PBS, the sections were mounted in a glycerol/PBS-based antifade solution (Citifluor AF1; Agar Scientific, London, UK) and observed on an Olympus BH-2 microscope equipped with epifluorescence irradiation.
Sections were tested with monoclonal antibodies (mAbs) specific for homogalacturonan (HG) with a low (JIM5) or high (JIM7) degree of methyl esterification, 1,4-β-galactan (LM5), 1,5-α-arabinan (LM6), or arabinogalactan proteins (AGPs) (LM2, JIM8, and MAC207). These mAbs were purchased from PlantProbes (Leeds, UK), except JIM8 and MAC207 which were obtained from the John Innes Institute (Norwich, UK).
Statistical analysis
Experiments were set up in completely randomized design and repeated three times. When indicated, ANOVA followed by a Tukey test (P < 0.05) was used for variance analysis. All analyses were performed using Statistica (Stat-Soft) software.
Results and discussion
Effect of NaCl concentration on explants survival, callus formation, and calluses growth
The Stylosanthes guianensis CIAT-184 hypocotyls explants survival and calluses formation percentages were evaluated after growing in media with increasing NaCl concentrations. Both parameters were reduced when the NaCl content in cell culture medium was higher than 100 mM (Fig. 1a). Concentrations of 190 and 225 mM NaCl reduced in a 50 % with respect to the controls the calluses formation and survival, respectively (I 50). These values are in the same range in relation to other species, such as the maize Tripsacum hybrid, in which organogenic calluses survival and regeneration percentages were reduced to half in 140 mM NaCl (Pesqueira et al. 2006), or different tomato varieties in which the I 50 for calluses formation was 83.5 mM NaCl (El-Aref 2002).
Percentages of calluses formation (filled circle) and calluses survival (open square) (a), and growth inhibition curves of Stylosanthes guianensis CIAT-184 hypocotyl explants by increasing the concentrations of NaCl after 30 days (b). Relative increase in fresh weight of Stylosanthes guianensis calluses during culture in different NaCl concentrations: 0 (open circle), 50 (filled square), 100 (filled diamond), 150 (filled hexagon), 200 (black up pointing triangle), and 250 (black down pointing triangle) mM (c). Values are mean ± SD of three measurements
The inhibitory-growth effect of NaCl on stylosanthes calluses was tested on fresh weight gain (Fig. 1b). This fresh weight gain gradually decreased as NaCl concentration increased, 160 mM being the required concentration to reduce it by 50 %, with respect to the control. So, stylosanthes calluses were more resistant to saline stress than those from other legumes, i.e., they were 6.4-fold more resistant than bean calluses (I 50: 25 mM; García-Angulo, personal communication). In this sense, they were more resistant to salinity than calluses of other species, such as the maizeTripsacum hybrid, in which the relative weight increment was strongly affected by NaCl concentrations higher than 70 mM (Pesqueira et al. 2006). On the other hand, stylosanthes calluses were resistant in the same range as that of cell cultures of different halophytes, such as calluses of Suaeda maritima (L.) Dumort. (I 50: 170 mM) (von Hedenström and Breckle 1974), or cell suspensions of Atriplex nummularia Lindl. (I 50: 200 mM) (Casas et al. 1991). Curiously, they were even more resistant than calluses cultures of other halophytes, such as Suaeda nudiflora (Willd.) Moq. (I 50: 63 mM) (Cherian and Reddy 2003).
The relative weight increase was reduced in the presence of NaCl throughout the culture cycle (Fig. 1c), especially at concentrations higher than 100 mM. As expected, seed germination and seedling growth processes were less affected by salt stress than calluses growth; it was previously reported that the I 50 for the reduction in root and shoot fresh weight gain of Stylosanthes guianensis seedlings in presence of NaCl reached values higher than 200 mM NaCl (Fuentes et al. 2010). In addition, the estimated concentration which reduced the germination percentage to 50 % in different Stylosanthes humilis Kunth seed populations varied between 200 and 295 mM NaCl (Lovato et al. 1994), and the concentrations which reduced shoot dry mass by 50 % during the initial growth stage varied from 84 to 108 mM NaCl (Lovato et al. 1999). The differences in sensitivity toward salt stress between cellular level and whole plant level showed by plants have been frequently described in several species, and it depends on physiological specializations operating in both levels (for a review, see Sahi et al. 2006).
Monitoring of cell wall changes during callogenesis in presence of NaCl
In order to monitor cell wall changes during the callogenesis process in presence of NaCl, three approaches were followed: FTIR spectroscopy and cellulose assay, both in cell walls, and immunolocation of cell wall proteins and pectins, in calluses pieces.
FTIR spectroscopy
The FTIR spectra from cell walls showed a notable variability regarding NaCl concentration, especially for two regions: the fingerprint (800–1200 cm−1) and 1575–1450 cm−1 regions (Fig. 2). The fingerprint area of spectra from cell walls of calluses grown in the presence of 50 mM NaCl increased with respect to that of the controls one. However, in other NaCl concentrations, the area of this region decreased, especially for cell wall spectra from calluses grown in the presence of 200 mM NaCl.
Area-normalized and baseline-corrected FTIR spectra of cell walls obtained from Stylosanthes guianensis CIAT-184 calluses growing in different NaCl concentrations: 0 (solid line), 50 (long dash), 100 (dotted), and 200 (dash–dot) mM. Lines show bands assigned to different cell wall components: pectins, cellulose, proteins, lignin, and galactose
The difference spectra obtained by digital subtraction constitute a useful tool for unravelling differences between two FTIR spectra as well as for establishing the contribution of certain wavenumbers to them (Largo-Gosens et al. 2014). Marked differences among FTIR spectra obtained from cell walls of calluses formed in the presence of 50, 100, and 200 mM NaCl and control were observed (Fig. 3). Cell wall spectra from calluses exposed to 50 mM NaCl showed a broader fingerprint area than control spectra (Fig. 3). In spectra from 100 mM NaCl-grown calluses, there appeared some changes that were emphasized in calluses formed at 200 mM NaCl. First, cell wall spectra from 100 to 200 mM NaCl-calluses showed much narrower fingerprint area than control spectra. Second, cell walls from calluses formed in the presence of 200 mM NaCl showed a decrease of bands assigned to cellulose, such as 900, 1040, 1060, 1160, 1320, 1370 cm−1 (Alonso-Simón et al. 2011; Largo-Gosens et al. 2014), and 1430 cm−1 (Wilson et al. 2000). Additionally, increases of bands assigned to polyphenols (1515 cm−1 –aromatic rings– and 1470 cm−1 –methyl groups of lignin–) and proteins (1550 cm−1) were observed. These results are in concordance with those observed in NaCl-adapted tobacco cells, which exhibit a substantial increase of bands corresponding to protein- (amide stretches at 1650 and 1550 cm−1) and polyphenols (1620 and 1515 cm−1)-assigned bands (McCann et al. 1994). This observed increase of bands corresponding to polyphenols-assigned bands points to an initial lignification process. Recently, a set of works have established a consistent correlation between cellulose reduction and lignin or a lignin-like polymer enrichment, not only in secondary cell walls, but also in cell walls of cell cultures, indicating a contribution of this phenolic polymer in cell wall stiffening (Shen et al. 2013; Mélida et al. 2015). It is significant that the transcriptomic analysis of roots of Salicornia europaea L. under saline conditions showed a down-regulation of genes involved in cellulose synthesis, joined to an up-regulation of many of the genes implied in lignin biosynthesis (Fan et al. 2013).
Difference spectra generated by digital subtraction of FTIR spectra of cell walls obtained from Stylosanthes guianensis CIAT-184 calluses growing in different NaCl concentrations: 50 mM NaCl–control (a), 100 mM NaCl–control (b), and 200 mM NaCl–control (c). Lines show bands assigned to different cell wall components: pectins, cellulose, proteins, lignin, and galactose
A clear trend regarding diagnostic band of pectins was not observed, because some bands assigned to them, such as 1104 and 1250 cm−1, or those corresponding to unesterified uronic acids (1610 and 1420 cm−1) and galactose (950 cm−1) decreased, whereas other ones, such as those assigned to esters (1725 cm−1) tended to increase, pointing to different contributions of pectic polysaccharides according to their particular composition. Comparing the opposite trend of bands corresponding to unesterified uronic acids and esters, a higher degree of methyl esterification would be deduced in calluses under higher NaCl concentrations.
A part of the bands assigned to xyloglucan, the most abundant hemicellulose in dicot cell walls (i.e., 1040, 1317, 1371 cm−1, Largo-Gosens et al. 2014), are usually masked by those of cellulose making it difficult to recognize their contribution to saline stress; however, other bands specific for xyloglucan, such as 1078 or 1120 cm−1 (Largo-Gosens et al. 2014), are not clearly apparent in cell walls of calluses subjected to saline stress, pointing to a minor contribution of hemicelluloses to cell walls modifications during callogenesis in the presence of saline concentrations.
Assay of the cellulose content
Cellulose content in the cell walls of control calluses, assessed by the Updegraff method, (13 %), was somewhat lower than that reported for other in vitro cultures of species with type I cell walls such as bean (25 % Encina et al. 2001), tomato (18 % Shedletzky et al. 1990), or tobacco (26 % Shedletzky et al. 1992).
Cellulose content significantly increased in cell walls of calluses growing in the presence of 50 mM NaCl. This cellulose increase was correlated with an increment in the area corresponding to the fingerprint region of the FTIR spectra (Figs. 2, 3a), and also of a set of bands assigned to cellulose, namely 1040, 1060, and 1160 cm−1. However, calluses formed in 100 and 200 mM NaCl showed a reduction in cellulose amount of 23 and 44 %, respectively, regarding control (Fig. 4), that correlated with a reduction in the fingerprint area of FTIR spectra from these cell walls (Figs. 2, 3b, c), and also of bands assigned to cellulose. A similar reduction in cellulose content promoted by saline conditions has been previously reported in tobacco cells adapted to grow in 0.428 M NaCl, in which the proportion of cellulose was only 50 % that of unadapted cells (Iraki et al. 1989), as well as in cell walls of cotton seedlings (Zhong and Läuchli 1993) and maize leaves (Uddin et al. 2013) under saline stress. The change of trend in the cellulose content observed when calluses are subjected to concentrations of NaCl well below the I 50 (in this case, 50 mM, whereas the I 50 is 160 mM) might be attributed to a subtoxic effect promoted by the saline conditions. Serra et al. (2015) have shown that subtoxic levels of chemical stressors are able to cause unexpected metabolic shifts, with different impacts on growth and metabolic composition of plant organs.
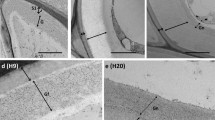
The differences in cellulose content were not appreciated in calluses sections by means of their staining with calcofluor (Figs. 17 vs. 18). In both analyzed lines (controls and 200 mM NaCl-calluses), the fluorescence due to calcofluor appeared in a continuous layer outlining cell wall. However, it is not appropriate to quantify changes in fluorescence intensity if the fluorochrome saturates the cellulose.
It has been repeatedly observed that cell cultures subjected or adapted to high NaCl concentrations, such as tobacco (Iraki et al. 1989; Bressan et al. 1990), or Suaeda nudifolia (Cherian and Reddy 2003) reduce their volume in order to maintain the turgor pressure, in a process mediated by changes in cell wall properties, affecting cell wall extensibility and as a consequence, cell expansion. However, microscopy analysis of our cells did not reveal differences in size or volume between control calluses and those obtained and maintained at 200 mM NaCl (Figs. 5–18). A similar lack of reduction in cell volume or shape has been described in other calluses cultures, such as citrus ones (Ben-Hayyim 1987), pointing to NaCl effects on calluses growth are not necessarily correlated with a reduction in cell volume. However, our cells maintained at 200 mM NaCl seem a bit more heterogeneous both in size and shape. This observation is also extended to the cell walls: walls from cells maintained at 200 mM NaCl had a more variable width than controls, and showed occasional thickenings (see i.e., Figs. 6, 8, 12).
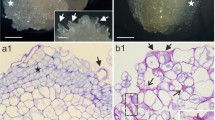
Immunolocation of cell wall pectin epitopes in sections of resin-embedded control calluses (5, 7, 9, 11) and calluses growing in the presence of 200 mM NaCl (6, 8, 10, 12). Sections were probed with monoclonal antibodies specific for homogalacturonan with a low (JIM5, 5 and 6) and high (JIM7, 7 and 8) degree of methyl esterification, 1,4-β-galactan (LM5, 9, and 10), and 1,5-α-arabinan (LM6, 11, and 12). Bars 10 µm (8), all others, 20 µm. Arrows point to cell wall appositions
Immunolocation of cell wall arabinogalactan protein epitopes in sections of resin-embedded control calluses (13, 15) and calluses growing in the presence of 200 mM NaCl (14, 16). Sections were probed with monoclonal antibodies LM2 (13, 14) and MAC207 (15, 16), or stained with calcofluor (17, 18). Bars 20 µm
Immunolocation of cell wall proteins and pectins
Since both FTIR spectra and cellulose content analysis of cell walls showed strong differences with respect to the control in calluses formed in 200 mM NaCl, and since previous studies have reported salt stress-related changes in several cell wall components, mainly pectins and proteins (Iraki et al. 1989; McCann et al. 1994), in order to examine further changes in cell wall components, sections and immunolocation with different antibodies (mAbs) for pectins (Figs. 5–12) and arabinogalactan proteins (Figs. 13–16) from stylosanthes calluses were made.
Pectin domains were probed with four mAbs with specificity for homogalacturonan with different degrees of methyl esterification: low (JIM5 Figs. 5, 6) and high (JIM7 Figs. 7, 8), respectively, whereas the side chains of rhamnogalacturonan I were probed with mAbs LM5 (Figs. 9, 10) and LM6 (Figs. 11, 12), with specificity for 1,4-β-galactan and 1,5-α-arabinan side chains, respectively (Jones et al. 1997; Willats et al. 1999).
It has been proposed that homogalacturonan polymers are deposited in the cell wall in a highly methyl-esterified form. Then, the homogalacturonan is demethylated by the action of pectin methylesterases, and this increases the capacity to form calcium cross-links (Grant et al. 1973).
In control calluses, JIM5 epitopes (Fig. 5) were dispersed throughout the cell wall whereas JIM7 epitopes (Fig. 7) were homogeneously localized, especially in cell junctions. Immunofluorescence labeling of calluses both with JIM5 (Figs. 5 vs 6) and JIM7 (Figs. 7 vs 8) probes showed a more intense and homogeneous labeling in 200 mM NaCl-calluses than in controls. In cell walls from NaCl-adapted tobacco calluses, immune-gold detection of JIM7 epitopes showed a stronger labeling compared with unadapted cells, but that of JIM5 showed a weaker labeling (McCann et al. 1994), whereas in petioles of aspen plants treated with saline solutions, stronger labeling with JIM7 and no differences in the labeling with JIM5 were observed (Muszynska et al. 2014), which contrasts with our results. However, we have to keep in mind that tobacco calluses were adapted to NaCl conditions, whereas our stylosanthes calluses were only treated with, but not adapted to salt. FTIR monitoring showed that our cell walls of stylosanthes calluses in the presence of 200 mM NaCl exhibited a higher degree of methylesterification. This is correlated to the more intense labeling with JIM7 observed in 200 mM NaCl-treated calluses, but it is not necessary an objection to the observed intense labeling with JIM5 in the same calluses. In fact, cellulose-impoverished cell walls as those of tomato dichlobenil-habituated are characterized by a general increment of pectic polysaccharides (both esterified and non-esterified) (Wells et al. 1994), and those of different maize hybrids subjected to salt stress (Uddin et al. 2013).
The labeling with LM5 −with specificity for 1,4-β-galactan− was weak and no differences were detected between control and 200 mM NaCl-grown calluses (Figs. 9 vs. 10). However, LM6 arabinan epitopes were more abundant in calluses formed in 200 mM NaCl than in controls (Figs. 12 vs. 11). Both epitopes have been correlated with cell expansion and proliferation processes in carrot cell suspensions (Kikuchi et al. 1996; Iwai et al. 2001). When carrot cells were induced to elongation in a cell culture medium lacking 2,4-D, LM6 arabinan epitopes disappeared whereas LM5 galactan epitopes increased (Willats et al. 1999). Our calluses formed in the presence of NaCl grew and elongated lesser than controls, which is consistent with the higher amount of LM6 epitopes.
Stylosanthes calluses formed in 200 mM NaCl showed cell wall appositions containing homogalacturonan with low (JIM5) and high (JIM7) degree of methyl esterification, and rhamnogalacturonan I (LM6) (Figs. 6, 8, 12). Cell wall appositions have been described in other cell cultures with reduced cellulose content, such as tobacco (Sabba et al. 1999), arabidopsis (Manfield et al. 2004), and French bean (García-Angulo et al. 2006) cultured cells habituated to different cellulose biosynthesis inhibitors, suggesting that impairment in cellulose deposition affects the correct assembly of matrix polysaccharides: these habituated cell cultures have in common a decreased amount of hemicelluloses, and an increased amount of esterified and unesterified pectins. Moreover, in tobacco cells, pectins are crosslinked with extensions to form the main cell wall network (Sabba et al. 1999), whereas in tomato cells, pectins are crosslinked via phenolic-ester and/or phenolic ether linkages (Shedletzky et al. 1990, 1992).
AGPs have been described as important signaling molecules in different developmental and stress processes (Ellis et al. 2010; Tan et al. 2012) including salt stress (Zagorchev et al. 2014). AGPs were probed with mAbs LM2 (Smallwood et al. 1996), JIM8 (Pennell et al. 1991), and MAC207 (Pennell et al. 1989) (Figs. 13–16). No differences in JIM8 epitopes were detected (data not shown). In other cases, the binding of mAbs to the glycan epitopes of AGPs appeared as discrete dots located throughout the cell wall and the outer zone of cytoplasm. Calluses formed in 200 mM NaCl showed more intense labeling, especially with MAC207 (Figs. 15 vs 16).
As glycoproteins of the plant cell surface, AGPs are glycosylated in Golgi apparatus and then are transported by vesicles to the cell wall (Gaspar et al. 2001). In stylosanthes calluses growing in the presence of 200 mM NaCl, the LM2 and MAC207 epitopes seem to be localized in the cytoplasm vesicles during their transport (Figs. 13–16). In salt-stressed rice plants (Ma et al. 2010) as well as in tobacco cell suspensions (Lamport et al. 2006) a set of AGPs were up-regulated; however, tobacco cells contained lower levels of plasma membrane-bound AGPs because the AGP release rate was much higher than in control cells (Lamport et al. 2006). Our cells grown on a solid medium, and probably their AGP release rate was lower than in tobacco cell suspension, in which the culture medium could be considered as an extension of the apoplast. These could explain the presence of more transport vesicles and more AGP epitopes in the cell walls of 200 mM NaCl-grown calluses.
AGPs are implicated in cell expansion (Park et al. 2003), and it has been proposed that the up-regulation of AGPs should enhance cell expansion by affecting the porosity of the pectin network in muro, decreasing pectin alignment and cross-linking (Lamport et al. 2006).
Occasional thickenings were observed in walls from cells maintained at 200 mM NaCl (see Figs. 6, 8, 12), and these seem to be mainly due to localized cellulose accumulations (Figs. 17, 18), but also to the accumulation of the same components of appositions, that is homogalacturonan −both with low and high level of methylesterification−, rhamnogalacturonan I as well as AGPs components (Figs. 13–16).
Taking into account the bulk of results, our study reflects that the structure and composition of cell walls were modified in stylosanthes calluses when they were maintained at high (200 mM) concentrations of NaCl. Modifications in cell walls have been regarded as a common feature of the response to salinity in plants (Le Gall et al. 2015). A reduction of cellulose levels was compensated with other cell wall components, such as pectins like homogalacturonan and rhamnogalacturonan I as well as AGPs.
Other components may also play important roles in the modifications experienced by stylosanthes cell walls in saline conditions, such as lignin or other lignin-like phenolic polymers as well as other proteins −evidenced by FTIR monitoring– without excluding hemicellulosic polysaccharides like xyloglucan, given the inherent limitations to FTIR monitoring.
References
Alonso-Simón A, García-Angulo P, Mélida H, Encina A, Álvarez JM, Acebes JL (2011) The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Sign Behav 6:1104–1110
Benderradji L, Bouzerzou H, Djekoun A, Benmahammed A (2007) Effects of NaCl stress on callus proliferation and plant regeneration from mature embryos of bread wheat (Triticum aestivum L.) cultivar Mahom Demias and Hidhab. Plant Tissue Cult Biotech 17:19–27
Ben-Hayyim G (1987) Relationship between salt tolerance and resistance to polyethylene glycol-induced water stress in cultured citrus cells. Plant Physiol 85:430–433
Bressan RA, Nelson DE, Iraki NM, LaRosa PC, Singh NK, Hasegawa PM, Carpita NC (1990) Reduced cell expansion and changes in cell walls of plant cells adapted to NaCl. In: Kattermann F (ed) Environmental injury to plants. Academic Press, New York, pp 137–171
Casas AM, Bressan RA, Hasegawa PM (1991) Cell growth and water relations of the halophyte, Atriplex nummularia L., in response to NaCl. Plant Cell Rep 10:81–84
Chandra A (2006) A partial genetic map of Stylosanthes developed using inter-specific F2 cross. Cytologia 71:353–358
Cherian S, Reddy MP (2003) Evaluation of NaCl tolerance in the callus cultures of Suaeda nudiflora Moq. Biol Plant 46:193–198
Consoli L, Vieira MLC, de Souza Lopes, Jr C, Garcia AAF (1996) Tissue culture effects on quantitative traits in Stylosanthes guianensis (Leguminosae). Braz J Genet 19:469–474
Dische Z (1962) Color reaction of carbohydrates. In: Whistler RL, Wolfrom ML (eds) Methods in carbohydrate chemistry. Academic Press, New York, pp 475–514
Dornelas MC, Vieira MLC, Appezzato-da-Glória B (1992) Histological analysis of organogenesis and somatic embryogenesis induced in immature tissues of Stylosanthes scabra. Ann Bot 70:477–482
El-Aref HM (2002) In vitro selection of salt-tolerant tomato plants and the changes in gene expression under salinity stress. Assiut J Agric Sci 33:23–46
Ellis M, Egelund J, Schultz CJ, Bacic A (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 153:403–419
Encina A, Moral R, Acebes JL, Alvarez J (2001) Characterization of cell walls in bean (Phaseolus vulgaris L.) callus cultures tolerant to 2,6-dichlorobenzonitrile. Plant Sci 160:331–339
Fan P, Nie L, Jiang P, Feng J, Lv S et al (2013) Transcriptome analysis of Salicornia europaea under saline conditions revealed the adaptive primary metabolic pathways as early events to facilitate salt adaptation. PLoS One 8:e80595
Fuentes L, Pérez Y, Alemán S, Sosa D, Sosa M, González S, Infante D, Domínguez D, Mesa AR (2010) Physiological and biochemical response of Stylosanthes guianensis cv. CIAT-184 and Centrosema molle to sodium chloride stress. Pastos y Forrajes 3:173–186
Gandonou CB, Errabii T, Abrini J, Idaomar M, Senhaji NS (2006) Selection of callus cultures of sugarcane (Saccharum sp.) tolerant to NaCl and their response to salt tolerance. Plant Cell Tissue Organ Cult 87:9–16
García-Angulo P, Willats WGT, Encina AE, Alonso-Simón A, Álvarez JM, Acebes JL (2006) Immunocytochemical characterization of the cell walls of bean cell suspensions during habituation and dehabituation to dichlobenil. Physiol Plant 27:87–99
Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ (2001) The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol 47:161–176
Gonela A, Lemos EGM, Rodrigues TJD, Paterniani MLS (2004) Notas Científicas Reação enzimática ao estresse salino durante a germinação de estilosantes. Pesq Agropec Bras 39:93–95
González LM, López RC, Fonseca I, Ramírez R (2000) Growth stomatal frequency, DM yield and accumulation of ions in nine species of grassland legumes grown under saline conditions. Pastos y Forrajes 23:299–308
Grant GT, Morris ER, Rees DA, Smith PJC, Thorn D (1973) Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 32:195–198
Iraki NM, Singh N, Bressan RA, Carpita NC (1989) Cell walls of tobacco cells and changes in composition associated with reduced growth upon adaptation to water and saline stress. Plant Physiol 91:48–53
Iwai H, Ishii T, Satoh S (2001) Absence of arabinan in the side chains of the pectic polysaccharides strongly associated with cell walls of Nicotiana plumbaginifolia non-organic callus with loosely attached constituent cell. Planta 213:907–915
Jones L, Seymour GB, Knox JP (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1-4)-β-D-galactan. Plant Physiol 113:1405–1412
Kaviani B (2008) Proline accumulation and growth of soybean callus under salt and water stress. Int J Agric Biol 10:221–223
Kikuchi A, Edashige Y, Ishii T, Fujii T, Satoh S (1996) Variations in the structure of neutral sugar chains in the pectic polysaccharides of morphologically different carrot calli and correlation with the size of cell clusters. Planta 198:634–639
Lamport DTA, Kieliszewski MJ, Showalter AM (2006) Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyse AGP function. New Phytol 169:479–492
Largo-Gosens A, Hernandez-Altamirano M, Garcia-Calvo L, Alonso-Simón A, Álvarez JM, Acebes JL (2014) Fourier transform mid infrared spectroscopy applications for monitoring the structural plasticity of plant cell walls. Front Plant Sci 5:303
Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166
Liu T-H, Nada K, Handa C, Kitashiba H, Wen X-P, Pan X-M, Moriguchi T (2006) Polyamine biosynthesis of apple callus under salts stress: importance of arginine decarboxylase pathway in stress response. J Exp Bot 57:2589–2599
Lovato MB, Martins PS (1997) Genetic variability in salt tolerance during germination of Stylosanthes humilis H.B.K. and association between salt tolerance and isozymes. Braz J Genet 20:25–33
Lovato MB, Martins PS, Lemos Filho JPD (1994) Germination in Stylosanthes humilis populations in the presence of NaCl. Aust J Bot 42:717–723
Lovato MB, Lemos Filho JP, Martins PS (1999) Growth responses of Stylosanthes humilis (Fabaceae) populations to saline stress. Environ Exp Bot 41:145–153
Ma H, Zhao J (2010) Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.). J Exp Bot 61:2647–2668
Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, Gilmartin PM, Mikkelsen J, Knox JP, Willats WGT (2004) Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant J 40:260–275
McCann MC, Shi J, Roberts K, Carpita NC (1994) Changes in pectin structure and localization during the growth of unadapted and NaCl-adapted tobacco-cells. Plant J 5:773–785
Mélida H, Largo-Gosens A, Novo-Uzal E, Santiago R, Pomar F, García P, García-Angulo P, Acebes JL, Alvarez J, Encina A (2015) Ectopic lignification in primary cellulose-deficient cell walls of maize cell suspension cultures. J Integr Plant Biol 57:357–372
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Muszynska A, Jarocka K, Kurczynska EU (2014) Plasma membrane and cell wall properties of an aspen hybrid (Populus tremula x tremuloides) parenchyma cells under the influence of salt stress. Acta Physiol Plant 36:1155–1165
Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I (2003) CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiol 131:1450–1459
Partridge I (1996) Protecting stylos from anthracnose. Rural Res 171:29–32
Pennell RI, Knox JP, Scofield GN, Selvendran R, Roberts K (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108:1967–1977
Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3:1371–1376
Pesqueira J, Garcia MD, Staltari S, Molina MC (2006) NaCl effects in Zea mays L. x Tripsacum dactyloides (L.) L. hybrid calli and plants. Electron J Biotechnol 9:286–290
Quecini VM, Alves AC, Oliveira CA, Aragao FJL, Rech EL, Almeida ERP, Gander ES, Vieira MLC (2006) Microparticle bombardment of Stylosanthes guianensis: transformation parameters and expression of a methionine-rich 2S albumin gene. Plant Cell Tissue Organ Cult 87:167–179
Sabba RP, Durso NA, Vaughn KC (1999) Structural and immunocytochemical characterization of the walls of DCB-habituated BY-2 tobacco cells. Int J Plant Sci 160:272–290
Saeman JF, Moore WE, Millet MA (1963) Sugar units present. In: Whistler RL (ed) Methods in carbohydrate chemistry, vol 3., CelluloseAcademic Press, New York, pp 54–69
Sahi C, Singh A, Kumar K, Blumwald E, Grover A (2006) Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genom 6:263–284
Serra AA, Couée I, Renault D, Gouesbet G, Sulmon C (2015) Metabolic profiling of Lolium perenne shows functional integration of metabolic responses to diverse subtoxic conditions of chemical stress. J Exp Bot 66:1801–1816
Shedletzky E, Shmuel M, Delmer DP, Lamport DTA (1990) Adaptation and growth of tomato cells on the herbicide 2,6-dichlorobenzonitrile leads to production of unique cell walls virtually lacking a cellulose-xyloglucan network. Plant Physiol 94:980–987
Shedletzky E, Shmuel M, Trainin T, Kalman S, Delmer DP (1992) Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile. Plant Physiol 100:120–130
Shen H, Mazarei M, Hisano H et al (2013) A genomics approach to deciphering lignin biosynthesis in switchgrass. Plant Cell 25:4342–4361
Silva PO, Medina EF, Barros RS, Ribeiro DM (2014) Germination of salt-stressed seeds as related to the ethylene biosynthesis ability in three Stylosanthes species. J Plant Physiol 171:14–22
Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP (1996) Immunochemical comparison of membrane associated and secreted arabinogalactan proteins in rice and carrot. Planta 198:452–459
Talmadge KM, Keegstra K, Bauer WD, Albersheim P (1973) The structure of plant cell walls I. The macromolecular components of the walls of suspension-cultured sycamore cells with a detailed analyses of the pectic polysaccharides. Plant Physiol 51:159–173
Tan L, Showalter AM, Egelund J, Hernandez-Sanchez A, Doblin MS, Bacic A (2012) Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front Plant Sci 3:140
Uddin MN, Hanstein S, Leubner R, Schubert S (2013) Leaf cell-wall components as influenced in the first phase of salt stress in three maize (Zea mays L.) hybrids differing in salt resistance. J Agro Crop Sci 199:405–415
Updegraff DM (1969) Semi-micro determination of cellulose in biological materials. Anal Biochem 32:420–424
Valarini MJ, Otsuk IP, Vieira MLC (1997) Changes in N2 fixation in Stylosanthes scabra derived from tissue culture. Braz J Genet 20:713–716
von Hedenström H, Breckle S-W (1974) Obligate halophytes? A test with tissue culture methods. Z Pflanzenphysiol 4:183–185
Wells B, McCann MC, Shedletzky E, Delmer D, Roberts K (1994) Structural features of cell walls from tomato cells adapted to grow on the herbicide 2,6-dichlorobenzonitrile. J Microsc 173:155–164
Willats WGT, Steele-King CG, Marcus SE, Knox JP (1999) Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J 20:619–628
Wilson RH, Smith AC, Kacuráková M, Saunders PK, Wellner N, Waldron KW (2000) The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol 124:397–405
Zagorchev L, Kamenova P, Odjakova M (2014) The role of plant cell wall proteins in response to salt stress. Sci World J ID 764089. doi:10.1155/2014/764089
Zhong H, Läuchli A (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44:773–778
Acknowledgments
The authors thank Dr. Antonio Encina for his helpful scientific discussion and Denise P for his help with the English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuentes, L., Domínguez, A., Pérez, Y. et al. Monitoring of cell wall modifications during callogenesis in Stylosanthes guianensis (Fabaceae) under salt stress conditions. Braz. J. Bot 38, 783–793 (2015). https://doi.org/10.1007/s40415-015-0196-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-015-0196-0